Influence of Tumor Site on Survival in Young Patients with Head and Neck Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Inclusion Criteria
2.2. Data Collection and Processing
2.3. p16 Status
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
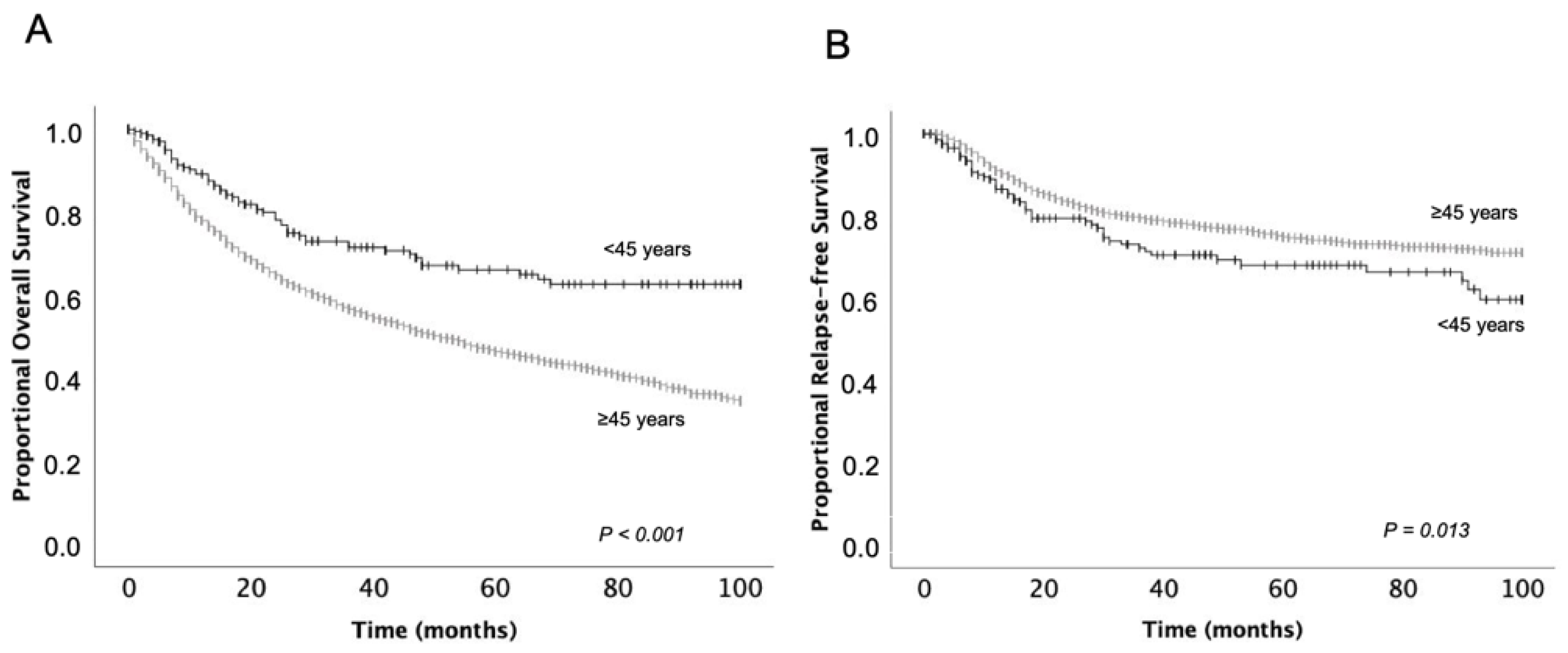
3.2. Survival Outcomes
3.3. Factors Affecting Survival in Patients under 45 Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Monsjou, H.S.; Wreesmann, V.B.; van den Brekel, M.W.; Balm, A.J. Head and neck squamous cell carcinoma in young patients. Oral Oncol. 2013, 49, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.M.; Ingarfield, K.; McMahon, A.D.; Savage, S.A.; Conway, D.I.; MacKenzie, K. Presenting symptoms and long-term survival in head and neck cancer. Clin. Otolaryngol. 2018, 43, 795–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.H.; Kapp, D.S. Oral cavity and oropharyngeal cancer in a younger population. Review of literature and experience at Yale. Cancer 1985, 55, 441–444. [Google Scholar] [CrossRef]
- Llewellyn, C.D.; Johnson, N.W.; Warnakulasuriya, K.A.A.S. Risk factors for squamous cell carcinoma of the oral cavity in young people—A comprehensive literature review. Oral Oncol. 2001, 37, 401–418. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.-L.; Liang, X.-H.; Tang, Y.-L. The etiologic spectrum of head and neck squamous cell carcinoma in young patients. Oncotarget 2016, 7, 66226–66238. [Google Scholar] [CrossRef] [Green Version]
- Shiboski, C.H.; Schmidt, B.L.; Jordan, R.C.K. Tongue and tonsil carcinoma. Cancer 2005, 103, 1843–1849. [Google Scholar] [CrossRef]
- Annertz, K.; Anderson, H.; Biörklund, A.; Möller, T.; Kantola, S.; Mork, J.; Olsen, J.H.; Wennerberg, J. Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. Int. J. Cancer 2002, 101, 95–99. [Google Scholar] [CrossRef]
- Singh, B.; Alfonso, A.; Sabin, S.; Poluri, A.; Shaha, A.R.; Sundaram, K.; Lucente, F.E. Outcome differences in younger and older patients with laryngeal cancer: A retrospective case-control study. Am. J. Otolaryngol. 2000, 21, 92–97. [Google Scholar] [CrossRef]
