Small Bowel Adenocarcinoma: From Molecular Insights to Clinical Management
Abstract
1. Introduction
2. Epidemiology
3. Etiology
4. Molecular Alterations
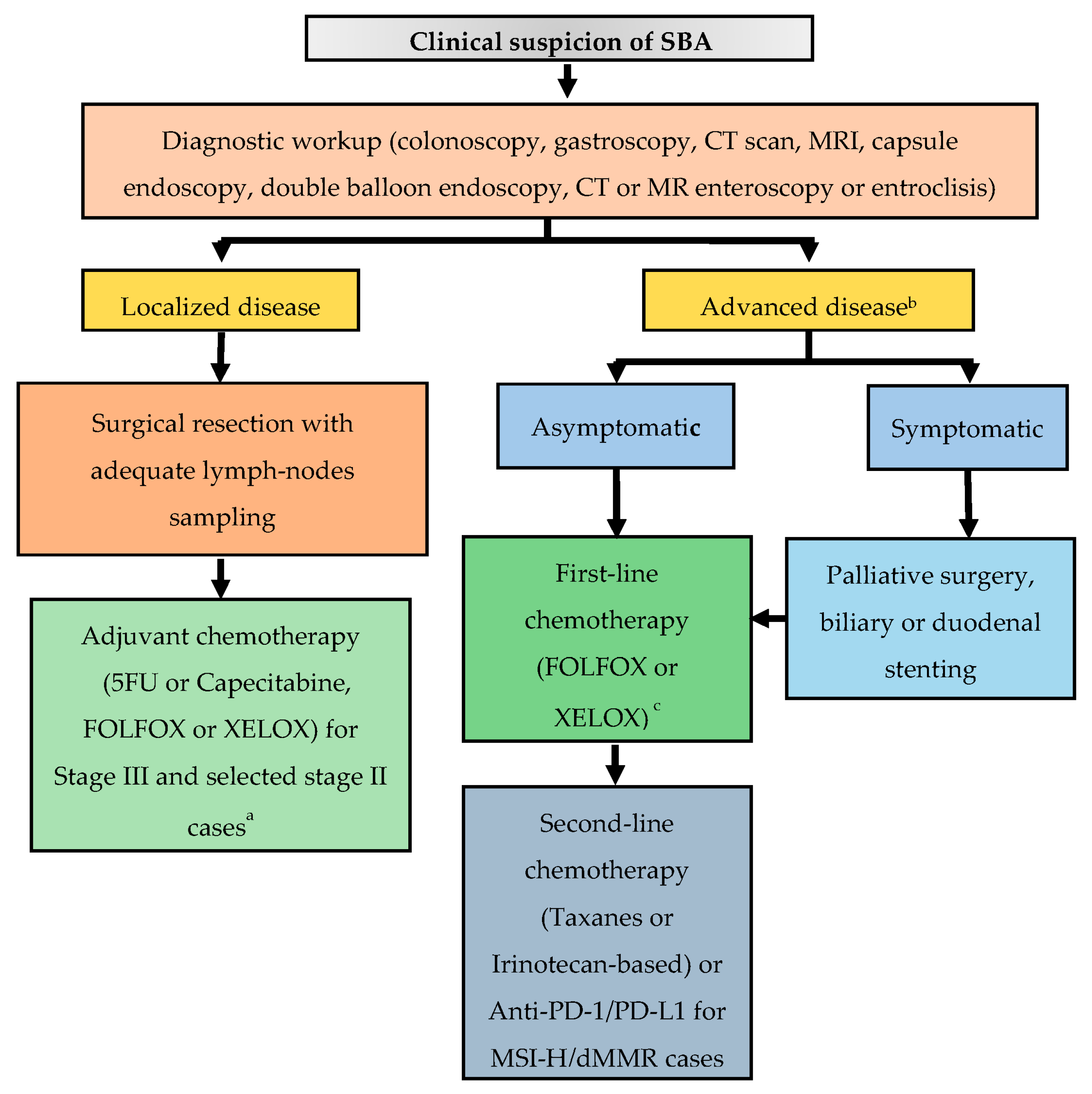
5. Clinical Presentation and Initial Diagnostic Workup
6. Staging
7. Management of Locoregional Disease
7.1. Surgery
7.2. Adjuvant Treatment
8. Management of Advanced Disease
8.1. Systemic Therapy
8.2. Surgery of Primary Tumor and Metastatic Disease
9. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lepage, C.; Bouvier, A.M.; Phelip, J.M.; Hatem, C.; Vernet, C.; Faivre, J. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut 2004, 53, 549–553. [Google Scholar] [CrossRef]
- Neugut, A.I.; Jacobson, J.S.; Suh, S.; Mukherjee, R.; Arber, N. The epidemiology of cancer of the small bowel. Cancer Epidemiol. Biomark. Prev. 1998, 7, 243–251. [Google Scholar]
- Pedersen, K.S.; Raghav, K.; Overman, M.J. Small Bowel Adenocarcinoma: Etiology, Presentation, and Molecular Alterations. J. Natl. Compr. Cancer Netw. 2019, 17, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.S.; Chen, C.C.; Ahsan, H.; Neugut, A.I. A population-based study of the incidence of malignant small bowel tumours: SEER, 1973–1990. Int. J. Epidemiol. 1996, 25, 722–728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lowenfels, A.B. Does bile promote extra-colonic cancer? Lancet 1978, 2, 239–241. [Google Scholar] [CrossRef]
- Kaerlev, L.; Teglbjaerg, P.S.; Sabroe, S.; Kolstad, H.A.; Ahrens, W.; Eriksson, M.; Guénel, P.; Hardell, L.; Launoy, G.; Merler, E.; et al. Is there an association between alcohol intake or smoking and small bowel adenocarcinoma? Results from a European multi-center case-control study. Cancer Causes Control 2000, 11, 791–797. [Google Scholar] [CrossRef]
- Chow, W.H.; Linet, M.S.; McLaughlin, J.K.; Hsing, A.W.; Co Chien, H.T.; Blot, W.J. Risk factors for small intestine cancer. Cancer Causes Control 1993, 4, 163–169. [Google Scholar] [CrossRef]
- Palascak-Juif, V.; Bouvier, A.M.; Cosnes, J.; Flourié, B.; Bouché, O.; Cadiot, G.; Lémann, M.; Bonaz, B.; Denet, C.; Marteau, P.; et al. Small bowel adenocarcinoma in patients with Crohn′s disease compared with small bowel adenocarcinoma de novo. Inflamm. Bowel Dis. 2005, 11, 828–832. [Google Scholar] [CrossRef]
- Piton, G.; Cosnes, J.; Monnet, E.; Beaugerie, L.; Seksik, P.; Savoye, G.; Cadiot, G.; Flourie, B.; Capelle, P.; Marteau, P.; et al. Risk factors associated with small bowel adenocarcinoma in Crohn’s disease: A case-control study. Am. J. Gastroenterol. 2008, 103, 1730–1736. [Google Scholar] [CrossRef]
- Swinson, C.M.; Slavin, G.; Coles, E.C.; Booth, C.C. Coeliac disease and malignancy. Lancet 1983, 1, 111–115. [Google Scholar] [CrossRef]
