Clinical Outcomes and Prognosis Analysis of Younger Bladder Cancer Patients
Abstract
:1. Introduction
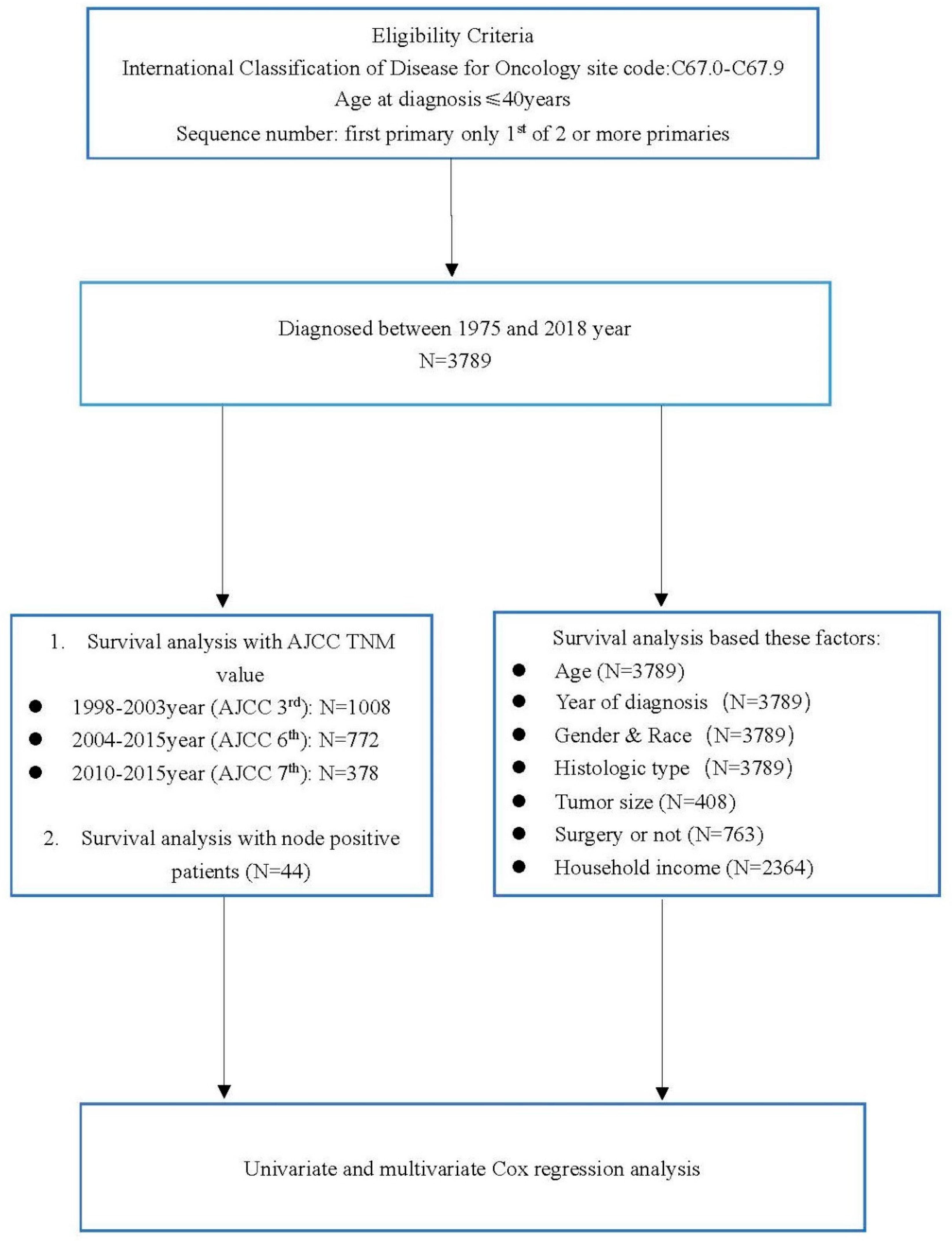
