Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer
Abstract
:1. Introduction
2. Lymph Node Assessment in Endometrial Cancer
3. Is Lymphadenectomy Therapeutic?
4. Sentinel Lymph Node Mapping in Endometrial Cancer
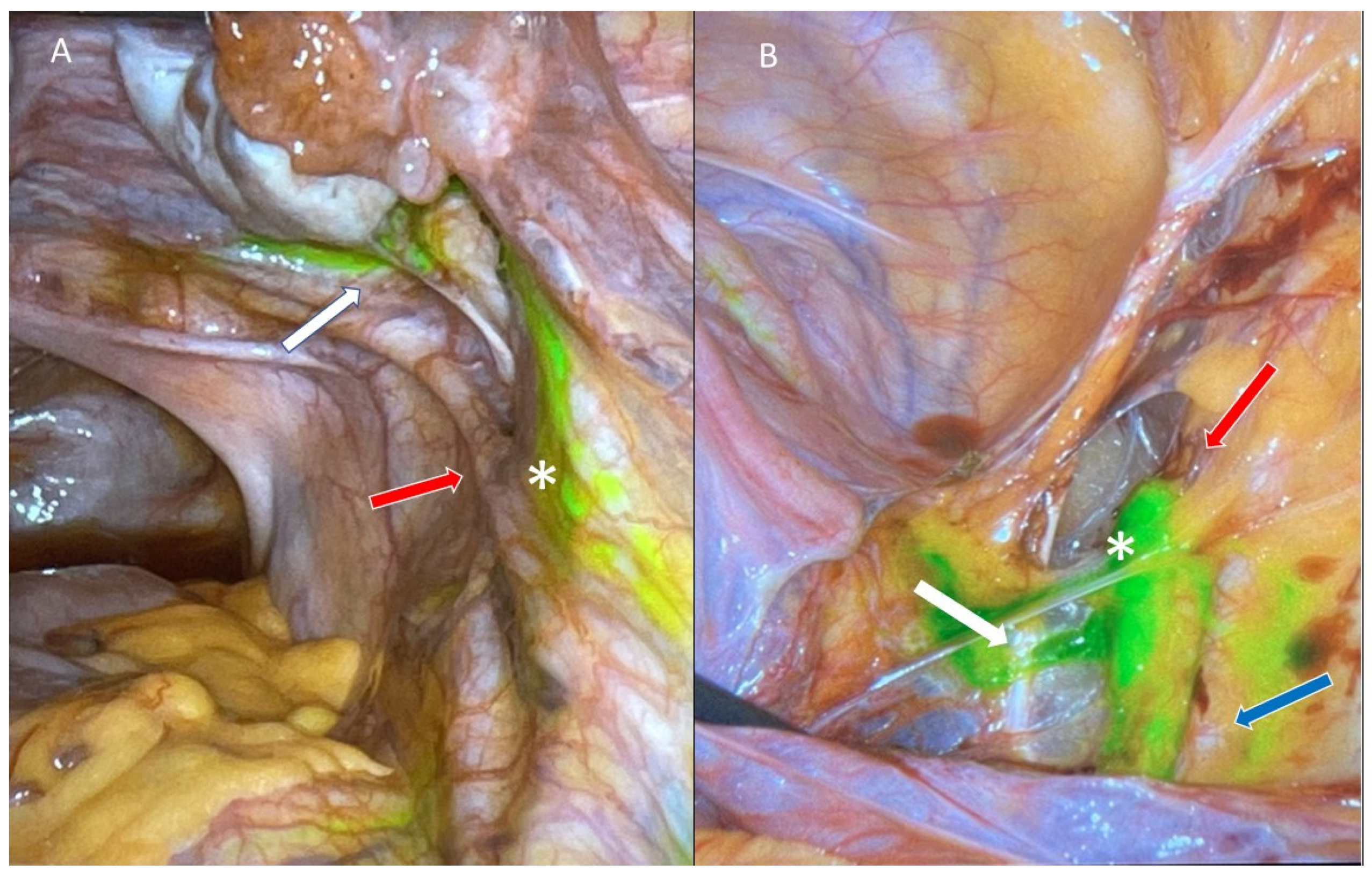
4.1. Injection Sites and Tracers
4.2. Sentinel Lymph Node Algorithm and Ultra-Staging
4.3. Benefits of Sentinel Lymph Node Mapping
4.4. Detection Rate and Sensitivity
5. Accuracy of Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer
5.1. Sentinel Lymph Node in High-Grade Histology
5.2. Isolated Para-Aortic Lymph Node Metastases
5.3. Oncologic Outcome following Sentinel Lymph Node Mapping
6. Sentinel Lymph Mapping in the Era of Molecular Classification
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanis, P.J.; Nieweg, O.E.; Olmos, R.A.V.; Rutgers, E.J.T.; Kroon, B.B.R. History of sentinel node and validation of the technique. Breast Cancer Res. 2001, 3, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Abu-Rustum, N.R. Sentinel Lymph Node Mapping for Endometrial Cancer. Pelvic Cancer Surg. Mod. Break. Futur. Adv. 2015, 12, 305–313. [Google Scholar] [CrossRef]
- Glaser, G.; Dinoi, G.; Multinu, F.; Yost, K.; Al Hilli, M.; Larish, A.; Kumar, A.; McGree, M.; Weaver, A.L.; Cheville, A.; et al. Reduced lymphedema after sentinel lymph node biopsy versus lymphadenectomy for endometrial cancer. Int. J. Gynecol. Cancer 2021, 31, 85–91. [Google Scholar] [CrossRef]
- Swart, A.M. The Writing Committee ASTEC Study Group. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Van Der Zee, A.G.J.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.; Baldwin, P.J.; Van Dorst, E.B.; et al. Sentinel Node Dissection Is Safe in the Treatment of Early-Stage Vulvar Cancer. J. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef]
- Gould, E.A.; Winship, T.; Philbin, P.H.; Kerr, H.H. Observations on a “sentinel node” in cancer of the parotid. Cancer 1960, 13, 77–78. [Google Scholar] [CrossRef]
- Cabanas, R.M. An approach for the treatment of penile carcinoma. Cancer 1977, 39, 456–466. [Google Scholar] [CrossRef]
- Kim, T.; Giuliano, A.E.; Lyman, G.H. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: A metaanalysis. Cancer 2006, 106, 4–16. [Google Scholar] [CrossRef]
- Valsecchi, M.E.; Silbermins, D.; de Rosa, N.; Wong, S.L.; Lyman, G.H. Lymphatic Mapping and Sentinel Lymph Node Biopsy in Patients with Melanoma: A Meta-Analysis. J. Clin. Oncol. 2011, 29, 1479–1487. [Google Scholar] [CrossRef]
- Levenback, C.F.; Ali, S.; Coleman, R.L.; Gold, M.A.; Fowler, J.M.; Judson, P.L.; Bell, M.C.; De Geest, K.; Spirtos, N.M.; Potkul, R.K.; et al. Lymphatic Mapping and Sentinel Lymph Node Biopsy in Women with Squamous Cell Carcinoma of the Vulva: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2012, 30, 3786–3791. [Google Scholar] [CrossRef]
- Krag, D.N.; Anderson, S.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Costantino, J.P.; Ashikaga, T.; Weaver, D.L.; Mamounas, E.P.; Jalovec, L.M.; et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010, 11, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Burke, T.W.; Levenback, C.; Tornos, C.; Morris, M.; Wharton, J.; Gershenson, D.M. Intraabdominal Lymphatic Mapping to Direct Selective Pelvic and Paraaortic Lymphadenectomy in Women with High-Risk Endometrial Cancer: Results of a Pilot Study. Gynecol. Oncol. 1996, 62, 169–173. [Google Scholar] [CrossRef]