- Yamada, A.; Komaki, Y.; Komaki, F.; Micic, D.; Zullow, S.; Sakuraba, A. Risk of gastrointestinal cancers in patients with cystic fibrosis: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 758–767. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Chen, Z.M.E.; Wang, H.L. Immunohistochemical investigation of tumorigenic pathways in small intestinal adenocarcinoma: A comparison with colorectal adenocarcinoma. Mod. Pathol. 2006, 19, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz-Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, N.A.; Halverson, A.; Fazio, V.W.; Rybicki, L.A.; Goldblum, J.R. Adenocarcinoma of the small bowel: A study of 37 cases with emphasis on histologic prognostic factors. Dis. Colon Rectum 2002, 45, 1496–1502. [Google Scholar] [CrossRef]
- Hänninen, U.A.; Katainen, R.; Tanskanen, T.; Plaketti, R.M.; Laine, R.; Hamberg, J.; Ristimäki, A.; Pukkala, E.; Taipale, M.; Mecklin, J.P.; et al. Exome-wide somatic mutation characterization of small bowel adenocarcinoma. PLoS Genet. 2018, 14, e1007200. [Google Scholar] [CrossRef]
- Schrock, A.B.; Devoe, C.E.; McWilliams, R.; Sun, J.; Aparicio, T.; Stephens, P.J.; Ross, J.S.; Wilson, R.; Miller, V.A.; Ali, S.M.; et al. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol. 2017, 3, 1546–1553. [Google Scholar] [CrossRef]
- Haan, J.C.; Buffart, T.E.; Eijk, P.P.; van de Wiel, M.A.; van Wieringen, W.N.; Howdle, P.D.; Mulder, C.J.; van de Velde, C.J.; Quirke, P.; Nagtegaal, I.D.; et al. Small bowel adenocarcinoma copy number profiles are more closely related to colorectal than to gastric cancers. Ann. Oncol. 2012, 23, 367–374. [Google Scholar] [CrossRef]
- Caputo, F.; Santini, C.; Bardasi, C.; Cerma, K.; Casadei-Gardini, A.; Spallanzani, A.; Andrikou, K.; Cascinu, S.; Gelsomino, F. BRAF-Mutated Colorectal Cancer: Clinical and Molecular Insights. Int. J. Mol. Sci. 2019, 20, 5369. [Google Scholar] [CrossRef]
- Gelsomino, F.; Barbolini, M.; Spallanzani, A.; Pugliese, G.; Cascinu, S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat. Rev. 2016, 51, 19–26. [Google Scholar] [CrossRef]
- Zaaimi, Y.; Aparicio, T.; Laurent-Puig, P.; Taieb, J.; Zaanan, A. Advanced small bowel adenocarcinoma: Molecular characteristics and therapeutic perspectives. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 154–160. [Google Scholar] [CrossRef]
- Maglinte, D.D.T.; O’Connor, K.; Bessette, J.; Chernish, S.M.; Kelvin, F.M. The Role of the Physician in the Late Diagnosis of Primary Malignant Tumors of the Small Intestine. Am. J. Gastroenterol. 1991, 86, 304–308. [Google Scholar] [PubMed]
- Puccini, A.; Battaglin, F.; Lenz, H.J. Management of Advanced Small Bowel Cancer. Curr. Treat. Options Oncol. 2018, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Jasti, R.; Carucci, L.R. Small bowel neoplasms: A pictorial review. Radiographics 2020, 40, 1020–1038. [Google Scholar] [CrossRef] [PubMed]
- Cobrin, G.M.; Pittman, R.H.; Lewis, B.S. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer 2006, 107, 22–27. [Google Scholar] [CrossRef]
- Sunada, K.; Yamamoto, H.; Kita, H.; Yano, T.; Sato, H.; Hayashi, Y.; Miyata, T.; Sekine, Y.; Kuno, A.; Iwamoto, M.; et al. Clinical outcomes of enteroscopy using the double-balloon method for strictures of the small intestine. World J. Gastroenterol. 2005, 11, 1087–1089. [Google Scholar] [CrossRef]
