Endoscopic Diagnosis and Treatment of Superficial Esophageal Squamous Cell Cancer: Present Status and Future Perspectives
Abstract
1. Introduction
2. Detection and Diagnosis of Esophageal SCC
2.1. Modalities Used for Detection
2.2. Endoscopic Diagnosis of SCC
3. Endoscopic Diagnosis of Cancer Invasion Depth
3.1. Modalities Used for Diagnosis of Cancer Invasion Depth
3.2. Diagnosis of Cancer Invasion Depth by White-Light Endoscopy and Magnifying Endoscopy
4. Indications for ER
4.1. Cancer Invasion Depth
4.2. Lateral Spread of SCC
4.3. ER Procedure
5. Curability Assessment
5.1. pT1a-EP/LPM Cancer without Lymphovascular Invasion
5.2. pT1a mm Cancer without Lymphovascular Invasion
5.3. pSM Cancer or pMM with Lymphovascular Invasion
6. Comparison of ER and Surgical Resection
7. Future Perspectives
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Toh, Y.; Ishihara, R.; Kono, K.; Matsubara, H.; Murakami, K.; Muro, K.; Numasaki, H.; Oyama, T.; Ozawa, S.; et al. Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Comprehensive registry of esophageal cancer in Japan. Esophagus, 2014; Online ahead of print. [Google Scholar] [CrossRef]
- Igaki, H.; Kato, H.; Tachimori, Y.; Daiko, H.; Fukaya, M.; Yajima, S.; Nakanishi, Y. Clinicopathologic characteristics and survival of patients with clinical Stage I squamous cell carcinomas of the thoracic esophagus treated with three-field lymph node dissection. Eur. J. Cardio-Thoracic Surg. 2001, 20, 1089–1094. [Google Scholar] [CrossRef]
- Yamashina, T.; Ishihara, R.; Nagai, K.; Matsuura, N.; Matsui, F.; Ito, T.; Fujii, M.; Yamamoto, S.; Hanaoka, N.; Takeuchi, Y.; et al. Long-Term Outcome and Metastatic Risk After Endoscopic Resection of Superficial Esophageal Squamous Cell Carcinoma. Am. J. Gastroenterol. 