- Schantz, S.P.; Yu, G.-P. Head and Neck Cancer Incidence Trends in Young Americans, 1973-1997, With a Special Analysis for Tongue Cancer. Arch. Otolaryngol.-Head Neck Surg. 2002, 128, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Young, D.; Xiao, C.C.; Murphy, B.; Moore, M.; Fakhry, C.; Day, T.A. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol. 2015, 51, 727–730. [Google Scholar] [CrossRef]
- Trizna, Z.; Schantz, S.P. Hereditary and environmental factors associated with risk and progression of head and neck cancer. Otolaryngol. Clin. N. Am. 1992, 25, 1089–1103. [Google Scholar] [CrossRef]
- Lee, C.-C.; Ho, H.-C.; Chen, H.-L.; Hsiao, S.-H.; Hwang, J.-H.; Hung, S.-K. Squamous cell carcinoma of the oral tongue in young patients: A matched-pair analysis. Acta Oto-Laryngol. 2007, 127, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P.D.; Piccirillo, J.F.; Merritt, M.G.; Zequeira, M.R. Head and neck squamous cell carcinoma: Better to be young. Otolaryngol. –Head Neck Surg. 2000, 122, 253–258. [Google Scholar] [CrossRef]
- Pytynia, K.B.; Grant, J.R.; Etzel, C.J.; Roberts, D.; Wei, Q.; Sturgis, E.M. Matched Analysis of Survival in Patients With Squamous Cell Carcinoma of the Head and Neck Diagnosed Before and After 40 Years of Age. Arch. Otolaryngol.-Head Neck Surg. 2004, 130, 869–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilroy, J.S.; Morris, C.G.; Amdur, R.J.; Mendenhall, W.M. Impact of young age on prognosis for head and neck cancer: A matched-pair analysis. Head Neck 2005, 27, 269–273. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual; Springer International Publishing: Basel, Switzerland, 2018. [Google Scholar]
- Edge, S.B.; Byrd, D.R.; Carducci, M.A.; Compton, C.C.; Fritz, A.; Greene, F. AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2010; Volume 7. [Google Scholar]
- Shaw, R.; Beasley, N. Aetiology and risk factors for head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S9–S12. [Google Scholar] [CrossRef] [PubMed]
- Neckel, N.; Michael, M.; Troeltzsch, D.; Wüster, J.; Koerdt, S.; Doll, C.; Jöhrens, K.; Neumann, K.; Heiland, M.; Raguse, J.D. Rediscussing the Role of Traditional Risk Factors in Young Adults With Oral Squamous Cell Carcinoma. Anticancer. Res. 2020, 40, 6987–6995. [Google Scholar] [CrossRef]