- Soyer, P.; Aout, M.; Hoeffel, C.; Vicaut, E.; Placé, V.; Boudiaf, M. Helical CT-enteroclysis in the detection of small-bowel tumours: A meta-analysis. Eur. Radiol. 2013, 23, 388–399. [Google Scholar] [CrossRef]
- Masselli, G.; Casciani, E.; Polettini, E.; Laghi, F.; Gualdi, G. Magnetic resonance imaging of small bowel neoplasms. Cancer Imaging 2013, 13, 92–99. [Google Scholar]
- Chen, E.Y.; Vaccaro, G.M. Small Bowel Adenocarcinoma. Clin. Colon Rectal Surg. 2018, 31, 267–277. [Google Scholar] [CrossRef]
- Young, J.I.; Mongoue-Tchokote, S.; Wieghard, N.; Mori, M.; Vaccaro, G.M.; Sheppard, B.C.; Tsikitis, V.L. Treatment and Survival of Small-bowel Adenocarcinoma in the United States: A Comparison with Colon Cancer. Dis. Colon Rectum 2016, 59, 306–315. [Google Scholar] [CrossRef]
- Locher, C.; Batumona, B.; Afchain, P.; Carrère, N.; Samalin, E.; Cellier, C.; Aparicio, T.; Becouarn, Y.; Bedenne, L.; Michel, P.; et al. Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. 2018, 50, 15–19. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Norton, J.A.; Visser, B.C.; Poultsides, G.A. Does the extent of resection impact survival for duodenal adenocarcinoma? Analysis of 1611 cases. Ann. Surg. Oncol. 2015, 22, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Bakaeen, F.G.; Murr, M.M.; Sarr, M.G.; Thompson, G.B.; Farnell, M.B.; Nagorney, D.M.; Farley, D.R.; van Heerden, J.A.; Wiersema, L.M.; Schleck, C.D.; et al. What prognostic factors are important in duodenal adenocarcinoma? Arch. Surg. 2000, 135, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Tocchi, A.; Mazzoni, G.; Puma, F.; Miccini, M.; Cassini, D.; Bettelli, E.; Tagliacozzo, S. Adenocarcinoma of the third and fourth portions of the duodenum: Results of surgical treatment. Arch. Surg. 2003, 138, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Hu, C.Y.; Kopetz, S.; Abbruzzese, J.L.; Wolff, R.A.; Chang, G.J. A population-based comparison of adenocarcinoma of the large and small intestine: Insights into a rare disease. Ann. Surg. Oncol. 2012, 19, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Hu, C.Y.; Wolff, R.A.; Chang, G.J. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: Analysis of the surveillance, epidemiology, and end results database. Cancer 2010, 116, 5374–5382. [Google Scholar] [CrossRef]
- Tran, T.B.; Qadan, M.; Dua, M.M.; Norton, J.A.; Poultsides, G.A.; Visser, B.C. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery 2015, 158, 486–493. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J.N.; Zhang, Q.W.; Tang, C.T.; Zhang, X.T.; Tang, M.Y.; Li, X.B.; Ge, Z.Z. A New Metastatic Lymph Node Classification-based Survival Predicting Model in Patients with Small Bowel Adenocarcinoma: A Derivation and Validation Study. eBioMedicine 2018, 32, 134–141. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Guidelines for Small Bowel Adenocarcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/small_bowel.pdf (accessed on 20 January 2022).