2013, 108, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Minashi, K.; Nihei, K.; Mizusawa, J.; Takizawa, K.; Yano, T.; Ezoe, Y.; Tsuchida, T.; Ono, H.; Iizuka, T.; Hanaoka, N.; et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019, 157, 382–390.e3. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ishihara, R.; Motoori, M.; Kawaguchi, Y.; Uedo, N.; Takeuchi, Y.; Higashino, K.; Yano, M.; Nakamura, S.; Iishi, H. Comparison between Definitive Chemoradiotherapy and Esophagectomy in Patients With Clinical Stage I Esophageal Squamous Cell Carcinoma. Am. J. Gastroenterol. 2011, 106, 1048–1054. [Google Scholar] [CrossRef]
- Muto, M.; Minashi, K.; Yano, T.; Saito, Y.; Oda, I.; Nonaka, S.; Omori, T.; Sugiura, H.; Goda, K.; Kaise, M.; et al. Early Detection of Superficial Squamous Cell Carcinoma in the Head and Neck Region and Esophagus by Narrow Band Imaging: A Multicenter Randomized Controlled Trial. J. Clin. Oncol. 2010, 28, 1566–1572. [Google Scholar] [CrossRef]
- Gono, K.; Yamazaki, K.; Doguchi, N.; Nonami, T.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; et al. Endoscopic Observation of Tissue by Narrowband Illumination. Opt. Rev. 2003, 10, 211–215. [Google Scholar] [CrossRef]
- Iwatsubo, T.; Ishihara, R.; Yamasaki, Y.; Tonai, Y.; Hamada, K.; Kato, M.; Suzuki, S.; Kono, M.; Fukuda, H.; Shimamoto, Y.; et al. Narrow band imaging under less-air condition improves the visibility of superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2020, 20, 389. [Google Scholar] [CrossRef]
- Ishihara, R.; Inoue, T.; Uedo, N.; Yamamoto, S.; Kawada, N.; Tsujii, Y.; Kanzaki, H.; Hanafusa, M.; Hanaoka, N.; Takeuchi, Y.; et al. Significance of each narrow-band imaging finding in diagnosing squamous mucosal high-grade neoplasia of the esophagus. J. Gastroenterol. Hepatol. 2009, 25, 1410–1415. [Google Scholar] [CrossRef]