2. Patients and Methods
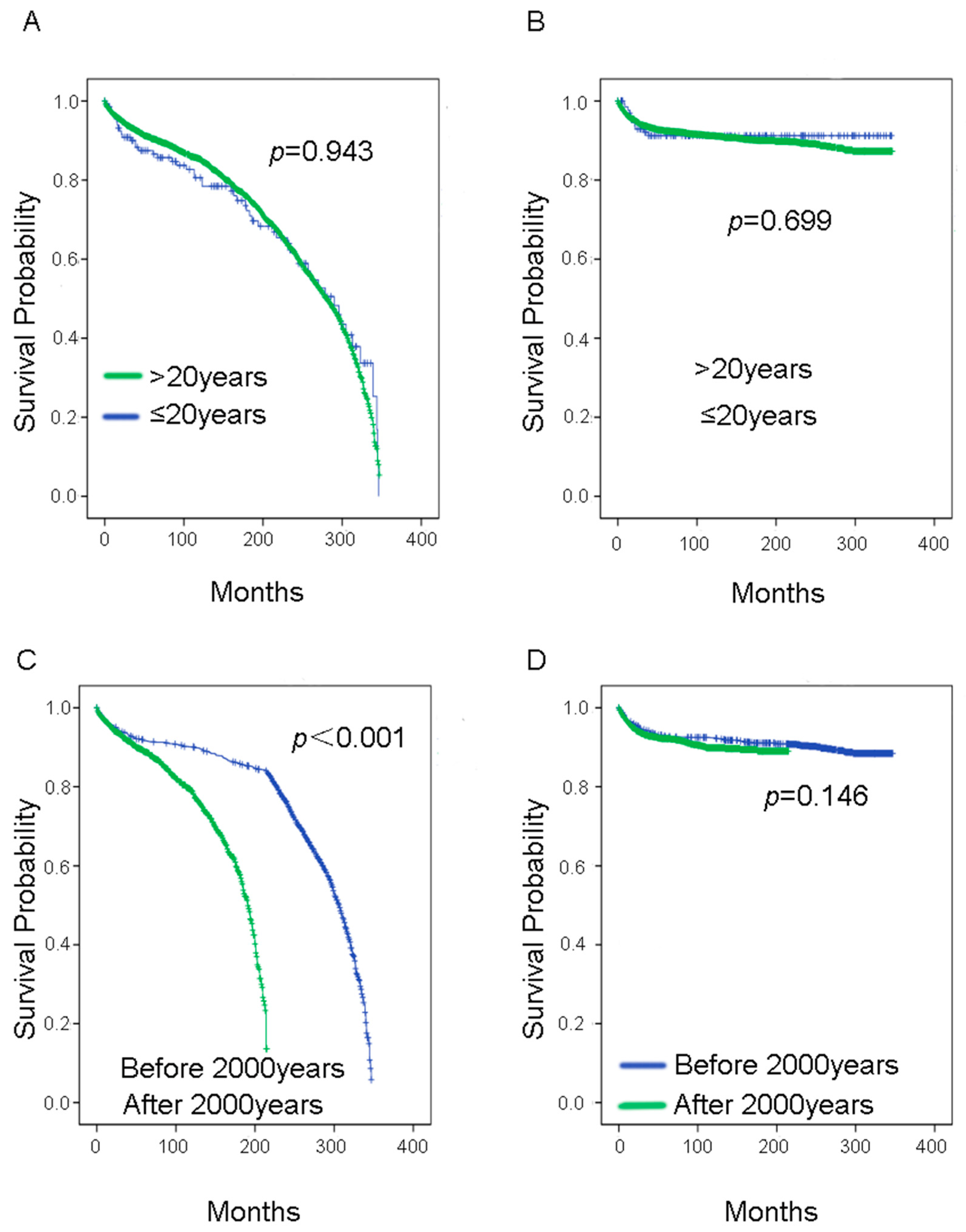
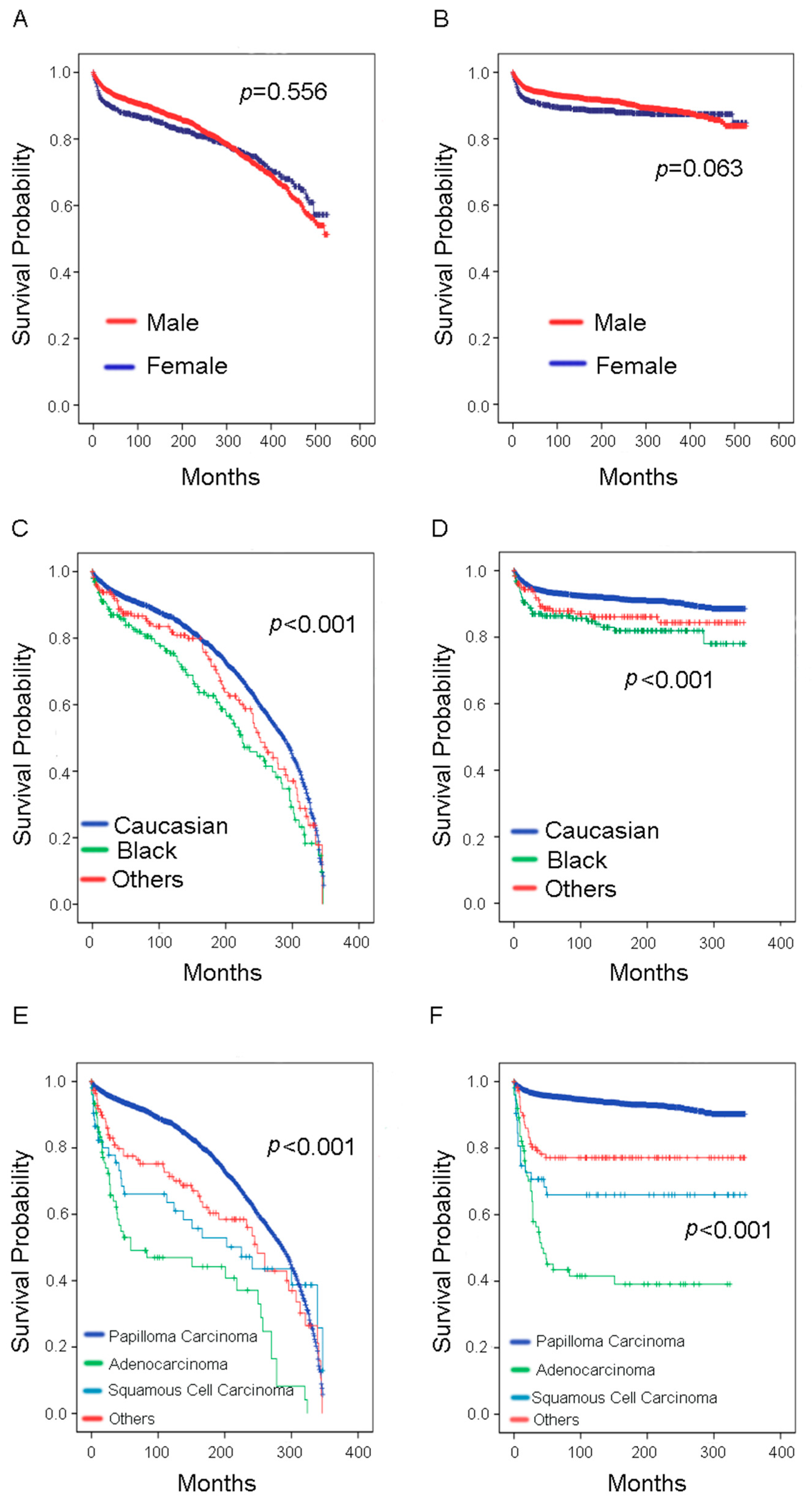