- Ecker, B.L.; McMillan, M.T.; Datta, J.; Dempsey, D.T.; Karakousis, G.C.; Fraker, D.L.; Drebin, J.A.; Mamtani, R.; Giantonio, B.J.; Roses, R.E. Lymph node evaluation and survival after curative-intent resection of duodenal adenocarcinoma: A matched cohort study. Eur. J. Cancer 2016, 69, 135–141. [Google Scholar] [CrossRef]
- Sarela, A.I.; Brennan, M.F.; Karpeh, M.S.; Klimstra, D.; Conlon, K.C. Adenocarcinoma of the duodenum: Importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann. Surg. Oncol. 2004, 11, 380–386. [Google Scholar] [CrossRef]
- Ecker, B.L.; McMillan, M.T.; Datta, J.; Mamtani, R.; Giantonio, B.J.; Dempsey, D.T.; Fraker, D.L.; Drebin, J.A.; Karakousis, G.C.; Roses, R.E. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: A propensity score-matched analysis. Cancer 2016, 122, 693–701. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Aydin, D.; Sendur, M.A.; Kefeli, U.; Unal, O.U.; Tastekin, D.; Akyol, M.; Tanrikulu, E.; Ciltas, A.; Ustaalioglu, B.B.; Uysal, M.; et al. Evaluation of Prognostic Factors and Adjuvant Chemotherapy in Patients with Small Bowel Adenocarcinoma Who Underwent Curative Resection. Clin. Colorectal Cancer 2017, 16, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, G.; Chen, H.; Li, Y. Meta-analysis of postoperative adjuvant therapy for small bowel adenocarcinoma. PLoS ONE 2018, 13, e0200204. [Google Scholar]
- De Bree, E.; Rovers, K.P.; Stamatiou, D.; Souglakos, J.; Michelakis, D.; de Hingh, I.H. The evolving management of small bowel adenocarcinoma. Acta Oncol. 2018, 57, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Kopetz, S.; Lin, E.; Abbruzzese, J.L.; Wolff, R.A. Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol. 2010, 49, 474–479. [Google Scholar] [CrossRef]
- Dabaja, B.S.; Suki, D.; Pro, B.; Bonnen, M.; Ajani, J. Adenocarcinoma of the small bowel: Presentation, prognostic factors, and outcome of 217 patients. Cancer 2004, 101, 518–526. [Google Scholar] [CrossRef]
- Howe, J.R.; Karnell, L.H.; Menck, H.R.; Scott-Conner, C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: Review of the National Cancer Data Base, 1985–1995. Cancer 1999, 86, 2693–2706. [Google Scholar] [CrossRef]
- Ecker, B.L.; McMillan, M.T.; Datta, J.; Lee, M.K.; Karakousis, G.C.; Vollmer, C.M.J.; Drebin, J.A.; Fraker, D.L.; Roses, R.E. Adjuvant chemotherapy versus chemoradiotherapy in the management of patients with surgically resected duodenal adenocarcinoma: A propensity score-matched analysis of a nationwide clinical oncology database. Cancer 2017, 123, 967–976. [Google Scholar] [CrossRef]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Small Intestine Cancer. 2019. Available online: https://seer.cancer.gov/statfacts/html/smint.html (accessed on 20 January 2022).