- Harris, S.L.; Kimple, R.J.; Hayes, D.N.; Couch, M.E.; Rosenman, J.G. Never-smokers, never-drinkers: Unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck 2010, 32, 499–503. [Google Scholar] [CrossRef]
- Garavello, W.; Spreafico, R.; Gaini, R.M. Oral tongue cancer in young patients: A matched analysis. Oral Oncol. 2007, 43, 894–897. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Sanabria, A.; Carvalho, A.L.; Vartanian, J.G.; Magrin, J.; Ikeda, M.K.; Kowalski, L.P. Comorbidity is a prognostic factor in elderly patients with head and neck cancer. Ann. Surg. Oncol. 2007, 14, 1449–1457. [Google Scholar] [CrossRef]
- Tsukuda, M.; Ooishi, K.; Mochimatsu, I.; Sato, H. Head and Neck Carcinomas in Patients under the Age of Forty Years. Jpn. J. Cancer Res. 1993, 84, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Soudry, E.; Preis, M.; Hod, R.; Hamzany, Y.; Hadar, T.; Bahar, G.; Strenov, Y.; Shpitzer, T. Squamous cell carcinoma of the oral tongue in patients younger than 30 years: Clinicopathologic features and outcome. Clin. Otolaryngol. 2010, 35, 307–312. [Google Scholar] [CrossRef]
- Mafi, N.; Kadivar, M.; Hosseini, N.; Ahmadi, S.; Zare-Mirzaie, A. Head and neck squamous cell carcinoma in Iranian patients and risk factors in young adults: A fifteen-year study. Asian Pac. J. Cancer Prev. 2012, 13, 3373–3378. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, S.; Yue, K.; Wang, X.-D. The recurrence and survival of oral squamous cell carcinoma: A report of 275 cases. Chin. J. Cancer 2013, 32, 614–618. [Google Scholar] [CrossRef] [Green Version]
- Guibert, M.; Lepage, B.; Woisard, V.; Rives, M.; Serrano, E.; Vergez, S. Quality of life in patients treated for advanced hypopharyngeal or laryngeal cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.; Yang, X.; Song, X.; Yan, L. Radiotherapy vs surgery for T1-2N0M0 laryngeal squamous cell carcinoma: A population-based and propensity score matching study. Cancer Med. 2018, 7, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; le Maître, A.; Bourhis, J. Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, S112–S114. [Google Scholar] [CrossRef]
