Diffusion-Weighted MRI in Patients with Testicular Tumors—Intra- and Interobserver Variability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI
2.3. Observers
2.4. Statistical Analysis
3. Results
3.1. Interobserver Agreement (between Two Observers)
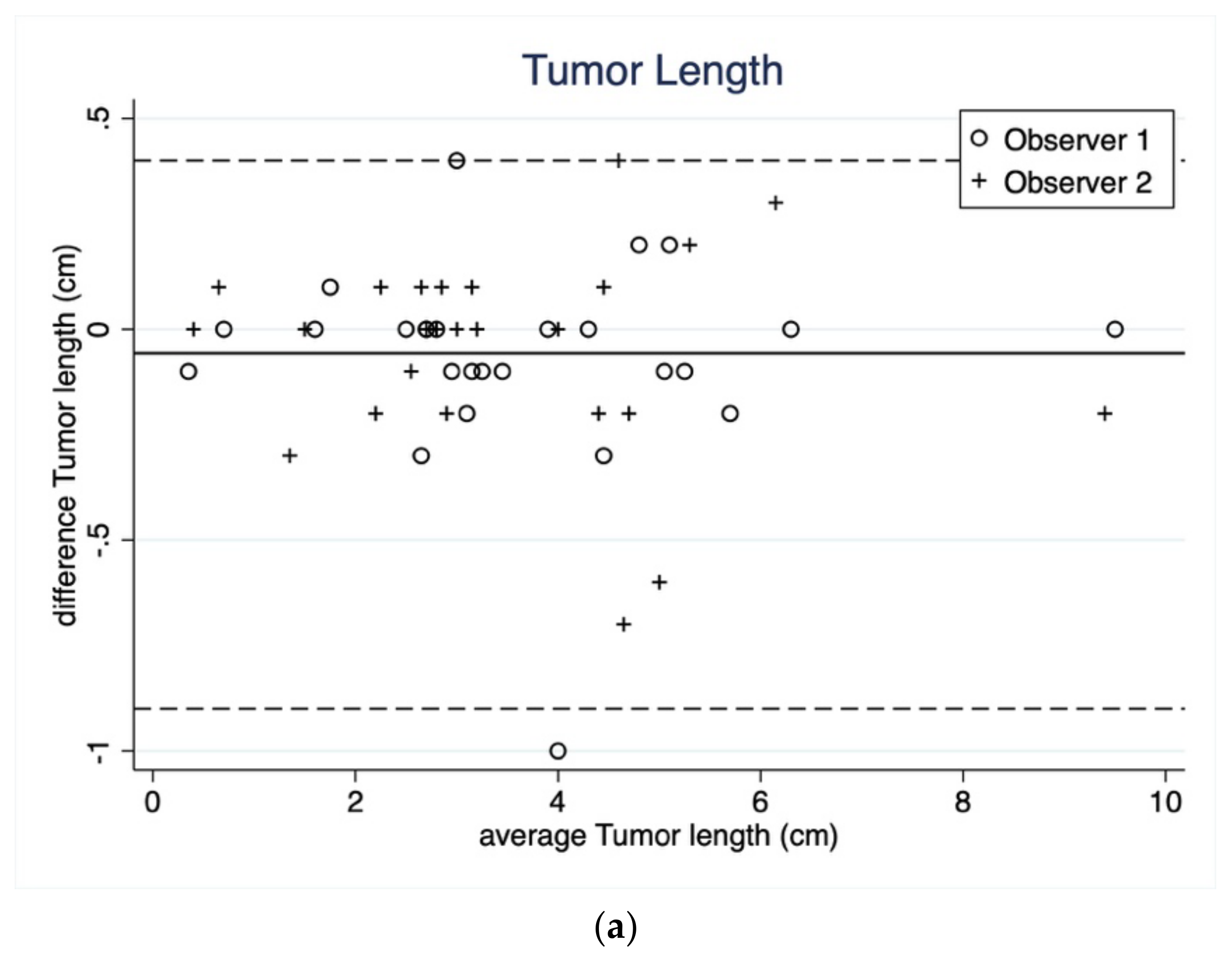
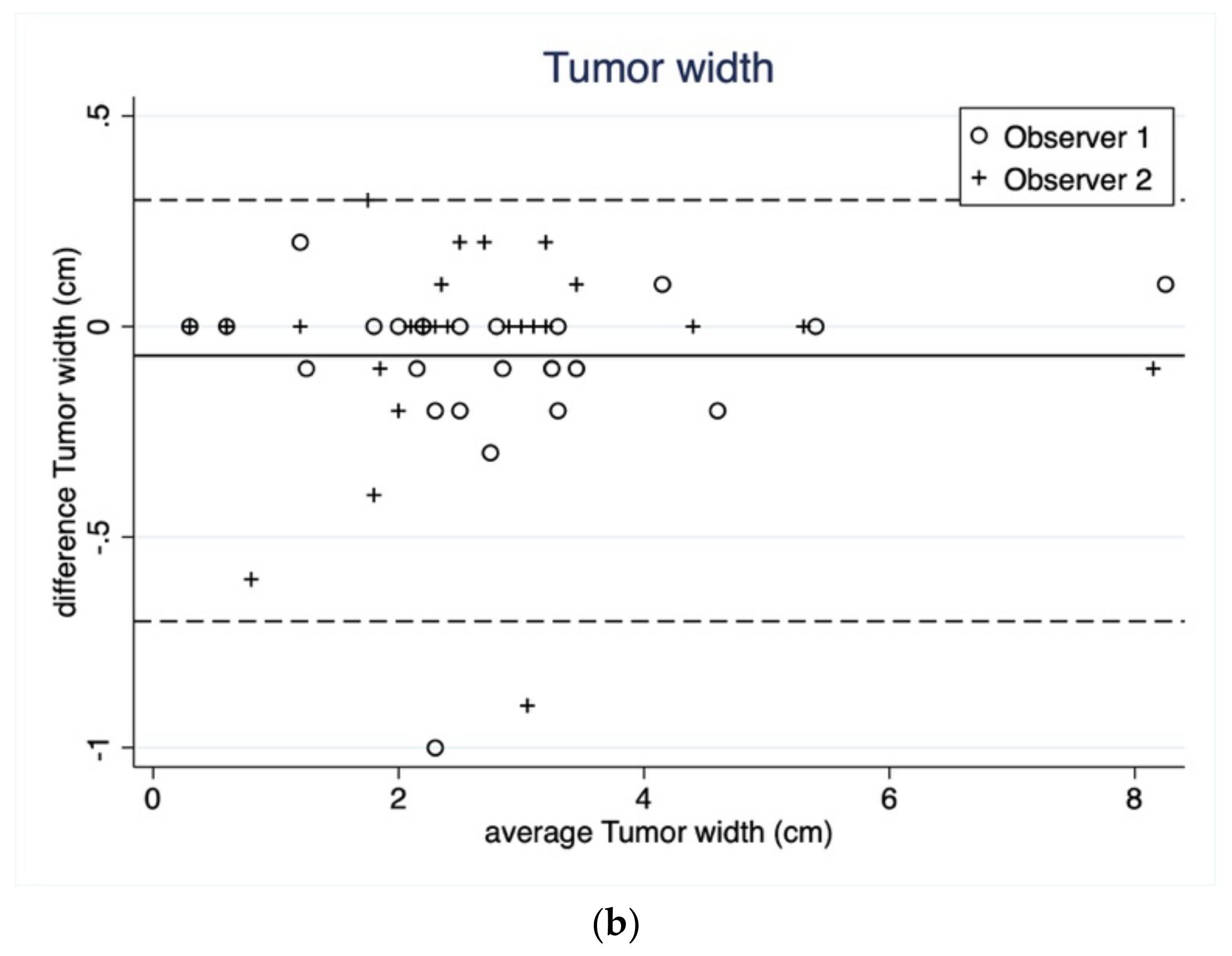
3.1.1. Tumor Length and Width
3.1.2. Whole, Round, Small ROI
3.2. Intraobserver Agreement (within Observers)
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen, M.R.; Osther, P.J.; Nissen, H.D.; Vedsted, P.; Møller, H.; Rafaelsen, S.R. Elastography and diffusion-weighted MRI in patients with testicular microlithiasis, normal testicular tissue, and testicular cancer: An observational study. Acta Radiol. 2019, 60, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Schueller-Weidekamm, C.; Kaserer, K.; Schueller, G.; Scheuba, C.; Ringl, H.; Weber, M.; Czerny, C.; Herneth, A.M. Can Quantitative Diffusion-Weighted MR Imaging Differentiate Benign and Malignant Cold Thyroid Nodules? Initial Results in 25 Patients. Am. J. Neuroradiol. 2009, 30, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Nerad, E.; Pizzi, A.D.; Lambregts, D.M.J.; Maas, M.; Wadhwani, S.; Bakers, F.C.H.; Bosch, H.C.M.V.D.; Beets-Tan, R.G.H.; Lahaye, M.J. The Apparent Diffusion Coefficient (ADC) is a useful biomarker in predicting metastatic colon cancer using the ADC-value of the primary tumor. PLoS ONE 2019, 14, e0211830. [Google Scholar] [CrossRef] [PubMed]
- Wichtmann, B.D.; Zöllner, F.G.; Attenberger, U.I.; Schönberg, S.O. Multiparametric MRI in the Diagnosis of Prostate Cancer: Physical Foundations, Limitations, and Prospective Advances of Diffusion-Weighted MRI. Rofo 2021, 193, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Nissan, N.; Rahbar, H.; Kitsch, A.E.; Sigmund, E.E. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging 2017, 45, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Menias, C.O.; Quazi, R.; Hara, A.K.; Verma, S.; Shaaban, A.; Siegel, C.L.; Radmanesh, A.; Sandrasegaran, K. MR Imaging of the Penis and Scrotum. Radiographics 2015, 35, 1033–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wibmer, A.; Vargas, H. Imaging of Testicuolar and Scrotal Masses: The Essentials; Hodler, J., Kubik-Huch, R., von Schulthess, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Parenti, G.C.; Feletti, F.; Brandini, F.; Palmarini, D.; Zago, S.; Ginevra, A.; Campioni, P.; Mannella, P. Imaging of the scrotum: Role of MRI. Radiol. Med. 2009, 114, 414–424. [Google Scholar] [CrossRef]