- Czaykowski, P.; Hui, D. Chemotherapy in small bowel adenocarcinoma: 10-year experience of the British Columbia Cancer Agency. Clin. Oncol. Coll. Radiol. 2007, 19, 143–149. [Google Scholar] [CrossRef]
- Fishman, P.N.; Pond, G.R.; Moore, M.J.; Oza, A.; Burkes, R.L.; Siu, L.L.; Feld, R.; Gallinger, S.; Greig, P.; Knox, J.J. Natural history and chemotherapy effectiveness for advanced adenocarcinoma of the small bowel: A retrospective review of 113 cases. Am. J. Clin. Oncol. 2006, 29, 225–231. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; McWilliams, R.R.; Donohue, J.H.; Quevedo, J.F. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am. J. Surg. 2010, 199, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Aydin, D.; Sendur, M.A.; Kefeli, U.; Umut Unal, O.; Tastekin, D.; Akyol, M.; Tanrikulu, E.; Ciltas, A.; Bala Ustaalioglu, B.; Sener Dede, D.; et al. Evaluation of prognostic factors and treatment in advanced small bowel adenocarcinoma: Report of a multi-institutional experience of Anatolian Society of Medical Oncology (ASMO). J BUON 2016, 21, 1242–1249. [Google Scholar]
- Gibson, M.K.; Holcroft, C.A.; Kvols, L.K.; Haller, D. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist 2005, 10, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Zaanan, A.; Costes, L.; Gauthier, M.; Malka, D.; Locher, C.; Mitry, E.; Tougeron, D.; Lecomte, T.; Gornet, J.M.; Sobhani, I.; et al. Chemotherapy of advanced small-bowel adenocarcinoma: A multicenter AGEO study. Ann. Oncol. 2010, 21, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, T.; Taguri, M.; Honma, Y.; Takahashi, H.; Ueda, S.; Nishina, T.; Kawai, H.; Kato, S.; Suenaga, M.; Tamura, F.; et al. Multicenter retrospective study of 132 patients with unresectable small bowel adenocarcinoma treated with chemotherapy. Oncologist 2012, 17, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.J.; Liu, Y.W.; Zhang, L.; Qiu, F.; Yu, F.; Zhan, Z.Y.; Feng, M.; Yan, J.; Zhao, J.G.; Xiong, J.P. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs 2012, 23, 561–566. [Google Scholar] [CrossRef]
- Horimatsu, T.; Nakayama, N.; Moriwaki, T.; Hirashima, Y.; Fujita, M.; Asayama, M.; Moriyama, I.; Nakashima, K.; Baba, E.; Kitamura, H.; et al. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int. J. Clin. Oncol. 2017, 22, 905–912. [Google Scholar] [CrossRef]
- Overman, M.J.; Varadhachary, G.R.; Kopetz, S.; Adinin, R.; Lin, E.; Morris, J.S.; Eng, C.; Abbruzzese, J.L.; Wolff, R.A. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J. Clin. Oncol. 2009, 27, 2598–2603. [Google Scholar] [CrossRef]
- Takayoshi, K.; Kusaba, H.; Uenomachi, M.; Mitsugi, K.; Makiyama, C.; Makiyama, A.; Uchino, K.; Shirakawa, T.; Shibata, Y.; Shinohara, Y.; et al. Suggestion of added value by bevacizumab to chemotherapy in patients with unresectable or recurrent small bowel cancer. Cancer Chemother. Pharmacol. 2017, 80, 333–342. [Google Scholar] [CrossRef]