- Pointreau, Y.; Garaud, P.; Chapet, S.; Sire, C.; Tuchais, C.; Tortochaux, J.; Faivre, S.; Guerrif, S.; Alfonsi, M.; Calais, G. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J. Natl. Cancer Inst. 2009, 101, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Petersen, J.F.; Timmermans, A.J.; van Dijk, B.A.C.; Overbeek, L.I.H.; Smit, L.A.; Hilgers, F.J.M.; Stuiver, M.M.; van den Brekel, M.W.M. Trends in treatment, incidence and survival of hypopharynx cancer: A 20-year population-based study in the Netherlands. Eur. Arch. Otorhinolaryngol. 2018, 275, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Lee, R. Surgery vs. radiotherapy for locally advanced hypopharyngeal cancer in the contemporary era: A population-based study. Cancer Med. 2018, 7, 5889–5900. [Google Scholar] [CrossRef] [Green Version]
- Eckel, H.E.; Bradley, P.J. Treatment Options for Hypopharyngeal Cancer. Adv. Otorhinolaryngol. 2019, 83, 47–53. [Google Scholar] [CrossRef]
- Ayan, I.; Kaytan, E.; Ayan, N. Childhood nasopharyngeal carcinoma: From biology to treatment. Lancet Oncol. 2003, 4, 13–21. [Google Scholar] [CrossRef]
- Qiu, W.Z.; Peng, X.S.; Xia, H.Q.; Huang, P.Y.; Guo, X.; Cao, K.J. A retrospective study comparing the outcomes and toxicities of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy for the treatment of children and adolescent nasopharyngeal carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 1563–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claude, L.; Jouglar, E.; Duverge, L.; Orbach, D. Update in pediatric nasopharyngeal undifferentiated carcinoma. Br. J. Radiol. 2019, 92, 20190107. [Google Scholar] [CrossRef] [PubMed]
- Deschler, D.G.; Richmon, J.D.; Khariwala, S.S.; Ferris, R.L.; Wang, M.B. The “New” Head and Neck Cancer Patient—Young, Nonsmoker, Nondrinker, and HPV Positive: Evaluation. Otolaryngol.-Head Neck Surg. 2014, 151, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [Green Version]
| Variable | Age < 45 n (%) | Age ≥ 45 n (%) | p |
|---|---|---|---|
| Median age (range) | 41.0 (14–44) | 64.0 (45–106) | |
| All patients | 215 (4.8) | 4251 (95.2) | |
| Females | 68 (31.6) | 1154 (27.1) | |
| Males | 147 (68.4) | 3097 (72.9) | |
| Distribution of sex | 0.150 | ||
| Tumor site | |||
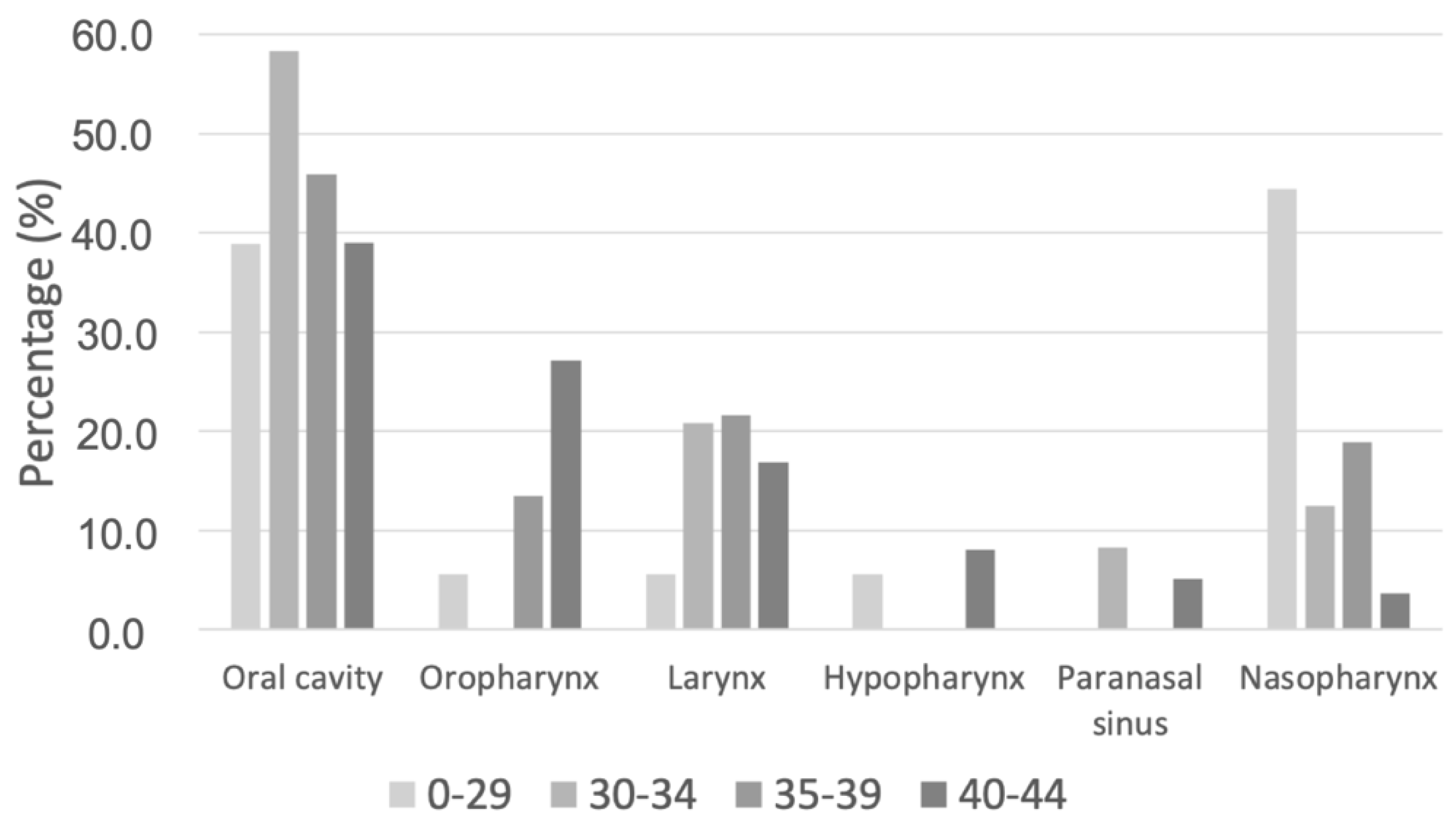
| Oropharynx | 43 (20.0) | 1344 (31.6) | |