- Maki, D.; Watanabe, Y.; Nagayama, M.; Ishimori, T.; Okumura, A.; Amoh, Y.; Nakashita, S.; Terai, A.; Dodo, Y. Diffusion-weighted magnetic resonance imaging in the detection of testicular torsion: Feasibility study. J. Magn. Reson. Imaging 2011, 34, 1137–1142. [Google Scholar] [CrossRef]
- Kantarci, M.; Doganay, S.; Yalcin, A.; Aksoy, Y.; Yilmaz-cankaya, B.; Salman, B. Diagnostic performance of diffusion-Weighted MRI in the detection of nonpalpable undescended testes: Comparison with conventional MRI and surgical findings. Am. J. Roentgenol. 2010, 195, W268–W273. [Google Scholar] [CrossRef]
- Emad-Eldin, S.; Salim, A.M.A.; Wahba, M.; Elahwany, A.; Abdelaziz, O. The use of diffusion-weighted MR imaging in the functional assessment of the testes of patients with clinical varicocele. Andrologia 2019, 51, e13197. [Google Scholar] [CrossRef]
- Tsili, A.C.; Bertolotto, M.; Turgut, A.T.; Dogra, V.; Freeman, S.; Rocher, L.; Belfield, J.; Studniarek, M.; Ntorkou, A.; Derchi, L.E.; et al. MRI of the scrotum: Recommendations of the ESUR scrotal and penile imaging working group. Eur. Radiol. 2018, 28, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Tsili, A.C.; Argyropoulou, M.I.; Dolciami, M.; Ercolani, G.; Catalano, C.; Manganaro, L. When to ask for an MRI of the scrotum. Andrology 2021, 9, 1395–1409. [Google Scholar] [CrossRef] [PubMed]
- Tsili, A.C.; Ntorkou, A.; Astrakas, L.; Xydis, V.; Tsampalas, S.; Sofikitis, N.; Argyropoulou, M.I. Diffusion-weighted magnetic resonance imaging in the characterization of testicular germ cell neoplasms: Effect of ROI methods on apparent diffusion coefficient values and interobserver variability. Eur. J. Radiol. 2017, 89, 1–6. [Google Scholar] [CrossRef]
- Pedersen, M.R.; Graumann, O.; Hørlyck, A.; Duus, L.A.; Jørgensen, M.M.B.; Vagn-Hansen, C.; Holst, R.; Rafaelsen, S.R. Inter- and intraobserver agreement in detection of testicular microlithiasis with ultrasonography. Acta Radiol. 2016, 57, 767–772. [Google Scholar] [CrossRef]