- Aydin, D.; Sendur, M.A.; Kefeli, U.; Ustaalioglu, B.B.; Aydin, O.; Yildirim, E.; Isik, D.; Ozcelik, M.; Surmeli, H.; Oyman, A.; et al. Evaluation of Bevacizumab in Advanced Small Bowel Adenocarcinoma. Clin. Colorectal Cancer 2017, 16, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Legué, L.M.; van Erning, F.N.; Bernards, N.; Lemmens, V.E.P.P.; de Hingh, I.H.J.T.; Creemers, G.J. Addition of Bevacizumab to First-Line Palliative Chemotherapy in Patients with Metastatic Small Bowel Adenocarcinoma: A Population-Based Study. Target. Oncol. 2019, 14, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Gulhati, P.; Raghav, K.; Shroff, R.T.; Varadhachary, G.R.; Kopetz, S.; Javle, M.; Qiao, W.; Wang, H.; Morris, J.; Wolff, R.A.; et al. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: A single-center, open-label, phase 2 study. Cancer 2017, 123, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Iijima, H.; Shinzaki, S.; Tashiro, T.; Iwatani, S.; Tani, M.; Otake, Y.; Yoshihara, T.; Sugimoto, A.; Egawa, S.; et al. Vascular endothelial growth factor-A is an Immunohistochemical biomarker for the efficacy of bevacizumab-containing chemotherapy for duodenal and jejunal adenocarcinoma. BMC Cancer 2021, 21, 978. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- McWilliams, R.R.; Foster, N.R.; Mahoney, M.R.; Smyrk, T.C.; Murray, J.A.; Ames, M.M.; Horvath, L.E.; Schneider, D.J.; Hobday, T.J.; Jatoi, A.; et al. North Central Cancer Treatment Group N0543 (Alliance): A phase 2 trial of pharmacogenetic-based dosing of irinotecan, oxaliplatin, and capecitabine as first-line therapy for patients with advanced small bowel adenocarcinoma. Cancer 2017, 123, 3494–3501. [Google Scholar] [CrossRef]
- Zaanan, A.; Gauthier, M.; Malka, D.; Locher, C.; Gornet, J.M.; Thirot-Bidault, A.; Tougeron, D.; Taïeb, J.; Bonnetain, F.; Aparicio, T.; et al. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: A multicenter AGEO study. Cancer 2011, 117, 1422–1428. [Google Scholar] [CrossRef]
- Aldrich, J.D.; Raghav, K.P.S.; Varadhachary, G.R.; Wolff, R.A.; Overman, M.J. Retrospective Analysis of Taxane-Based Therapy in Small Bowel Adenocarcinoma. Oncologist 2019, 24, e384–e386. [Google Scholar] [CrossRef]
- Overman, M.J.; Adam, L.; Raghav, K.; Wang, J.; Kee, B.; Fogelman, D.; Eng, C.; Vilar, E.; Shroff, R.; Dasari, A.; et al. Phase II study of nab-paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)-high colorectal cancer. Ann. Oncol. 2019, 30, 495. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Foster, N.R.; Overman, M.J.; Boland, P.M.; Kim, S.S.; Arrambide, K.A.; Jaszewski, B.L.; Bekaii-Saab, T.; Graham, R.P.; Welch, J.; et al. ZEBRA: A Multicenter Phase II Study of Pembrolizumab in Patients with Advanced Small-Bowel Adenocarcinoma. Clin. Cancer Res. 2021, 27, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Cardin, D.B.; Whisenant, J.; Ayers, G.D.; Gilbert, J.; Dahlman, K.B.; Shi, C.; Berlin, J. Pilot study to test safety and efficacy of avelumab in small bowel adenocarcinoma (SBA). J. Clin. Oncol. 2020, 38, 797. [Google Scholar] [CrossRef]