| Oral cavity | 91 (42.3) | 1568 (36.9) | |
| Larynx | 37 (17.2) | 766 (18.0) | |
| Hypopharynx | 12 (5.6) | 354 (8.3) | |
| Paranasal sinus | 9 (4.2) | 142 (3.3) | |
| Nasopharynx | 23 (10.7) | 77 (1.8) | |
| Distribution of tumor site | <0.001 | ||
| Disease stage | |||
| AJCC I/II | 73 (36.7) | 754 (26.5) | |
| AJCC III/IV | 126 (63.3) | 2090 (73.5) | |
| Distribution of disease stages | 0.002 |
| Variable | Overall Survival | Relapse-Free Survival | ||||
|---|---|---|---|---|---|---|
| Mean OS age <45 (months, SD) | Mean OS age ≥45 (months, SD) | p | Mean RFS age <45 (months, SD) | Mean RFS age ≥45 (months, SD) | p | |
| All patients | 72.1 (3.0) | 55.1 (0.7) | <0.001 | 73.3 (3.1) | 79.5 (0.7) | 0.013 |
| Females | 72.4 (5.6) | 57.8 (1.3) | 0.016 | 67.2 (6.0) | 79.6 (1.4) | 0.015 |
| Males | 71.9 (3.5) | 54.1 (0.8) | <0.001 | 75.7 (3.6) | 79.5 (0.8) | 0.172 |
| Tumor site | ||||||
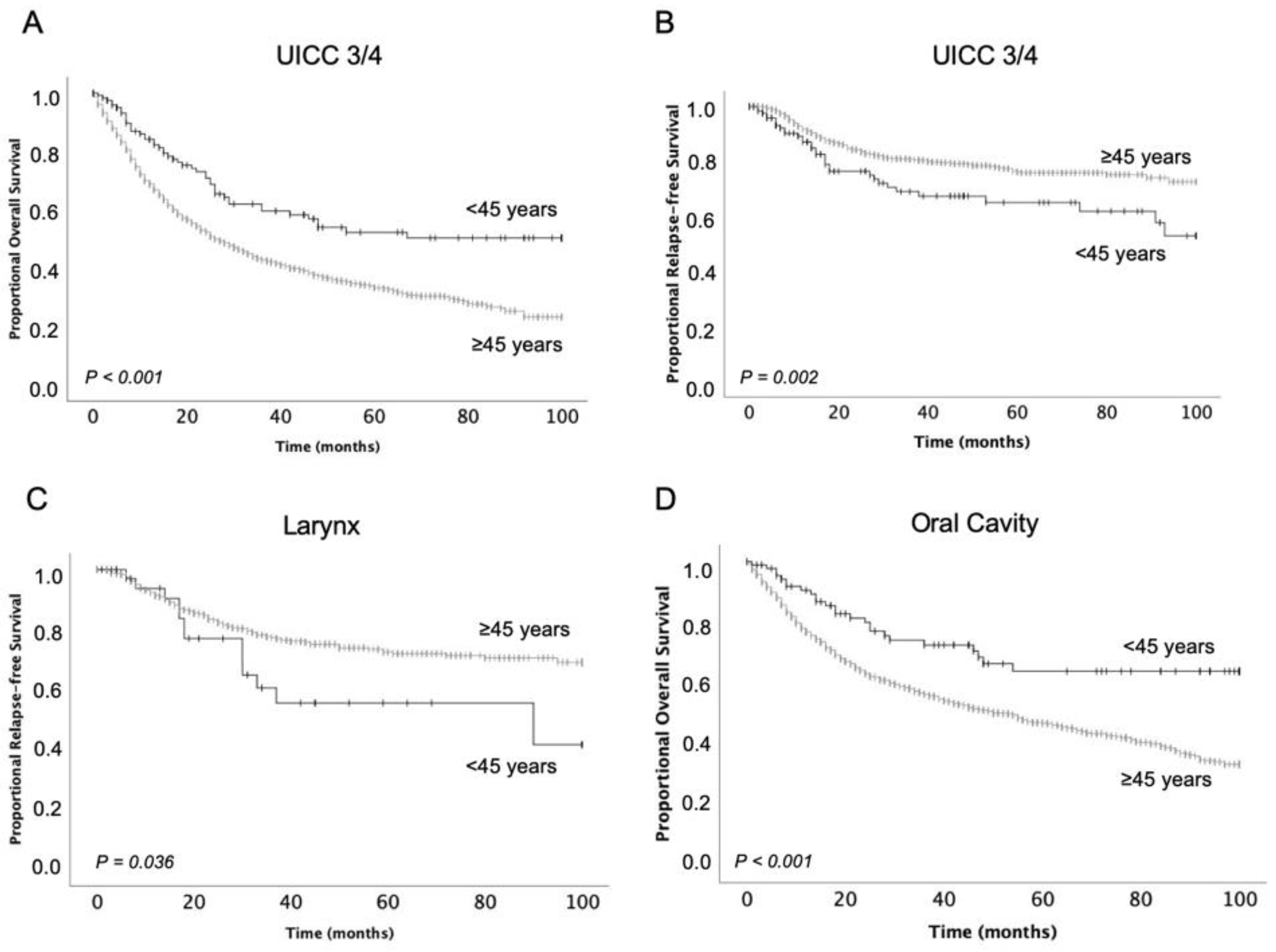
| Oropharynx | 75.4 (6.3) | 55.2 (1.3) | 0.006 | 84.9 (5.5) | 83.7 (1.2) | 0.900 |
| Oral cavity | 72.8 (4.1) | 54.7 (1.1) | <0.001 | 70.3 (4.8) | 76.8 (1.2) | 0.122 |
| Larynx | 72.3 (6.8) | 64.3 (1.6) | 0.308 | 64.3 (7.4) | 78.1 (1.7) | 0.036 |
| Hypopharynx | 29.8 (9.2) | 37.6 (2.1) | 0.624 | 54.2 (15.5) | 82.1 (2.6) | 0.065 |
| Paranasal sinus | 61.0 (13.5) | 56.6 (4.1) | 0.525 | 36.0 (13.0) | 72.6 (4.3) | 0.487 |
| Nasopharynx | 89.7 (6.9) | 53.8 (5.2) | 0.008 | 87.3 (7.7) | 88.9 (4.2) | 0.979 |
| Disease stage | ||||||
| AJCC I/II | 91.2 (3.4) | 67.6 (1.8) | <0.001 | 75.8 (5.0) | 74.8 (1.8) | 0.918 |
| AJCC III/IV | 61.8 (4.1) | 43.4 (1.0) | <0.001 | 70.2 (4.3) | 81.1 (1.1) | 0.002 |
| Variable | All | Age < 45 | Age ≥ 45 | p |
|---|---|---|---|---|
| Larynx + UICC 1/2 | 502 | 21 | 481 | 0.459 |
| Larynx + UICC 3/4 | 301 | 16 | 285 |
| Variable | 0–29 Years n (%) | 30–34 Years n (%) | 35–39 Years n (%) | 40–44 Years n (%) | p (Age Groups vs. Age Groups) | Univariate Analysis, P, HR (CI 95%) (Variable vs. Other Matching Variables Combining Age Groups) | Multivariate Analysis (Variable vs. Other Matching Variables Combining Age Groups), P, HR (CI 95%) |
|---|---|---|---|---|---|---|---|
| All patients, n (%) | 18 (8.4%) | 24 (11.2%) | 37 (17.2%) | 136 (63.3%) | |||