- Sorokin, I.; Welliver, C.; Elebyjian, L.; Feustel, P.J.; McCullough, A. Interinstitutional variability in testicular volumes and varicocele presence by ultrasound: Surprising discrepancies and implications for clinical decision making. Urology 2015, 85, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Welliver, C.; Cardona-Grau, D.; Elebyjian, L.; Feustel, P.J.; Kogan, B.A. Surprising interobserver and intra-observer variability in pediatric testicular ultrasound volumes. J. Pediatr. Urol. 2019, 15, 386.e1–386.e6. [Google Scholar] [CrossRef] [PubMed]
- Lung, P.F.C.; Fang, C.; Jaffer, O.S.; Deganello, A.; Shah, A.; Hedayati, V.; Obaro, A.; Yusuf, G.T.; Huang, D.Y.; Sellars, M.E.; et al. Vascularity of Intra-testicular Lesions: Inter-observer Variation in the Assessment of Non-neoplastic Versus Neoplastic Abnormalities After Vascular Enhancement With Contrast-Enhanced Ultrasound. Ultrasound Med. Biol. 2020, 46, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, B.; Larsen, O.B. The Danish Pathology Register. Scand. J. Public Health 2011, 39, 72–74. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, C.B. The Danish civil registration system. Scand. J. Public Health 2011, 39, 22–25. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Tsili, A.C.; Ntorkou, A.; Baltogiannis, D.; Goussia, A.; Astrakas, L.G.; Malamou-Mitsi, V.; Sofikitis, N.; Argyropoulou, M.I. The role of apparent diffusion coefficient values in detecting testicular intraepithelial neoplasia: Preliminary results. Eur. J. Radiol. 2015, 84, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, G.; Sivrioglu, A.K.; Velioglu, M.; Incedayi, M.; Soydan, H.; Ateş, F.; Saglam, M.; Kara, K. Optimized imaging techniques for testicular masses: Fast and with high accuracy. Wien. Klin. Wochenschr. 2012, 124, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Tsili, A.C.; Argyropoulou, M.I.; Giannakis, D.; Tsampalas, S.; Sofikitis, N.; Tsampoulas, K. Diffusion-weighted MR imaging of normal and abnormal scrotum: Preliminary results. Asian J. Androl. 2012, 14, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algebally, A.M.; Tantawy, H.I.; Yousef, R.R.; Szmigielski, W.; Darweesh, A. Advantage of adding diffusion weighted imaging to routine MRI examinations in the diagnostics of scrotal lesions. Pol. J. Radiol. 2015, 80, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Mussi, T.C.; Yamauchi, F.I.; Tridente, C.F.; Tachibana, A.; Tonso, V.M.; Recchimuzzi, D.Z.; Leão, L.R.S.; Luz, D.C.; Martins, T.; Baroni, R.H. Interobserver agreement of PI-RADS v. 2 lexicon among radiologists with different levels of experience. J. Magn. Reson. Imaging 2020, 51, 593–602. [Google Scholar] [CrossRef]