- Gulhati, P.; Raghav, K.; Shroff, R.; Varadhachary, G.; Javle, M.; Qiao, W.; Wang, H.; Morris, J.; Wolff, R.; Overman, M.J. Phase II Study of Panitumumab in RAS Wild-Type Metastatic Adenocarcinoma of Small Bowel or Ampulla of Vater. Oncologist 2018, 23, 277-e26. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Chiche, L.; Aloia, T.; Elias, D.; Salmon, R.; Rivoire, M.; Jaeck, D.; Saric, J.; Le Treut, Y.P.; Belghiti, J.; et al. Association Française de Chirurgie. Hepatic resection for noncolorectal nonendocrine liver metastases: Analysis of 1,452 patients and development of a prognostic model. Ann. Surg. 2006, 244, 524–535. [Google Scholar]
- Rompteaux, P.; Gagnière, J.; Gornet, J.M.; Coriat, R.; Baumgaertner, I.; Lecomte, T.; Afchain, P.; Zaanan, A.; Pocard, M.; Bachet, J.B.; et al. Resection of small bowel adenocarcinoma metastases: Results of the ARCAD-NADEGE cohort study. Eur. J. Surg. Oncol. 2019, 45, 331–335. [Google Scholar] [CrossRef]
- Rovers, K.P.; de Bree, E.; Yonemura, Y.; de Hingh, I.H. Treatment of peritoneal metastases from small bowel adenocarcinoma. Int. J. Hyperth. 2017, 33, 571–578. [Google Scholar] [CrossRef]
- Legué, L.M.; Simkens, G.A.; Creemers, G.M.; Lemmens, V.E.P.P.; de Hingh, I.H.J.T. Synchronous peritoneal metastases of small bowel adenocarcinoma: Insights into an underexposed clinical phenomenon. Eur. J. Cancer 2017, 87, 84–91. [Google Scholar] [CrossRef]
- Spiliotis, J.; Halkia, E.; de Bree, E. Treatment of peritoneal surface malignancies with hyperthermic intraperitoneal chemotherapy-current perspectives. Curr. Oncol. 2016, 23, 266–275. [Google Scholar] [CrossRef]
- Marchettini, P.; Sugarbaker, P.H. Mucinous adenocarcinoma of the small bowel with peritoneal seeding. Eur. J. Surg. Oncol. 2002, 28, 19–23. [Google Scholar] [CrossRef]
- Jacks, S.P.; Hundley, J.C.; Shen, P.; Russell, G.B.; Levine, E.A. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis from small bowel adenocarcinoma. J. Surg. Oncol. 2005, 91, 112–117. [Google Scholar] [CrossRef]
- Elias, D.; Glehen, O.; Pocard, M.; Quenet, F.; Goéré, D.; Arvieux, C.; Rat, P.; Gilly, F.; Association Française de Chirurgie. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann. Surg. 2010, 251, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, P.; Stewart, J.H.; Russell, G.B.; Levine, E.A. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from small bowel adenocarcinoma. Am. Surg. 2013, 79, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Van Oudheusden, T.R.; Lemmens, V.E.; Braam, H.J.; van Ramshorst, B.; Meijerink, J.; te Velde, E.A.; Mehta, A.M.; Verwaal, V.J.; de Hingh, I.H. Peritoneal metastases from small bowel cancer: Results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in The Netherlands. Surgery 2015, 157, 1023–1027. [Google Scholar] [CrossRef]