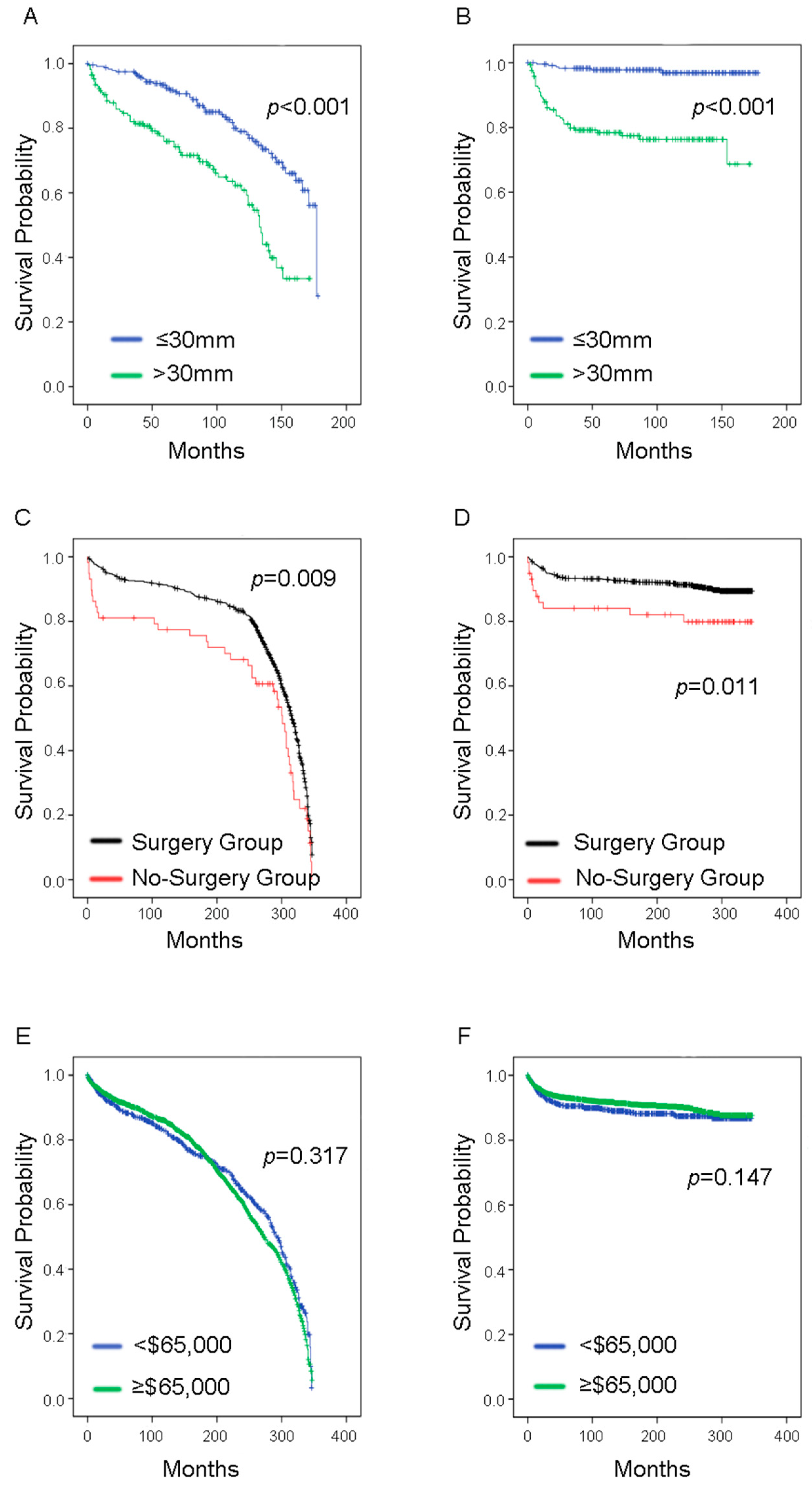
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Today. Available online: https://gco.iarc.fr/today/online-analysis-tble?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1 (accessed on 25 September 2021).