- Hauspy, J.; Beiner, M.; Harley, I.; Ehrlich, L.; Rasty, G.; Covens, A. Sentinel lymph nodes in early stage cervical cancer. Gynecol. Oncol. 2007, 105, 285–290. [Google Scholar] [CrossRef]
- De Cicco, C.; Sideri, M.; Bartolomei, M.; Grana, C.; Cremonesi, M.; Fiorenza, M.; Maggioni, A.; Bocciolone, L.; Mangioni, C.; Colombo, N.; et al. Sentinel node biopsy in early vulvar cancer. Br. J. Cancer 2000, 82, 295–299. [Google Scholar] [CrossRef]
- De Hullu, J.A.; Hollema, H.; Piers, D.; Verheijen, R.M.; Van Diest, P.; Mourits, M.E.; Aalders, J.; Van Der Zee, A.J. Sentinel Lymph Node Procedure Is Highly Accurate in Squamous Cell Carcinoma of the Vulva. J. Clin. Oncol. 2000, 18, 2811–2816. [Google Scholar] [CrossRef]
- Oonk, M.H.M.; Slomovitz, B.; Baldwin, P.J.W.; van Doorn, H.C.; van der Velden, J.; de Hullu, J.A.; Gaarenstroom, K.N.; Slangen, B.F.M.; Vergote, I.; Brännström, M.; et al. Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients with Micrometastases in the Sentinel Node: Results of GROINSS-V II. J. Clin. Oncol. 2021, 39, 3623–3632. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Koh, W.-J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 170–199. [Google Scholar] [CrossRef] [Green Version]
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Lowery, W.J.; Rossi, E.C.; Tanner, E.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415. [Google Scholar] [CrossRef]
- Ballester, M.; Dubernard, G.; Lécuru, F.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011, 12, 469–476. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Soliman, P.T.; Westin, S.N.; Dioun, S.; Sun, C.C.; Euscher, E.; Munsell, M.F.; Fleming, N.D.; Levenback, C.; Frumovitz, M.; Ramirez, P.T.; et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol. Oncol. 2017, 146, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)—The final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.C.; Vicus, D.; Pulman, K.; Maganti, M.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Hogen, L.F.; Covens, A.L.; et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg. 2021, 156, 157–164. [Google Scholar] [CrossRef]
- Creasman, W.T.; Kohler, M.F.; Odicino, F.; Maisonneuve, P.; Boyle, P. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol. Oncol. 2004, 95, 593–596. [Google Scholar] [CrossRef]
- Felix, A.S.; Brinton, L.A. Cancer Progress and Priorities: Uterine Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 985–994. [Google Scholar] [CrossRef] [Green Version]
- Canadian Cancer Society’s Advisory and Committee on Cancer Statistics. Canadian Cancer Statistics. 2021, pp. 10–144. Available online: https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2021-statistics/2021-pdf-en-final.pdf?rev=2b9d2be7a2d34c1dab6a01c6b0a6a32d&hash=01DE85401DBF0217F8B64F2B7DF43986 (accessed on 30 January 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Iasonos, A.; Zhou, Q.; Oke, E.; Soslow, R.; Alektiar, K.M.; Chi, D.S.; Barakat, R.R. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am. J. Obstet. Gynecol. 2008, 198, 457.e1–457.e6. [Google Scholar] [CrossRef]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. Cancer 1987, 60, 2035–2041. Available online: http://incan-mexico.org/wp_ginecologia/wp-content/uploads/GOG-33.pdf (accessed on 30 January 2022). [CrossRef]