| Mean OS (months) | 100 | 68.4 (10.3) | 70.8 (7.0) | 70.1 (3.7) | 0.146 | ||
| Mean RFS (months) | 80.0 (9.4) | 73.1 (10.4) | 63.5 (7.4) | 76.4 (3.7) | 0.315 | ||
| Females, n (%) | 6 (33.3%) | 17 (70.8%) | 11 (29.7%) | 34 (25.0%) | <0.001 | ||
| Mean OS (months) | 100 | 68.0 (11.4) | 38.2 (4.2) | 72.2 (7.7) | 0.615 | 0.985 | |
| Mean RFS (months) | 54.7 (21.5) | 74.1 (11.1) | 26.2 (5.0) | 76.1 (7.6) | 0.132 | 0.245 | |
| Males, n (%) | 12 (66.7%) | 7 (29.2%) | 26 (70.3%) | 102 (75.0%) | <0.001 | ||
| Mean OS (months) | 100 | 38.0 (7.1) | 72.4 (7.8) | 69.3 (4.3) | 0.291 | 0.985 | |
| Mean RFS (months) | 91.8 (7.8) | 37.0 (9.0) | 68.1 (8.4) | 76.8 (4.3) | 0.491 | 0.245 | |
| Oropharynx, n (%) | 1 (5.6%) | 0 (0.0%) | 5 (13.5%) | 37 (27.2%) | 0.003 | ||
| Mean OS (months) | 100 | N/A | 69.0 (12.5) | 76.1 (6.8) | 0.938 | 0.617 | |
| Mean RFS (months) | 100 | N/A | 74.4 (14.0) | 85.5 (5.9) | 0.718 | 0.101 | |
| Oral cavity, n (%) | 7 (38.9%) | 14 (58.3%) | 17 (45.9%) | 53 (39.0%) | 0.330 | ||
| Mean OS (months) | 100 | 49.0 (13.3) | 75.3 (10.4) | 73.8 (5.6) | 0.075 | 0.760 | |
| Mean RFS (months) | 64.7 (16.1) | 66.3 (13.9) | 58.8 (10.6) | 77.0 (5.7) | 0.385 | 0.440 | |
| Larynx, n (%) | 1 (5.6%) | 5 (20.8%) | 8 (21.6%) | 23 (16.9%) | 0.484 | ||
| Mean OS (months) | 100 | 100 | 37.3 (6.1) | 72.0 (8.6) | 0.359 | 0.975 | |
| Mean RFS (months) | 90 (0.0) | 44.7 (6.0) | 23.2 (3.8) | 68.4 (9.2) | 0.331 | 0.190 | |
| Hypopharynx, n (%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 11 (8.1%) | 0.156 | ||
| Mean OS (months) | 100 | N/A | N/A | 28.4 (9.1) | 0.489 | <0.001 | 0.011, 2.933 (1.285–6.692) |
| Mean RFS (months) | 100 | N/A | N/A | 52.4 (15.7) | 0.606 | 0.333 | |
| Paranasal sinus, n (%) | 0 (0.0%) | 2 (8.3%) | 0 (0.0%) | 7 (5.1%) | 0.290 | ||
| Mean OS (months) | N/A | 100 | N/A | 57.6 (15.6) | 0.617 | 0.868 | |
| Mean RFS (months) | N/A | 100 | N/A | 27.3 (14.2) | 0.362 | 0.385 | |
| Nasopharynx, n (%) | 8 (44.4%) | 3 (12.5%) | 7 (18.9%) | 5 (3.7%) | <0.001 | ||
| Mean OS (months) | 100 | 100 | 84.5 (14.1) | 43.0 (11.4) | 0.473 | 0.112 | |
| Mean RFS (months) | 87.6 (11.1) | 100 | 74 (0.0) | 100 | 0.823 | 0.108 | |
| Surgery only, n (%) | 8 (47.1%) | 11 (45.8%) | 11 (29.7%) | 42 (32.3%) | 0.363 | ||