- Ye, Z.; Fang, J.; Dai, S.; Xie, T.; Wang, F.; Wang, Z.; Li, K.; Fu, Z.; Wang, Y. Inter- and intra-observer reproducibility of ADC measurements in esophageal carcinoma primary tumors. Oncotarget 2017, 8, 92880–92889. [Google Scholar] [CrossRef] [Green Version]
- Clauser, P.; Marcon, M.; Maieron, M.; Zuiani, C.; Bazzocchi, M.; Baltzer, P.A.T. Is there a systematic bias of apparent diffusion coefficient (ADC) measurements of the breast if measured on different workstations? An inter- and intra-reader agreement study. Eur. Radiol. 2016, 26, 2291–2296. [Google Scholar] [CrossRef]
- Lambregts, D.M.J.; Beets, G.L.; Maas, M.; Curvo-Semedo, L.; Kessels, A.G.H.; Thywissen, T.; Beets-Tan, R.G.H. Tumour ADC measurements in rectal cancer: Effect of ROI methods on ADC values and interobserver variability. Eur. Radiol. 2011, 21, 2567–2574. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Guo, X.; Liu, L.; Zhan, Q.; Li, J.; Zhu, C.; Wang, L.; Zhang, J.; Fang, X.; Qu, J.; et al. Effect of region of interest size on ADC measurements in pancreatic adenocarcinoma. Cancer Imaging 2017, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Inoue, C.; Fujii, S.; Kaneda, S.; Fukunaga, T.; Kaminou, T.; Kigawa, J.; Harada, T.; Ogawa, T. Apparent diffusion coefficient (ADC) measurement in endometrial carcinoma: Effect of region of interest methods on ADC values. J. Magn. Reson. Imaging 2014, 40, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Ma, C.; Wang, Z.; Liu, J.-L.; Rui, Y.-P.; Li, Y.-H.; Peng, Y.-F. Effect of region of interest on ADC and interobserver variability in thyroid nodules. BMC Med. Imaging 2019, 19, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priola, A.M.; Priola, S.M.; Gned, D.; Giraudo, M.T.; Brundu, M.; Righi, L.; Veltri, A. Diffusion-weighted quantitative MRI of pleural abnormalities: Intra- and interobserver variability in the apparent diffusion coefficient measurements. J. Magn. Reson. Imaging 2017, 46, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Kuhnke, M.; Langner, S.; Khaw, A.V.; Angermaier, A.; Hosten, N.; Kirsch, M. Diffusion-weighted MRI—How many B-values are necessary? Rofo 2012, 184, 303–310. [Google Scholar] [CrossRef] [PubMed]



| MRI Sequence | TR (ms) | TE (ms) | FOV (mm2) | Matrix | Spacing (mm) | Slice (mm) |
|---|---|---|---|---|---|---|
| Axial T1 | 650 | 10 | 110 × 110 | 140 × 137 | 0.9 | 2 |