- Randall, M.E.; Filiaci, V.; Muss, H.; Spirtos, N.M.; Mannel, R.S.; Fowler, J.; Thigpen, J.T.; Benda, J.A. Randomized Phase III Trial of Whole-Abdominal Irradiation Versus Doxorubicin and Cisplatin Chemotherapy in Advanced Endometrial Carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 36–44. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. FIGO Cancer Report Cancer of the corpus uteri. Int. J. Gynecol. Obstet. 2018, 143 (Suppl. 2), 37–50. [Google Scholar] [CrossRef] [Green Version]
- Panici, P.B.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic Pelvic Lymphadenectomy vs No Lymphadenectomy in Early-Stage Endometrial Carcinoma: Randomized Clinical Trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Nayyar, N.; Lakhwani, P.; Goel, A.; Pande, P.K.; Kumar, K. The Futility of Systematic Lymphadenectomy in Early-Stage Low-grade Endometrial Cancer. Indian J. Surg. Oncol. 2018, 9, 204–210. [Google Scholar] [CrossRef]
- Rozenholc, A.; Samouelian, V.; Warkus, T.; Gauthier, P.; Provencher, D.; Gauthier, F.; Drakopoulos, P.; Cormier, B. Green versus blue: Randomized controlled trial comparing indocyanine green with methylene blue for sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2019, 153, 500–504. [Google Scholar] [CrossRef]
- Niikura, H.; Kaiho-Sakuma, M.; Tokunaga, H.; Toyoshima, M.; Utsunomiya, H.; Nagase, S.; Takano, T.; Watanabe, M.; Ito, K.; Yaegashi, N. Tracer injection sites and combinations for sentinel lymph node detection in patients with endometrial cancer. Gynecol. Oncol. 2013, 131, 299–303. [Google Scholar] [CrossRef]
- Rossi, E.C.; Jackson, A.; Ivanova, A.; Boggess, J.F. Detection of Sentinel Nodes for Endometrial Cancer with Robotic Assisted Fluorescence Imaging: Cervical Versus Hysteroscopic Injection. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2013, 23, 1704–1711. [Google Scholar] [CrossRef]
- Nagar, H.; Wietek, N.; Goodall, R.J.; Hughes, W.; Schmidt-Hansen, M.; Morrison, J. Sentinel node biopsy for diagnosis of lymph node involvement in endometrial cancer. Cochrane Database Syst. Rev. 2021, 2021, CD013021. [Google Scholar] [CrossRef]
- Rossi, E.C. Current state of sentinel lymph nodes for women with endometrial cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2019, 29, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, S.; Barahona-Orpinell, M.; Almansa-González, C.; Padilla-Iserte, P.; Bebia, V.; Martí, L.; Tejerizo-García, Á.; Domingo, S.; Gil-Moreno, A. Combined use of ICG and technetium does not improve sentinel lymph node detection in endometrial cancer: Results of the COMBITEC study. Gynecol. Oncol. 2021, 162, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Wang, X.; Jiang, J.; Chen, L. Sentinel lymph node mapping in high-risk endometrial cancer: A systematic review and meta-analysis. Gland Surg. 2020, 9, 2091–2105. [Google Scholar] [CrossRef] [PubMed]
- Bodurtha Smith, A.J.; Fader, A.N.; Tanner, E.J. Sentinel lymph node assessment in endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 459–476.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- How, J.A.; Patrick, O.; Zainab, A.; Susie, L.; Shannon, S.; Emily, H. Sentinel lymph node mapping in endometrial cancer: A systematic review and meta-analysis. Minerva Ginecol. 2018, 70, 194–214. [Google Scholar] [CrossRef]
