Preoperative C-Reactive Protein-to-Albumin Ratio Predicts Postoperative Pancreatic Fistula following Pancreatoduodenectomy: A Single-Center, Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
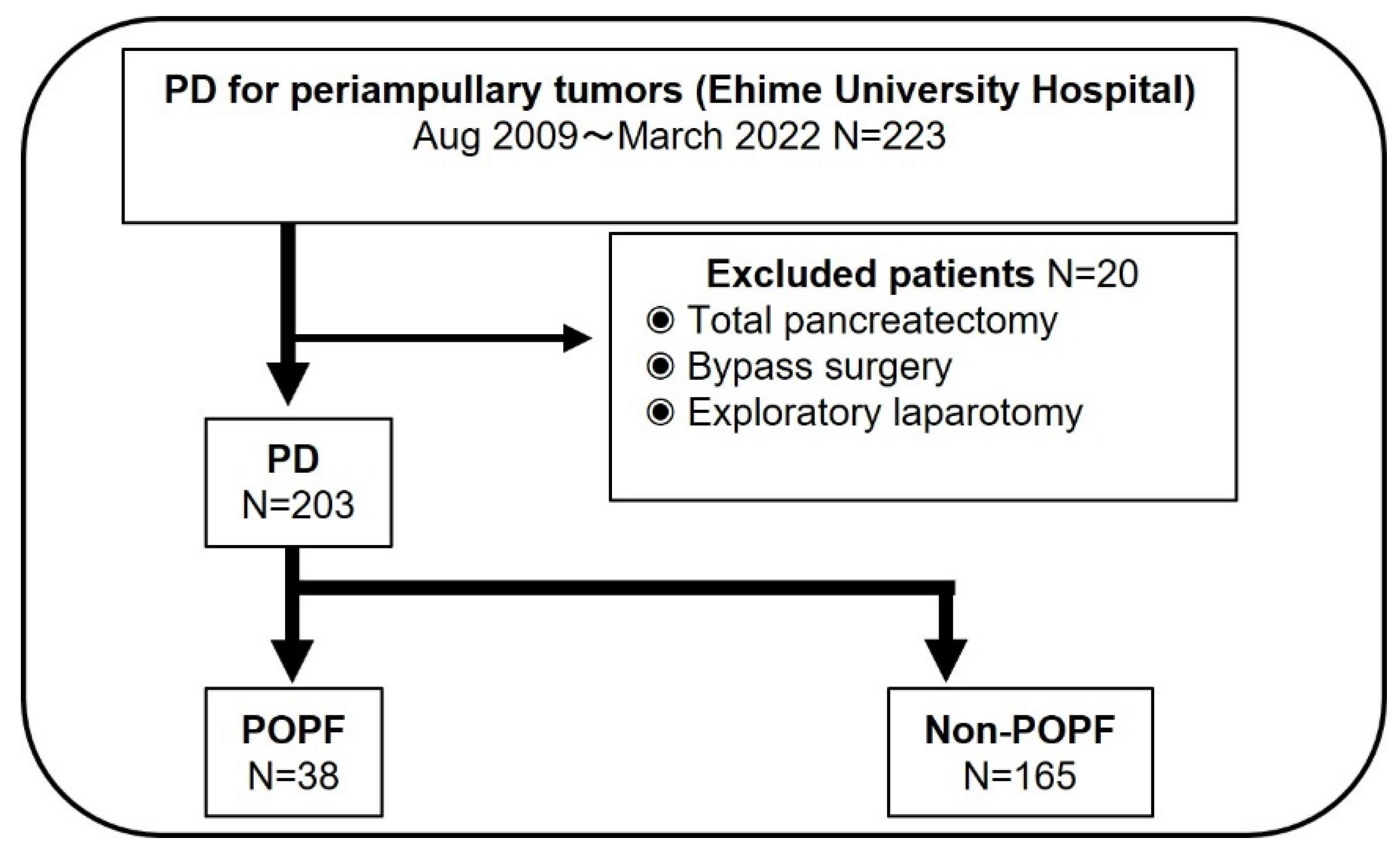
2.1. Patient Population
2.2. Collection of Clinical and Laboratory Data
2.3. Definition of CAR
2.4. Perioperative Management and Follow-Up Study
2.5. Statistical Analyses
3. Results
3.1. CAR and Clinicopathological Features
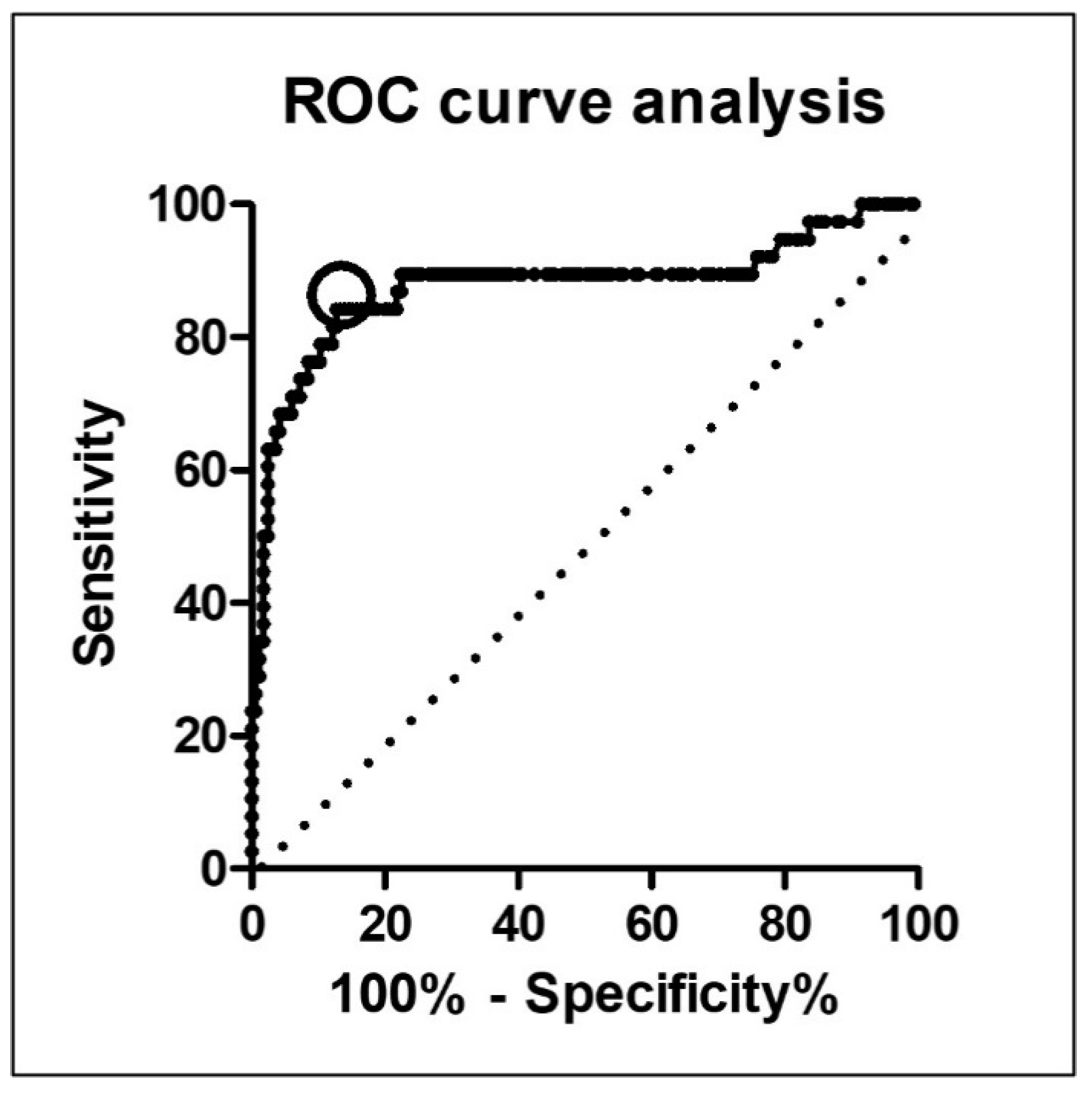
3.2. Optimal CAR Cut-Off Value Measured by ROC Curve Analysis
3.3. Multivariate Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linnemann, R.J.A.; Kooijman, B.J.L.; van der Hilst, C.S.; Sprakel, J.; Buis, C.I.; Kruijff, S.; Klaase, J.M. The Costs of Complications and Unplanned Readmissions after Pancreatoduodenectomy for Pancreatic and Periampullary Tumors: Results from a Single Academic Center. Cancers 2021, 13, 6271. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.; Shin, S.H.; Park, D.J.; Kim, N.; Heo, J.S.; Choi, D.W.; Han, I.W. Validation of Original and Alternative Fistula Risk Scores in Postoperative Pancreatic Fistula. J. Hepatobiliary Pancreat. Sci. 2019, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Fujii, T.; Kodera, Y.; Kanda, M.; Sahin, T.T.; Kanzaki, A.; Hayashi, M.; Sugimoto, H.; Nomoto, S.; Takeda, S.; et al. Recurrence Pattern and Prognosis of Pancreatic Cancer after Pancreatic Fistula. Ann. Surg. Oncol. 2011, 18, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Nakabayashi, Y.; Kurihara, K. Lower Geriatric Nutritional Risk Index Predicts Postoperative Pancreatic Fistula in Patients with Distal Pancreatectomy. Mol. Clin. Oncol. 2019, 12, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Omura, K.; Takada, Y.; Ozaki, T.; Mishima, K.; Igarashi, K.; Wakabayashi, G. Geriatric Nutritional Risk Index Less than 92 Is a Predictor for Late Postpancreatectomy Hemorrhage Following Pancreatoduodenectomy: A Retrospective Cohort Study. Cancers 2020, 12, 2779. [Google Scholar] [CrossRef]