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2020, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, T.; Han, D.; Li, C.; Xu, F.; Lyu, J.; Gao, Y. Incidence Trends and Survival Prediction of Urothelial Cancer of the Bladder: A Population-Based Study. World J. Surg. Oncol. 2021, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, M.; Xie, H.; Jia, Z.; Zhu, Y.; Zhu, Y.; Shi, G.; Zhang, H.; Dai, B.; Wan, F.; Shen, Y.; et al. Development and External Validation of a Novel 12-Gene Signature for Prediction of Overall Survival in Muscle-Invasive Bladder Cancer. Front. Oncol. 2019, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Larré, S.; Roupret, M.; Neuzillet, Y.; Pignot, G.; Quintens, H.; Houéde, N.; Roy, C.; Durand, X.; Varinot, J.; et al. Clinicopathological Characteristics of Urothelial Bladder Cancer in Patients Less Than 40 Years Old. Virchows Arch. 2015, 466, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Caione, P.; Patruno, G.; Pagliarulo, V.; Bulotta, A.L.; Salerno, A.; Camassei, F.D.; Lastilla, G.; Nappo, S.G. Nonmuscular Invasive Urothelial Carcinoma of the Bladder in Pediatric and Young Adult Patients: Age-Related Outcomes. Urology 2017, 99, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; He, W.; Yang, B.; Gou, X. Nomogram for Predicting Overall Survival of Patients with Bladder Cancer: A Population-Based Study. Int. J. Biol. Markers 2020, 35, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Tian, Z.; Meng, L.; Zhang, W.; Wang, J.; Liu, X.; Wang, X.; Zhang, Y. Young Age Increases the Risk of Lymph Node Positivity but Improves Prognosis in Patients with Bladder Cancer Treated via Cystectomy: A Population-Based Study. Transl. Androl. Urol. 2021, 10, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Schwalb, D.M.; Zhang, Z.F.; Sogani, P.C.; Fair, W.R.; Whitmore, W.F., Jr.; Oettgen, H.F. Intravesical Bacillus Calmette-Guerin Therapy Prevents Tumor Progression and Death from Superficial Bladder Cancer: Ten-Year Follow-up of a Prospective Randomized Trial. J. Clin. Oncol. 1995, 13, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab Monotherapy for the Treatment of High-Risk Non-Muscle-Invasive Bladder Cancer Unresponsive to Bcg (Keynote-057): An Open-Label, Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Catto, J.W.F.; Gordon, K.; Collinson, M.; Poad, H.; Twiddy, M.; Johnson, M.; Jain, S.; Chahal, R.; Simms, M.; Dooldeniya, M.; et al. Radical Cystectomy against Intravesical Bcg for High-Risk High-Grade Nonmuscle Invasive Bladder Cancer: Results from the Randomized Controlled Bravo-Feasibility Study. J. Clin. Oncol. 2021, 39, 202–214. [Google Scholar] [CrossRef]
- Tilki, D.; Reich, O.; Karakiewicz, P.I.; Novara, G.; Kassouf, W.; Ergün, S.; Fradet, Y.; Ficarra, V.; Sonpavde, G.; Stief, C.G.; et al. Validation of the Ajcc Tnm Substaging of Pt2 Bladder Cancer: Deep Muscle Invasion Is Associated with Significantly Worse Outcome. Eur. Urol. 2010, 58, 112–117. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Validation of the Eighth Ajcc New Substages for Bladder Cancer among Different Staging Contexts. Clin. Genitourin. Cancer 2017, 15, e1095–e1106. [Google Scholar] [CrossRef]