| Mean OS (months) | 100 | 89.1 (10.1) | 92.5 (7.1) | 86.5 (5.0) | 0.972 | <0.001 | 0.489 |
| Mean RFS (months) | 67.8 (14.1) | 75.1 (14.7) | 67.7 (13.0) | 76.3 (6.2) | 0.672 | 0.817 | |
| R(C)T + surgery, n (%) | 1 (5.9%) | 9 (37.5%) | 8 (21.6%) | 38 (29.2%) | 0.108 | ||
| Mean OS (months) | 100 | 27.1 (6.7) | 71.1 (9.0) | 76.8 (6.2) | N/A | 0.985 | |
| Mean RFS (months) | 100 | 31.1 (7.6) | 61.3 (13.1) | 79.6 (6.3) | 0.491 | 0.613 | |
| RCT, n (%) | 8 (47.1%) | 3 (12.5%) | 16 (43.2%) | 45 (34.6%) | 0.056 | ||
| Mean OS (months) | 100 | 36.3 (9.5) | 67.7 (11.3) | 52.0 (7.3) | 0.090 | 0.005 | 0.577 |
| Mean RFS (months) | 87.6 (11.1) | 100 | 57.1 (8.8) | 74.5 (7.6) | 0.380 | 0.799 | |
| T-classification 1–2, n (%) | 4 (25.0%) | 3 (14.3%) | 18 (50.0%) | 64 (48.9%) | 0.009 | ||
| Mean OS (months) | 100 | 64.0 (25.5) | 73.7 (9.7) | 53.9 (6.0) | 0.265 | <0.001 | 0.951 |
| Mean RFS (months) | 100 | 71.7 (23.1) | 51.2 (8.5) | 70.7 (6.3) | 0.537 | 0.164 | |
| T-classification 3–4, n (%) | 12 (75.0%) | 18 (85.7%) | 18 (50.0%) | 67 (51.1%) | 0.009 | ||
| Mean OS (months) | 100 | 38.4 (5.4) | 66.4 (10.2) | 85.1 (4.1) | 0.050 | <0.001 | 0.951 |
| Mean RFS (months) | 66.7 (11.9) | 45.2 (4.4) | 67.3 (10.6) | 79.7 (4.7) | 0.388 | 0.164 | |
| N-classification 0, n (%) | 8 (50.0%) | 8 (44.4%) | 15 (44.1%) | 53 (41.4%) | 0.922 | ||
| Mean OS (months) | 100 | 81.0 (16.5) | 80.7 (9.9) | 83.7 (4.9) | 0.700 | 0.001 | 0.930 |
| Mean RFS (months) | 67.8 (14.1) | 77.5 (19.5) | 57.2 (11.4) | 75.9 (5.7) | 0.312 | 0.623 | |
| N-classification >1, n (%) | 8 (50.0%) | 10 (55.6%) | 19 (55.9%) | 75 (58.6%) | 0.922 | ||
| Mean OS (months) | 100 | 25.8 (6.1) | 61.5 (8.1) | 62.8 (5.2) | 0.260 | 0.001 | 0.930 |
| Mean RFS (months) | 74.0 (14.7) | 35.8 (7.4) | 57.6 (9.3) | 76.7 (5.1) | 0.569 | 0.623 | |
| Grading (G) 1, n (%) | 2 (16.7%) | 1 (5.0%) | 2 (6.3%) | 7 (6.0%) | 0.422 | ||
| Mean OS (months) | 100 | 6.0 (0.0) | 100 | 100 | 0.019 | 0.139 | |
| Mean RFS (months) | 100 | 6.0 (0.0) | 74.0 (0.0) | 84.3 (14.3) | 0.146 | 0.662 | |
| Grading (G) 2, n (%) | 8 (66.7%) | 15 (75.0%) | 19 (59.4%) | 82 (70.7%) | 0.397 | ||
| Mean OS (months) | 100 | 66.4 (13.0) | 75.7 (9.1) | 65.8 (4.8) | 0.313 | 0.413 | |