- Funamizu, N.; Omura, K.; Ozaki, T.; Honda, M.; Mishima, K.; Igarashi, K.; Takada, Y.; Wakabayashi, G. Geriatric Nutritional Risk Index Serves as Risk Factor of Surgical Site Infection after Pancreatoduodenectomy: A Validation Cohort Ageo Study. Gland Surg. 2020, 9, 1982–1988. [Google Scholar] [CrossRef]
- Funamizu, N.; Nakabayashi, Y.; Iida, T.; Kurihara, K. Geriatric Nutritional Risk Index Predicts Surgical Site Infection after Pancreaticoduodenectomy. Mol. Clin. Oncol. 2018, 9, 274–278. [Google Scholar] [CrossRef]
- Kubo, T.; Ono, S.; Ueno, H.; Shinto, E.; Yamamoto, J.; Hase, K. Elevated Preoperative C-Reactive Protein Levels Are a Risk Factor for the Development of Postoperative Infectious Complications Following Elective Colorectal Surgery. Langenbecks Arch. Surg. 2013, 398, 965–971. [Google Scholar] [CrossRef]
- Rungsakulkij, N.; Vassanasiri, W.; Tangtawee, P.; Suragul, W.; Muangkaew, P.; Mingphruedhi, S.; Aeesoa, S. Preoperative Serum Albumin Is Associated with Intra-Abdominal Infection Following Major Hepatectomy. J. Hepatobiliary Pancreat. Sci. 2019, 26, 479–489. [Google Scholar] [CrossRef]
- Lee, B.; Han, H.-S.; Yoon, Y.-S.; Cho, J.Y.; Lee, J.S. Impact of Preoperative Malnutrition, Based on Albumin Level and Body Mass Index, on Operative Outcomes in Patients with Pancreatic Head Cancer. J. Hepatobiliary Pancreat. Sci. 2021, 28, 1069–1075. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, G.-L. C-Reactive Protein to Albumin Ratio Predicts the Outcome in Renal Cell Carcinoma: A Meta-Analysis. PLoS ONE 2019, 14, e0224266. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, K.; Guo, M.; Long, J.; Liu, L.; Liu, C.; Xu, J.; Ni, Q.; Luo, G.; Yu, X. Prognostic Value of the CRP/Alb Ratio, a Novel Inflammation-Based Score in Pancreatic Cancer. Ann. Surg. Oncol. 2017, 24, 561–568. [Google Scholar] [CrossRef]