- Dutta, R.; Abdelhalim, A.; Martin, J.W.; Vernez, S.L.; Faltas, B.; Lotan, Y.; Youssef, R.F. Effect of Tumor Location on Survival in Urinary Bladder Adenocarcinoma: A Population-Based Analysis. Urol. Oncol. 2016, 34, 531.e1–531.e6. [Google Scholar] [CrossRef]
- Rosiello, G.; Palumbo, C.; Knipper, S.; Pecoraro, A.; Dzyuba-Negrean, C.; Luzzago, S.; Tian, Z.; Gallina, A.; Montorsi, F.; Shariat, S.F.; et al. Unmarried Men Have Worse Oncologic Outcomes after Radical Cystectomy for Nonmetastatic Urothelial Bladder Cancer. Urol. Oncol. 2020, 38, 76.e1–76.e9. [Google Scholar] [CrossRef]
- Macleod, L.C.; Fam, M.M.; Yabes, J.G.; Hale, N.E.; Turner, R.M., II; Lopa, S.H.; Gingrich, J.R.; Borza, T.; Skolarus, T.A.; Davies, B.J.; et al. Comparison of Neoadjuvant and Adjuvant Chemotherapy in Muscle-Invasive Bladder Cancer. Clin. Genitourin. Cancer 2020, 18, 201–209.e2. [Google Scholar] [CrossRef] [PubMed]
- Noone, A.-M.; Lund, J.L.; Mariotto, A.; Cronin, K.; McNeel, T.; Deapen, D.; Warren, J.L. Comparison of Seer Treatment Data with Medicare Claims. Med. Care 2016, 54, e55–e64. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient Characteristics | Univariate Cox Regression Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Alive (N) | Dead (N) | p Value a | p Value | HR | 95% CI | ||
| Age at diagnosis | ≤20 years | 208 | 22 | 0.458 | 0.642 | 1.154 | 0.630–2.116 |
| >20 years | 3195 | 364 | |||||
| Year of diagnosis | ≤2000 year | 2203 | 271 | 0.174 | 0.153 | 1.223 | 0.928–1.613 |
| <2000 year | 1201 | 114 | |||||
| Gender | Female | 922 | 120 | 0.279 | 0.064 | 0.814 | 0.655–1.012 |
| Male | 2482 | 265 | |||||
| Race | Caucasian | 2961 | 305 | 0.347 | 0.001 | 1.388 | 1.147–1.678 |
| Black | 226 | 48 | |||||
| Others b | 216 | 33 | |||||
| Histological type | Papilloma carcinoma | 3130 | 254 | 0.364 | 0.001 | 1.758 | 1.574–1.964 |
| Adenocarcinoma | 52 | 59 | |||||
| Squamous cell carcinoma | 88 | 29 | |||||
| Others c | 134 | 43 | |||||
| AJCC d Cancer Stage Manual 7th Edition | 0a | 272 | 0 | 0.268 | 0.001 | 3.574 | 2.618–4.880 |
| 0is | 15 | 0 | |||||
| Ⅰ | 50 | 3 | |||||
| Ⅱ | 10 | 3 | |||||
| Ⅲ | 4 | 6 | |||||
| Ⅳ | 3 | 12 | |||||
| AJCC Cancer Stage Manual 6th Edition | 0a | 531 | 3 | 0.266 | 0.001 | 2.814 | 2.417–3.276 |
| 0is | 45 | 0 | |||||
| Ⅰ | 90 | 11 | |||||
| Ⅱ | 21 | 12 | |||||
| Ⅲ | 9 | 10 | |||||
| Ⅳ | 11 | 29 | |||||
| AJCC Cancer Stage Manual 3rd Edition | 0 | 773 | 25 | 0.316 | 0.001 | 3.027 | 2.641–3.469 |
| Ⅰ | 95 | 7 | |||||
| Ⅱ | 28 | 7 | |||||
| Ⅲ | 20 | 16 | |||||
| Ⅳ | 7 | 30 | |||||
| Node-positive rate | ≤50% | 17 | 16 | 0.626 | 0.316 | 1.547 | 0.659–3.629 |
| >50% | 3 | 8 | |||||