- Barlin, J.N.; Khoury-Collado, F.; Kim, C.; Leitao, M.M.; Chi, D.S.; Sonoda, Y.; Alektiar, K.; DeLair, D.F.; Barakat, R.R.; Abu-Rustum, N.R. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol. Oncol. 2012, 125, 531–535. [Google Scholar] [CrossRef]
- Leitao, M.M.J.; Khoury-Collado, F.; Gardner, G.; Sonoda, Y.; Brown, C.; Alektiar, K.; Hensley, M.; Soslow, R.; Barakat, R.; Abu-Rustum, N. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol. Oncol. 2013, 129, 38–41. [Google Scholar] [CrossRef]
- Kim, C.H.; Soslow, R.A.; Park, K.; Barber, E.L.; Khoury-Collado, F.; Barlin, J.N.; Sonoda, Y.; Hensley, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Pathologic Ultrastaging Improves Micrometastasis Detection in Sentinel Lymph Nodes During Endometrial Cancer Staging. Int. J. Gynecol. Cancer 2013, 23, 964–970. [Google Scholar] [CrossRef]
- Burg, L.C.; Hengeveld, E.M.; Bulten, J.; Bult, P.; Zusterzeel, P.L.M. Ultrastaging methods of sentinel lymph nodes in endometrial cancer—A systematic review. Int. J. Gynecol. Cancer 2021, 31, 647–655. [Google Scholar] [CrossRef]
- Helgers, R.J.A.; Winkens, B.; Slangen, B.F.M.; Werner, H.M. Lymphedema and Post-Operative Complications after Sentinel Lymph Node Biopsy versus Lymphadenectomy in Endometrial Carcinomas—A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 10, 120. [Google Scholar] [CrossRef]
- Accorsi, G.S.; Paiva, L.L.; Schmidt, R.; Vieira, M.; Reis, R.; Andrade, C. Sentinel Lymph Node Mapping vs Systematic Lymphadenectomy for Endometrial Cancer: Surgical Morbidity and Lymphatic Complications. J. Minim. Invasive Gynecol. 2020, 27, 938–945.e2. [Google Scholar] [CrossRef] [PubMed]
- Geppert, B.; Lönnerfors, C.; Bollino, M.; Persson, J. Sentinel lymph node biopsy in endometrial cancer—Feasibility, safety and lymphatic complications. Gynecol. Oncol. 2018, 148, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M.J.; Zhou, Q.C.; Gomez-Hidalgo, N.R.; Iasonos, A.; Baser, R.; Mezzancello, M.; Chang, K.; Ward, J.; Chi, D.S.; Roche, K.L.; et al. Patient-reported outcomes after surgery for endometrial carcinoma: Prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol. Oncol. 2020, 156, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, G.F.; Giuliano, A.E.; Veronesi, U.; Consensus Conference Committee. Proceedings of the Consensus Conference on the role of sentinel lymph node biopsy in carcinoma of the breast, 19–22 April 2001, Philadelphia, Pnsylvania. Cancer 2002, 94, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Khoury-Collado, F.; Glaser, G.E.; Zivanovic, O.; Sonoda, Y.; Levine, D.A.; Chi, D.S.; Gemignani, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecol. Oncol. 2009, 115, 453–455. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Walker, R.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Murphy, J.; Rosen, B.; Covens, A.; Clarke, B.; et al. Implementing a Cervical Sentinel Lymph Node Biopsy Program: Quality Improvement in Gynaecologic Oncology. J. Obstet. Gynaecol. Canada JOGC = J. D’obstetrique Gynecol. Canada JOGC 2017, 39, 659–667. [Google Scholar] [CrossRef]
- Cibula, D.; Oonk, M.H.M.; Abu-Rustum, N.R. Sentinel lymph node biopsy in the management of gynecologic cancer. Curr. Opin. Obstet. Gynecol. 2015, 27, 66–72. [Google Scholar] [CrossRef]