- Oyama, T.; Inoue, H.; Arima, M.; Momma, K.; Ishihara, R.; Hirasawa, D.; Takeuchi, M.; Tomori, A.; Goda, K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: Magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2016, 14, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Uno, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawamura, O.; Kusano, M.; Kuwano, H.; Takeuchi, H.; et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: Part 1. Esophagus 2019, 16, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Arima, M.; Iizuka, T.; Oyama, T.; Katada, C.; Kato, M.; Goda, K.; Goto, O.; Tanaka, K.; Yano, T.; et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig. Endosc. 2020, 32, 452–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Kim, G.H.; Hoseok, I.; Park, D.Y.; Baek, D.H.; Lee, B.E.; Song, G.A. Predicting the invasion depth of esophageal squamous cell carcinoma: Comparison of endoscopic ultrasonography and magnifying endoscopy. Scand. J. Gastroenterol. 2014, 49, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Mizusawa, J.; Kushima, R.; Matsuura, N.; Yano, T.; Kataoka, T.; Fukuda, H.; Hanaoka, N.; Yoshio, T.; Abe, S.; et al. Assessment of the Diagnostic Performance of Endoscopic Ultrasonography After Conventional Endoscopy for the Evaluation of Esophageal Squamous Cell Carcinoma Invasion Depth. JAMA Netw. Open 2021, 4, e2125317. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Yano, T.; Kato, T.; Imajoh, M.; Noguchi, M.; Morimoto, H.; Osera, S.; Yoda, Y.; Oono, Y.; Ikematsu, H.; et al. Prophylactic steroid administration for strictures after endoscopic resection of large superficial esophageal squamous cell carcinoma. Endosc. Int. Open 2016, 4, E1267–E1274. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kobayashi, M.; Takeuchi, M.; Sato, Y.; Narisawa, R.; Aoyagi, Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest. Endosc. 2011, 74, 1389–1393. [Google Scholar] [CrossRef]