- Liu, Y.; Ishibashi, H.; Takeshita, K.; Mizumoto, A.; Hirano, M.; Sako, S.; Takegawa, S.; Takao, N.; Ichinose, M.; Yonemura, Y. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Dissemination from Small Bowel Malignancy: Results from a Single Specialized Center. Ann. Surg. Oncol. 2016, 23, 1625–1631. [Google Scholar] [CrossRef]
- Saxena, A.; Valle, S.J.; Liauw, W.; Morris, D.L. Recurrence and Survival Outcomes after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Small Bowel Adenocarcinoma. Anticancer Res. 2017, 37, 5737–5742. [Google Scholar] [PubMed]
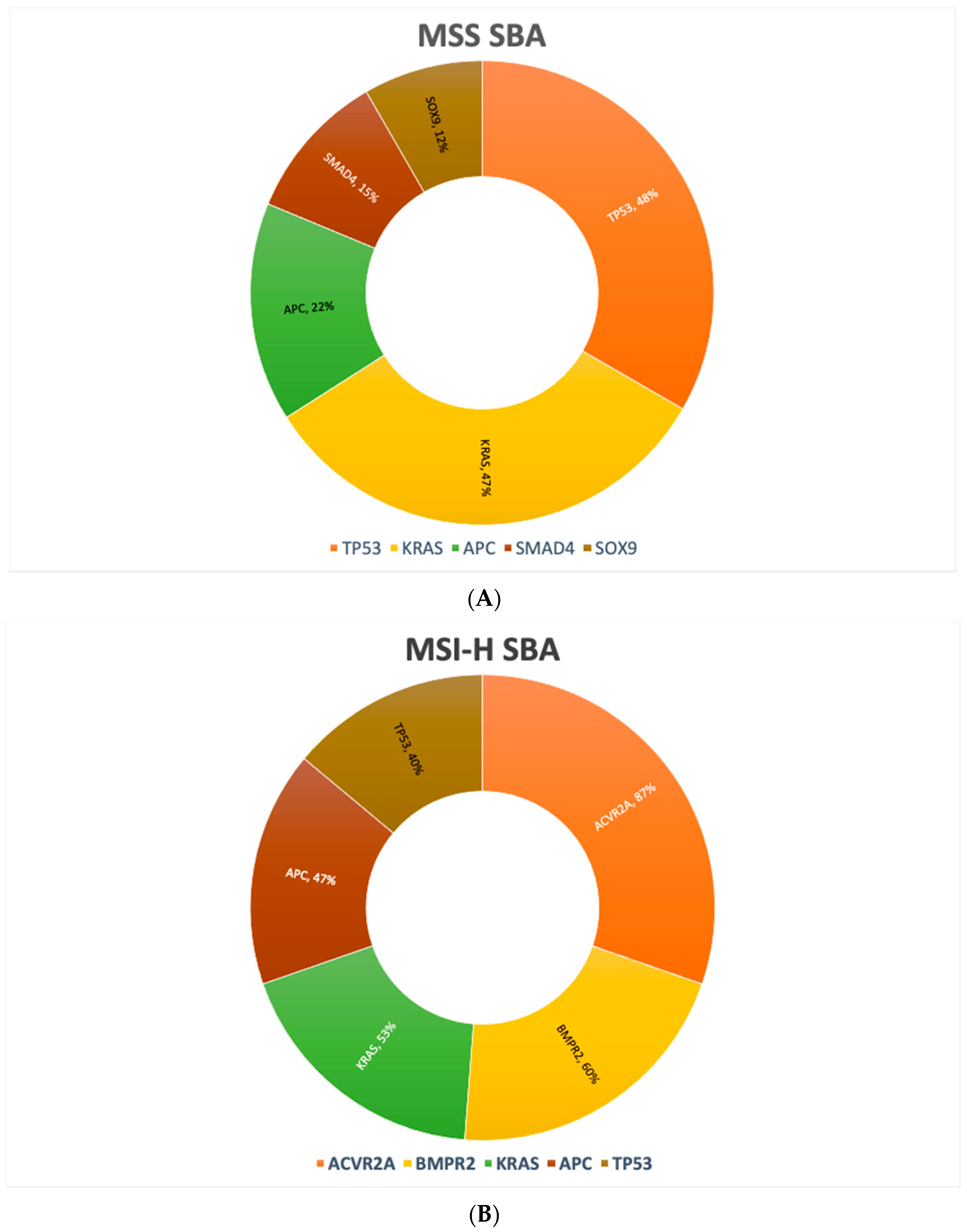
| Gene | SBA | GC | CRC |
|---|---|---|---|
| TP53 | 58.4% | 58.4% | 75% |
| KRAS | 53.6% | 14.2% | 52% |
| APC | 26.8% | 7.8% | 75.9% |
| SMAD4 | 17.4% | 5.2% | 18.9% |
| PIK3CA | 16.1% | 17.7% | 12.5% |
| CDKN2A | 14.5% | 14.7% | 2.6% |
| T | N | M | |
|---|---|---|---|
| Stage I | 1–2 | 0 | 0 |
| Stage IIA | 3 | 0 | 0 |
| Stage IIB | 4 | 0 | 0 |
| Stage IIIA | Any | 1 | 0 |
| Stage IIIB | Any | 2 | 0 |
| Stage IV | Any | Any | 1 |
| Author [Ref.] | N | Regimen | Line | Response Rate (%) | PFS (Months) | OS (Months) |
|---|---|---|---|---|---|---|
| Gibson [56] | 38 | FAM | ≥1 | 18.4 | 5 | 8 |
| Overman [61] | 30 a | XELOX | 1 | 50 | 11.3 | 20.4 |
| Xiang [59] | 33 | mFOLFOX | 1 | 48.5 | 7.8 | 15.2 |
| Horimatsu [60] | 24 | mFOLFOX | 1 | 45 | 5.9 | 17.3 |
| McWilliams [68] | 33 | XELIRINOX | 1 | 37.5 | 8.9 | 13.4 |
| Gulhati [65] | 30 b | XELOX + bev | 1 | 48.3 | 8.7 | 12.9 |
| Overman [71] | 13 c | Nab-Paclitaxel | ≥2 | 20 | 3.2 | 10.9 |
| Gulhati [75] | 9 d | Panitumumab | >1 | 0 | 2.4 | 5.7 |
| Pedersen [73] | 40 | Pembrolizumab | >1 | 8 | 2.8 | 7.1 |
| Cardin [74] | 8 e | Avelumab | >1 | 29 | 8 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelsomino, F.; Balsano, R.; De Lorenzo, S.; Garajová, I. Small Bowel Adenocarcinoma: From Molecular Insights to Clinical Management. Curr. Oncol. 2022, 29, 1223-1236. https://doi.org/10.3390/curroncol29020104
Gelsomino F, Balsano R, De Lorenzo S, Garajová I. Small Bowel Adenocarcinoma: From Molecular Insights to Clinical Management. Current Oncology. 2022; 29(2):1223-1236. https://doi.org/10.3390/curroncol29020104
Chicago/Turabian StyleGelsomino, Fabio, Rita Balsano, Stefania De Lorenzo, and Ingrid Garajová. 2022. "Small Bowel Adenocarcinoma: From Molecular Insights to Clinical Management" Current Oncology 29, no. 2: 1223-1236. https://doi.org/10.3390/curroncol29020104
APA StyleGelsomino, F., Balsano, R., De Lorenzo, S., & Garajová, I. (2022). Small Bowel Adenocarcinoma: From Molecular Insights to Clinical Management. Current Oncology, 29(2), 1223-1236. https://doi.org/10.3390/curroncol29020104