| Axial T2 | 2593 | 100 | 130 × 130 | 164 × 162 | 0.9 | 2 |
| Axial DWI | 3224 | 108 | 200 × 200 | 124 × 122 | 0.4 | 3 |
| Parameters | Whole Volume ROI | Round ROI | Small ROIs | ||||
|---|---|---|---|---|---|---|---|
| Observer 1 | Observer 2 | Observer 1 | Observer 2 | Observer 1 | Observer 2 | ||
| Reading 1 | ADC | 0.860 | 0.862 | 0.859 | 0.899 | 0.842 | 0.862 |
| Q1–Q3 | 0.764–1.111 | 0.740–1.081 | 0.708–1.135 | 0.759–1.016 | 0.688–1.013 | 0.728–1.016 | |
| Range | 0.590–1.554 | 0.600–1.552 | 0.579–1.545 | 0.592–1.549 | 0.575–1.469 | 0.586–1.518 | |
| Reading 2 | ADC | 0.870 | 0.841 | 0.894 | 0.873 | 0.855 | 0.851 |
| Q1–Q3 | 0.754–1.021 | 0.750–1.003 | 0.709–1.071 | 0.717–1.074 | 0.686–0.917 | 0.733–1.042 | |
| Range | 0.601–1.574 | 0.613–1.552 | 0.581–1.660 | 0.603–1.585 | 0.561–1.411 | 0.611–1.555 | |
| Observer 1 | Observer 2 | |||
|---|---|---|---|---|
| Length (cm) | Reading 1 | Reading 2 | Reading 1 | Reading 2 |
| Median | 3.3 | 3.2 | 3.1 | 3.1 |
| Q1–Q3 | 2.7–4.7 | 2.7–.4.8 | 2.6–4.5 | 2.6–4.5 |
| Range | 0.4–9.5 | 0.3–9.5 | 0.4–9.5 | 0.4–9.5 |
| Width (cm) | Reading 1 | Reading 2 | Reading 1 | Reading 2 |
| Median | 2.8 | 2.6 | 2.4 | 2.5 |
| Q1–Q3 | 2.2–3.4 | 2.0–3.3 | 2.0–3.1 | 1.9–3.1 |
| Range | 0.3–8.2 | 0.3–8.3 | 0.3–8.2 | 0.3–8.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedersen, M.R.V.; Loft, M.K.; Dam, C.; Rasmussen, L.Æ.L.; Timm, S. Diffusion-Weighted MRI in Patients with Testicular Tumors—Intra- and Interobserver Variability. Curr. Oncol. 2022, 29, 837-847. https://doi.org/10.3390/curroncol29020071
Pedersen MRV, Loft MK, Dam C, Rasmussen LÆL, Timm S. Diffusion-Weighted MRI in Patients with Testicular Tumors—Intra- and Interobserver Variability. Current Oncology. 2022; 29(2):837-847. https://doi.org/10.3390/curroncol29020071
Chicago/Turabian StylePedersen, Malene Roland Vils, Martina Kastrup Loft, Claus Dam, Lone Ærenlund Lohmann Rasmussen, and Signe Timm. 2022. "Diffusion-Weighted MRI in Patients with Testicular Tumors—Intra- and Interobserver Variability" Current Oncology 29, no. 2: 837-847. https://doi.org/10.3390/curroncol29020071
APA StylePedersen, M. R. V., Loft, M. K., Dam, C., Rasmussen, L. Æ. L., & Timm, S. (2022). Diffusion-Weighted MRI in Patients with Testicular Tumors—Intra- and Interobserver Variability. Current Oncology, 29(2), 837-847. https://doi.org/10.3390/curroncol29020071