| Mean RFS (months) | 67.0 (17.1) | 69.3 (14.3) | 67.1 (9.8) | 75.1 (4.9) | 0.578 | 0.762 | |
| Grading (G) 3, n (%) | 2 (16.7%) | 4 (20.0%) | 11 (34.4%) | 27 (23.3%) | 0.352 | ||
| Mean OS (months) | 100 | 100 | 42.0 (5.8) | 68.9 (8.9) | 0.796 | 0.969 | |
| Mean RFS (months) | 100 | 37.0 (9.5) | 35.0 (6.5) | 72.7 (9.0) | 0.879 | 0.532 | |
| AJCC I/II, n (%) | 10 (62.5%) | 11 (50.0%) | 10 (30.3%) | 42 (32.8%) | 0.053 | ||
| Mean OS (months) | 100 | 89.1 (10.1) | 91.8 (7.8) | 91.1 (4.2) | 0.829 | <0.001 | 0.007, 5.563 (1.590–19.462) |
| Mean RFS (months) | 71.4 (12.3) | 87.1 (11.9) | 64.5 (13.8) | 78.5 (6.2) | 0.397 | 0.396 | |
| AJCC III/IV, N (%) | 6 (37.5%) | 11 (50.0%) | 23 (69.7%) | 86 (67.2%) | 0.053 | ||
| Mean OS (months) | 100 | 29.6 (6.1) | 63.0 (8.8) | 60.6 (5.0) | 0.421 | <0.001 | 0.007, 5.563 (1.590–19.462) |
| Mean RFS (months) | 74.0 (14.7) | 37.7 (6.4) | 50.4 (7.7) | 74.3 (5.0) | 0.347 | 0.396 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffen, C.; Piwonski, I.; Heiland, M.; Stromberger, C.; Kofla, G.; Doll, C.; Coordes, A.; Beck-Broichsitter, B. Influence of Tumor Site on Survival in Young Patients with Head and Neck Squamous Cell Carcinoma. Curr. Oncol. 2022, 29, 969-980. https://doi.org/10.3390/curroncol29020082
Steffen C, Piwonski I, Heiland M, Stromberger C, Kofla G, Doll C, Coordes A, Beck-Broichsitter B. Influence of Tumor Site on Survival in Young Patients with Head and Neck Squamous Cell Carcinoma. Current Oncology. 2022; 29(2):969-980. https://doi.org/10.3390/curroncol29020082
Chicago/Turabian StyleSteffen, Claudius, Iris Piwonski, Max Heiland, Carmen Stromberger, Grzegorz Kofla, Christian Doll, Annekatrin Coordes, and Benedicta Beck-Broichsitter. 2022. "Influence of Tumor Site on Survival in Young Patients with Head and Neck Squamous Cell Carcinoma" Current Oncology 29, no. 2: 969-980. https://doi.org/10.3390/curroncol29020082
APA StyleSteffen, C., Piwonski, I., Heiland, M., Stromberger, C., Kofla, G., Doll, C., Coordes, A., & Beck-Broichsitter, B. (2022). Influence of Tumor Site on Survival in Young Patients with Head and Neck Squamous Cell Carcinoma. Current Oncology, 29(2), 969-980. https://doi.org/10.3390/curroncol29020082