| Tumor size | ≤30 mm | 232 | 6 | 0.249 | 0.001 | 10.564 | 4.458–25.034 |
| <30 mm | 132 | 38 | |||||
| Surgery | Yes | 635 | 69 | 0.458 | 0.013 | 2.244 | 1.187–4.242 |
| No | 48 | 11 | |||||
| Median income e | <$65,000/year | 595 | 73 | 0.272 | 0.148 | 0.812 | 0.613–1.076 |
| ≥$65,000/year | 1546 | 150 | |||||
| AJCC Manual 7th Edition (N = 203) | AJCC Manual 6th Edition (N = 380) | AJCC Manual 3rd Edition (N = 551) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | ||
| Race | Caucasian | 0.992 | Ref | - | 0.269 | Ref | - | 0.03 | Ref | - |
| Black | 0.985 | 0.001 | 0.001–∞ | 0.107 | 0.181 | 0.023–1.442 | 0.235 | 1.527 | 0.760–3.069 | |
| Others | 0.900 | 1.111 | 0.215–5.751 | 0.945 | 1.049 | 0.271–4.065 | 0.01 | 3.138 | 1.594–6.174 | |
| Histological type | Papilloma carcinoma | 0.448 | Ref | - | 0.602 | Ref | - | 0.093 | Ref | - |
| Adenocarcinoma | 0.737 | 1.268 | 0.317–5.074 | 0.748 | 1.169 | 0.450–3.036 | 0.102 | 0.565 | 0.285–1.120 | |
| Squamous cell carcinoma | 0.985 | 0.001 | 0.001–∞ | 0.993 | 0.995 | 0.336–2.944 | 0.309 | 0.579 | 0.202–1.661 | |
| Others | 0.108 | 5.308 | 0.693–40.674 | 0.184 | 2.843 | 0.610–13.264 | 0.022 | 0.348 | 0.141–0.857 | |
| Tumor size | ≤30 mm | - | Ref | - | - | Ref | - | - a | - | - |
| >30 mm | 0.666 | 1.424 | 0.286–7.090 | 0.096 | 2.425 | 0.854–6.887 | - | - | - | |
| Cancer stage | 0a/0 | 0.022 | Ref | - | <0.001 | Ref | - | <0.001 | Ref | - |
| 0is | 0.718 | <0.001 | 0.000–3.472 × 10−23 | 0.983 | <0.001 | 0.000–∞ | - b | - | - | |
| Ⅰ | 0.892 | <0.001 | 0.000–1.190 × 10−64 | 0.019 | 15.318 | 1.571–149.339 | 0.052 | 2.306 | 0.994–5.351 | |
| Ⅱ | 0.002 | 0.026 | 0.003–0.256 | <0.001 | 83.302 | 9.883–702.177 | <0.001 | 8.646 | 3.704–20.186 | |
| Ⅲ | 0.011 | 0.110 | 0.020–0.606 | <0.001 | 118.849 | 13.370–1056.495 | <0.001 | 33.407 | 16.747–66.639 | |
| Ⅳ | 0.107 | 0.294 | 0.066–1.304 | <0.001 | 215.479 | 25.280–1836.379 | <0.001 | 143.180 | 71.370–287.243 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudurexiti, M.; Ma, J.; Li, Y.; Hu, C.; Cai, Z.; Wang, Z.; Jiang, N. Clinical Outcomes and Prognosis Analysis of Younger Bladder Cancer Patients. Curr. Oncol. 2022, 29, 578-588. https://doi.org/10.3390/curroncol29020052
Abudurexiti M, Ma J, Li Y, Hu C, Cai Z, Wang Z, Jiang N. Clinical Outcomes and Prognosis Analysis of Younger Bladder Cancer Patients. Current Oncology. 2022; 29(2):578-588. https://doi.org/10.3390/curroncol29020052
Chicago/Turabian StyleAbudurexiti, Mierxiati, Jie Ma, Yao Li, Chuanyi Hu, Zhikang Cai, Zhong Wang, and Ning Jiang. 2022. "Clinical Outcomes and Prognosis Analysis of Younger Bladder Cancer Patients" Current Oncology 29, no. 2: 578-588. https://doi.org/10.3390/curroncol29020052
APA StyleAbudurexiti, M., Ma, J., Li, Y., Hu, C., Cai, Z., Wang, Z., & Jiang, N. (2022). Clinical Outcomes and Prognosis Analysis of Younger Bladder Cancer Patients. Current Oncology, 29(2), 578-588. https://doi.org/10.3390/curroncol29020052