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; et al. A Randomized Comparison of Sentinel-Node Biopsy with Routine Axillary Dissection in Breast Cancer. N. Engl. J. Med. 2003, 349, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Ballester, M.; Dubernard, G.; Rouzier, R.; Barranger, E.; Darai, E. Use of the Sentinel Node Procedure to Stage Endometrial Cancer. Ann. Surg. Oncol. 2008, 15, 1523–1529. [Google Scholar] [CrossRef]
- Lopes, L.A.F.; Nicolau, S.; Baracat, F.F.; Baracat, E.C.; Goncalves, W.J.; Santos, H.V.B.; Lopes, R.G.; Lippi, U.G. Sentinel lymph node in endometrial cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2007, 17, 1113–1117. [Google Scholar] [CrossRef]
- Frumovitz, M.; Bodurka, D.; Broaddus, R.R.; Coleman, R.L.; Sood, A.K.; Gershenson, D.M.; Burke, T.W.; Levenback, C.F. Lymphatic mapping and sentinel node biopsy in women with high-risk endometrial cancer. Gynecol. Oncol. 2007, 104, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.W.; Ahmad, S.; Kendrick, J.E.; Bigsby, G.E.; Brudie, L.A.; Ghurani, G.B.; Stavitzski, N.M.; Gise, J.L.; Ingersoll, S.B.; Pepe, J.W. A Prospective Cohort Study Comparing Colorimetric and Fluorescent Imaging for Sentinel Lymph Node Mapping in Endometrial Cancer. Ann. Surg. Oncol. 2017, 24, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Klar, M.; Khetan, V.U.; Violette, C.J.; Nusbaum, D.J.; Muderspach, L.I.; Roman, L.D.; Wright, J.D. Sentinel lymph node biopsy for stage II endometrial cancer: Recent utilization and outcome in the United States. Gynecol. Oncol. 2022, 164, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Davidson, B.; Fadare, O.; Carlson, J.; Crum, C.P.; Gilks, C.B.; Irving, J.A.; Malpica, A.; Matias-Guiu, X.; McCluggage, W.G.; et al. High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int. J. Gynecol. Pathol. 2019, 38, S40–S63. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Tschernichovsky, R.; Diver, E.J.; Schorge, J.O.; Goodman, A. The Role of Lymphadenectomy Versus Sentinel Lymph Node Biopsy in Early-stage Endometrial Cancer. Am. J. Clin. Oncol. Cancer Clin. Trials 2016, 39, 516–521. [Google Scholar] [CrossRef]
- Altman, A.D.; Ferguson, S.E.; Atenafu, E.; Köbel, M.; McAlpine, J.N.; Panzarella, T.; Lau, S.; Gien, L.T.; Gilks, B.; Clarke, B.; et al. Canadian high risk endometrial cancer (CHREC) consortium: Analyzing the clinical behavior of high risk endometrial cancers. Gynecol. Oncol. 2015, 139, 268–274. [Google Scholar] [CrossRef]
- Del Carmen, M.G.; Birrer, M.; Schorge, J.O. Uterine papillary serous cancer: A review of the literature. Gynecol. Oncol. 2012, 127, 651–661. [Google Scholar] [CrossRef]
- Tanner, E.J.; Ojalvo, L.; Stone, R.L.; Levinson, K.; Temkin, S.M.; Murdock, T.; Vang, R.; Sinno, A.; Fader, A.N. The Utility of Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer. Int. J. Gynecol. Cancer 2017, 27, 1416–1421. [Google Scholar] [CrossRef]
- Nasioudis, D.; Albright, B.B.; Roy, A.; Ko, E.M.; Giuntoli, R.L.; Haggerty, A.F.; Cory, L.; Kim, S.H.; Morgan, M.A.; Latif, N.A. Patterns of use and outcomes of sentinel lymph node mapping for patients with high-grade endometrial cancer. Gynecol. Oncol. 2020, 159, 732–736. [Google Scholar] [CrossRef]