- Takamori, S.; Toyokawa, G.; Shimokawa, M.; Kinoshita, F.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Hirai, F.; Seto, T.; et al. The C-Reactive Protein/Albumin Ratio Is a Novel Significant Prognostic Factor in Patients with Malignant Pleural Mesothelioma: A Retrospective Multi-Institutional Study. Ann. Surg. Oncol. 2018, 25, 1555–1563. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 Update of the International Study Group (ISGPS) Definition and Grading of Postoperative Pancreatic Fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Fairclough, E.; Cairns, E.; Hamilton, J.; Kelly, C. Evaluation of a Modified Early Warning System for Acute Medical Admissions and Comparison with C-Reactive Protein/Albumin Ratio as a Predictor of Patient Outcome. Clin. Med. 2009, 9, 30–33. [Google Scholar] [CrossRef]
- Uemura, S.; Endo, H.; Ichihara, N.; Miyata, H.; Maeda, H.; Hasegawa, H.; Kamiya, K.; Kakeji, Y.; Yoshida, K.; Yasuyuki, S.; et al. Day of Surgery and Mortality after Pancreatoduodenectomy: A Retrospective Analysis of 29 270 Surgical Cases of Pancreatic Head Cancer from Japan. J. Hepatobiliary Pancreat. Sci. 2022, 29, 778–784. [Google Scholar] [CrossRef]
- Satoi, S.; Yamamoto, T.; Yoshitomi, H.; Motoi, F.; Kawai, M.; Fujii, T.; Wada, K.; Arimitsu, H.; Sho, M.; Matsumoto, I.; et al. Developing Better Practices at the Institutional Level Leads to Better Outcomes after Pancreaticoduodenectomy in 3,378 Patients: Domestic Audit of the Japanese Society of Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2017, 24, 501–510. [Google Scholar] [CrossRef]
- Hirono, S.; Shimokawa, T.; Nagakawa, Y.; Shyr, Y.-M.; Kawai, M.; Matsumoto, I.; Satoi, S.; Yoshitomi, H.; Okabayashi, T.; Motoi, F.; et al. Risk Factors for Pancreatic Fistula Grade C after Pancreatoduodenectomy: A Large Prospective, Multicenter Japan-Taiwan Collaboration Study. J. Hepatobiliary Pancreat. Sci. 2020, 27, 622–631. [Google Scholar] [CrossRef]