- Sato, H.; Inoue, H.; Kobayashi, Y.; Maselli, R.; Santi, E.G.R.; Hayee, B.; Igarashi, K.; Yoshida, A.; Ikeda, H.; Onimaru, M.; et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: Oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest. Endosc. 2013, 78, 250–257. [Google Scholar] [CrossRef]
- Hanaoka, N.; Ishihara, R.; Uedo, N.; Takeuchi, Y.; Higashino, K.; Akasaka, T.; Kanesaka, T.; Matsuura, N.; Yamasaki, Y.; Hamada, K.; et al. Refractory strictures despite steroid injection after esophageal endoscopic resection. Endosc. Int. Open 2016, 4, E354–E359. [Google Scholar] [CrossRef][Green Version]
- Tajiri, A.; Ishihara, R.; Sakurai, H.; Nakamura, T.; Tani, Y.; Inoue, T.; Matsueda, K.; Miyake, M.; Waki, K.; Fukuda, H.; et al. Positive predictive value of the clinical diagnosis of T1a-epithelial/lamina propria esophageal cancer depends on lesion size. Dig. Endosc. 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Chen, T.; Zhang, X.; Chen, W.-F.; Li, Q.; Yao, L.; Korrapati, P.; Jin, X.-J.; Zhang, Y.-X.; et al. Outcomes of Endoscopic Submucosal Dissection vs Esophagectomy for T1 Esophageal Squamous Cell Carcinoma in a Real-World Cohort. Clin. Gastroenterol. Hepatol. 2019, 17, 73–81.e3. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Lee, H.; Song, B.G.; Min, B.-H.; Kim, H.K.; Choi, Y.S.; Lee, J.H.; Hwang, N.-Y.; Carriere, K.C.; Rhee, P.-L.; et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: A propensity score-matched analysis. Gastrointest. Endosc. 2018, 88, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.Y.; Kountouri, M.; Roth, A.; Frossard, J.-L.; Huber, O.; Mönig, S.; Zilli, T. Endoscopic resection with adjuvant chemo-radiotherapy for superficial esophageal squamous cell carcinoma: A critical review. Crit. Rev. Oncol. 