- Marchocki, Z.; Cusimano, M.C.; Clarfield, L.; Kim, S.R.; Fazelzad, R.; Espin-Garcia, O.; Bouchard-Fortier, G.; Rossi, E.C.; Stewart, K.I.; Soliman, P.T.; et al. Sentinel lymph node biopsy in high-grade endometrial cancer: A systematic review and meta-analysis of performance characteristics. Am. J. Obstet. Gynecol. 2021, 225, 367.e1–367.e39. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Gomez, J.D.; Alektiar, K.M.; Soslow, R.; Hensley, M.L.; Leitao, M.M.; Gardner, G.J.; Sonoda, Y.; Chi, D.S.; Barakat, R.R. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol. Oncol. 2009, 115, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-J.; Kong, T.W.; Kim, W.Y.; Yoo, S.-C.; Yoon, J.-H.; Chang, K.-H.; Ryu, H.-S. Lymph-Vascular Space Invasion as a Significant Risk Factor for Isolated Para-aortic Lymph Node Metastasis in Endometrial Cancer: A Study of 203 Consecutive Patients. Ann. Surg. Oncol. 2011, 18, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Multinu, F.; Ducie, J.A.; Eriksson, A.G.Z.; Schlappe, B.A.; Cliby, W.A.; Glaser, G.E.; Grassi, T.; Keeney, G.L.; Weaver, A.L.; Abu-Rustum, N.R.; et al. Role of lymphadenectomy in endometrial cancer with nonbulky lymph node metastasis: Comparison of comprehensive surgical staging and sentinel lymph node algorithm. Gynecol. Oncol. 2019, 155, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Byrne, M.; Ko, E.M.; Ii, R.L.G.; Haggerty, A.F.; Cory, L.; Kim, S.H.; Morgan, M.A.; Latif, N.A. The impact of sentinel lymph node sampling versus traditional lymphadenectomy on the survival of patients with stage IIIC endometrial cancer. Int. J. Gynecol. Cancer 2021, 31, 840–845. [Google Scholar] [CrossRef]
- Kogan, L.; Matanes, E.; Wissing, M.; Mitric, C.; How, J.; Amajoud, Z.; Abitbol, J.; Yasmeen, A.; López-Ozuna, V.; Eisenberg, N.; et al. The added value of sentinel node mapping in endometrial cancer. Gynecol. Oncol. 2020, 158, 84–91. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit from Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Van den Heerik, A.S.V.M.; Horeweg, N.; Nout, R.A.; Lutgens, L.C.H.W.; Van Der Steen-Banasik, E.M.; Westerveld, G.H.; Berg, H.A.V.D.; Slot, A.; Koppe, F.L.A.; Kommoss, S.; et al. PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 2002–2007. [Google Scholar] [CrossRef]
- Obermair, A.; Nicklin, J.; Gebski, V.; Hayes, S.C.; Graves, N.; Mileshkin, L.; Lin, M.Y.; Beale, P.; Baxter, E.; Robledo, K.; et al. A phase III randomized clinical trial comparing sentinel node biopsy with no retroperitoneal node dissection in apparent early-stage endometrial cancer—ENDO-3: ANZGOG trial 1911/2020. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 1595–1601. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, L.; Cusimano, M.C.; Marchocki, Z.; Ferguson, S.E. Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer. Curr. Oncol. 2022, 29, 1123-1135. https://doi.org/10.3390/curroncol29020096
Salman L, Cusimano MC, Marchocki Z, Ferguson SE. Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer. Current Oncology. 2022; 29(2):1123-1135. https://doi.org/10.3390/curroncol29020096
Chicago/Turabian StyleSalman, Lina, Maria C. Cusimano, Zibi Marchocki, and Sarah E. Ferguson. 2022. "Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer" Current Oncology 29, no. 2: 1123-1135. https://doi.org/10.3390/curroncol29020096
APA StyleSalman, L., Cusimano, M. C., Marchocki, Z., & Ferguson, S. E. (2022). Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer. Current Oncology, 29(2), 1123-1135. https://doi.org/10.3390/curroncol29020096