2018, 124, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-K.; Lee, C.-H.; Le, P.-H.; Chen, B.-H. Adjuvant therapy for pT1a-m3/pT1b esophageal squamous cell carcinoma after endoscopic resection: Esophagectomy or chemoradiotherapy? A critical review. Crit. Rev. Oncol. 2020, 147, 102883. [Google Scholar] [CrossRef] [PubMed]
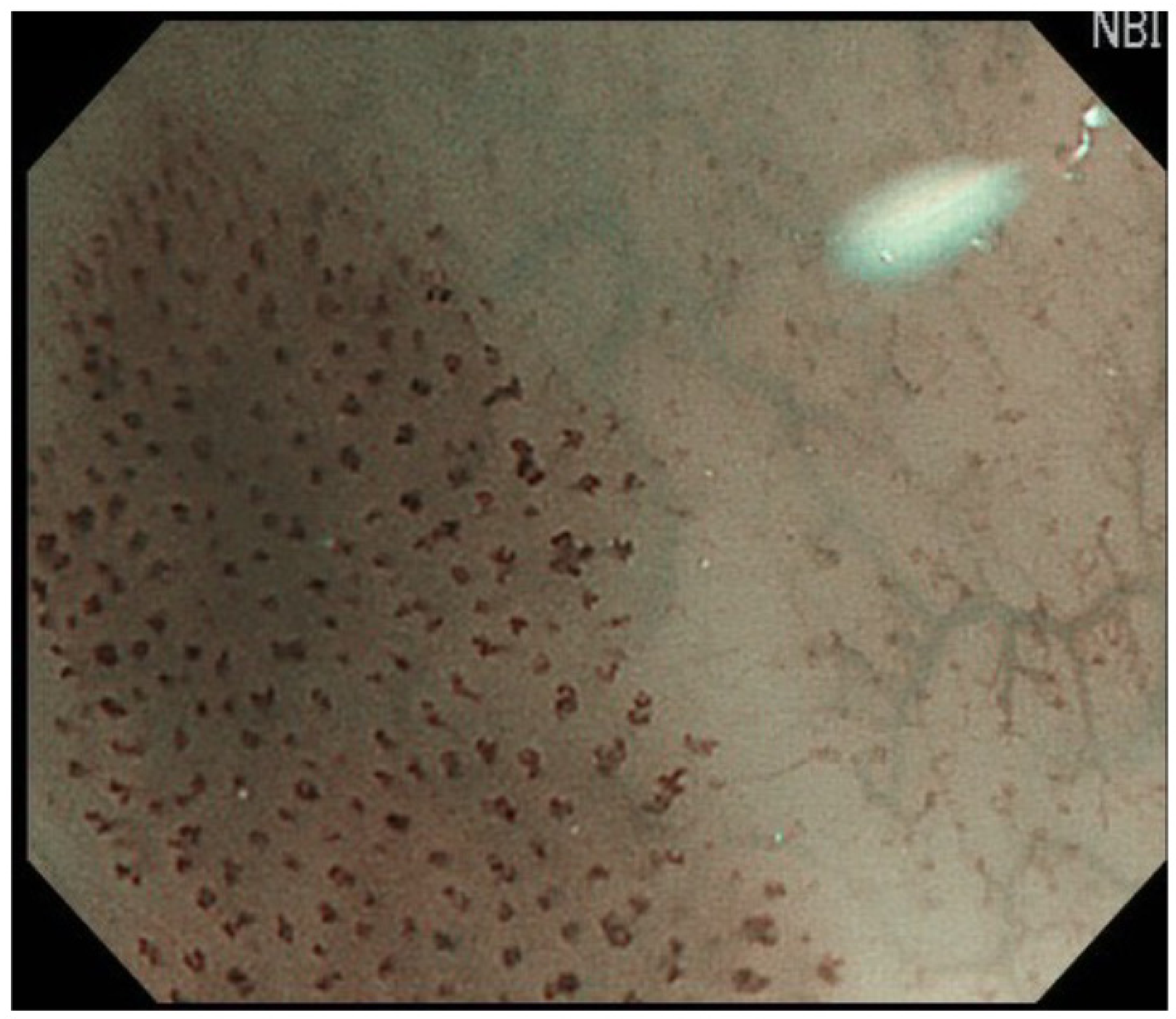

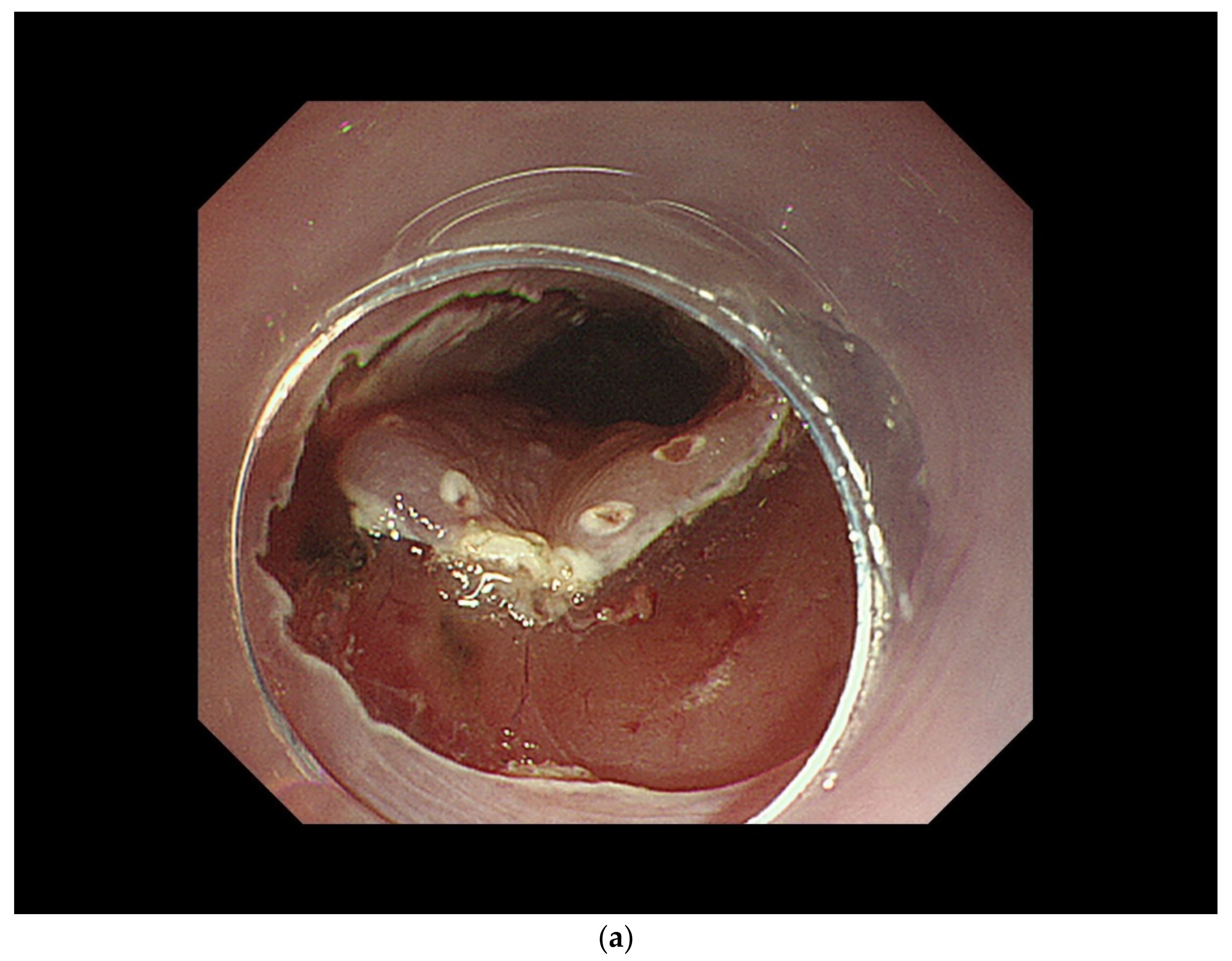

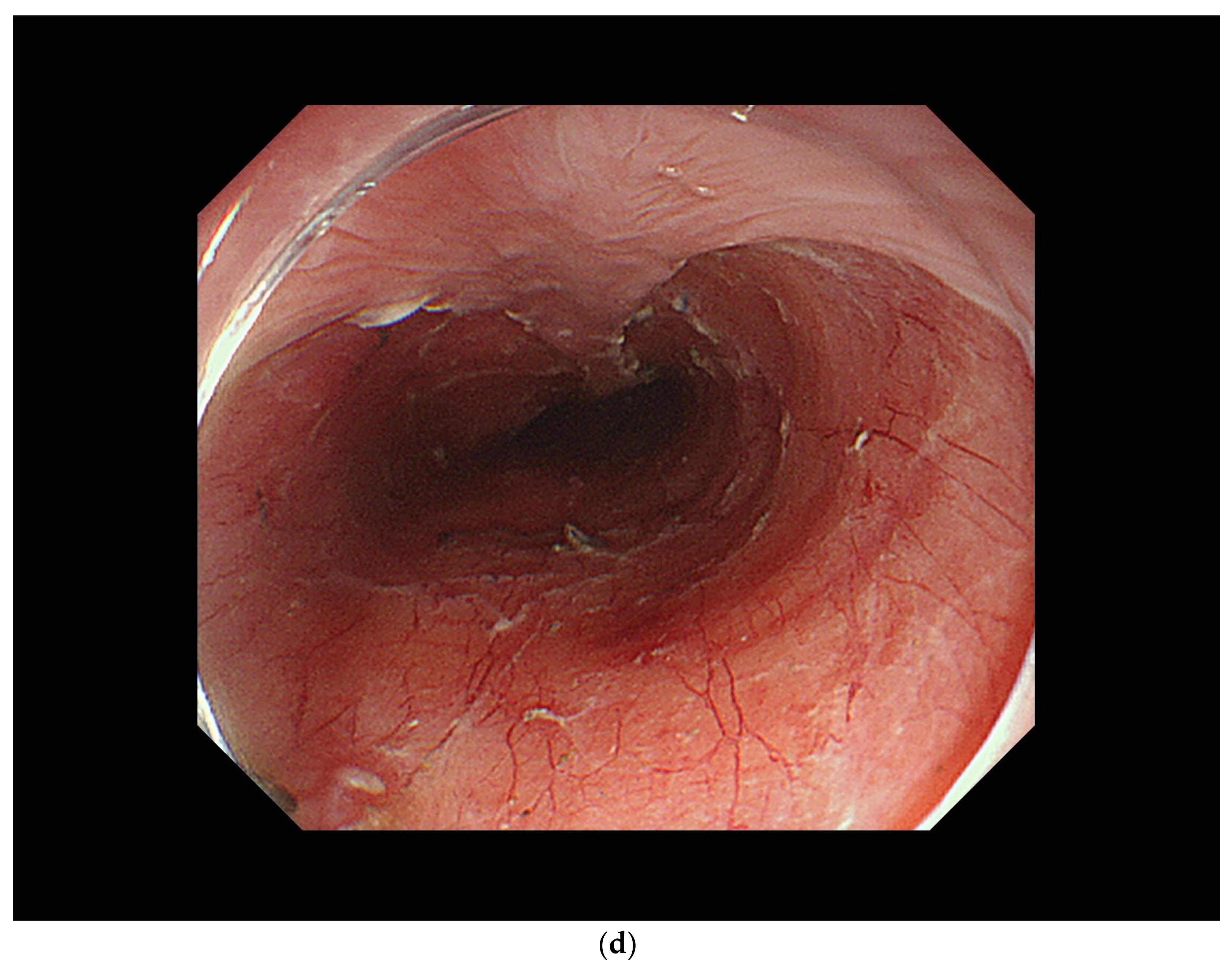
| cT1a-EP/LPM non-circumferential lesion |
| cT1a EP/LPM N0M0 circumferential lesion ≤ 50 mm |
| cT1a MM/T1b SM1 cancer non-circumferential lesion |
| Curative: pT1a-epithelial/lamina propria without lymphovascular invasion |
| Non-curative 1: pT1a MM without lymphovascular invasion |
| Non-curative 2: pT1b cancer invading the submucosa or lymphovascular invasion-positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, R. Endoscopic Diagnosis and Treatment of Superficial Esophageal Squamous Cell Cancer: Present Status and Future Perspectives. Curr. Oncol. 2022, 29, 534-543. https://doi.org/10.3390/curroncol29020048
Ishihara R. Endoscopic Diagnosis and Treatment of Superficial Esophageal Squamous Cell Cancer: Present Status and Future Perspectives. Current Oncology. 2022; 29(2):534-543. https://doi.org/10.3390/curroncol29020048
Chicago/Turabian StyleIshihara, Ryu. 2022. "Endoscopic Diagnosis and Treatment of Superficial Esophageal Squamous Cell Cancer: Present Status and Future Perspectives" Current Oncology 29, no. 2: 534-543. https://doi.org/10.3390/curroncol29020048
APA StyleIshihara, R. (2022). Endoscopic Diagnosis and Treatment of Superficial Esophageal Squamous Cell Cancer: Present Status and Future Perspectives. Current Oncology, 29(2), 534-543. https://doi.org/10.3390/curroncol29020048