- Utsumi, M.; Aoki, H.; Nagahisa, S.; Nishimura, S.; Une, Y.; Kimura, Y.; Taniguchi, F.; Arata, T.; Katsuda, K.; Tanakaya, K. Preoperative Nutritional Assessment Using the Controlling Nutritional Status Score to Predict Pancreatic Fistula after Pancreaticoduodenectomy. In Vivo 2020, 34, 1931–1939. [Google Scholar] [CrossRef]
- Coppola, A.; La Vaccara, V.; Caggiati, L.; Carbone, L.; Spoto, S.; Ciccozzi, M.; Angeletti, S.; Coppola, R.; Caputo, D. Utility of Preoperative Systemic Inflammatory Biomarkers in Predicting Postoperative Complications after Pancreaticoduodenectomy: Literature Review and Single Center Experience. World J. Gastrointest. Surg. 2021, 13, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Mori, Y.; Minagawa, N.; Tamura, T.; Shibao, K.; Higure, A.; Yamaguchi, K. Rapid Postoperative Reduction in Prognostic Nutrition Index Is Associated with the Development of Pancreatic Fistula Following Distal Pancreatectomy. Pancreatology 2014, 14, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Sakamoto, A.; Utsunomiya, T.; Uraoka, M.; Nagaoka, T.; Iwata, M.; Ito, C.; Tamura, K.; Sakamoto, K.; Ogawa, K.; et al. Geriatric Nutritional Risk Index as a Potential Prognostic Marker for Patients with Resectable Pancreatic Cancer: A Single-Center, Retrospective Cohort Study. Sci. Rep. 2022, 12, 13644. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Tian, C.; Wang, K.; Zhang, R.-J.; Zou, S.-B. Preoperative Platelet Lymphocyte Ratio as Independent Predictors of Prognosis in Pancreatic Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0178762. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Kopetz, S.; Chun, Y.S.; Palavecino, M.; Abdalla, E.K.; Vauthey, J.-N. Blood Neutrophil-to-Lymphocyte Ratio Predicts Survival in Patients with Colorectal Liver Metastases Treated with Systemic Chemotherapy. Ann. Surg. Oncol. 2009, 16, 614–622. [Google Scholar] [CrossRef]
- Jabłońska, B.; Pawlicki, K.; Mrowiec, S. Associations between Nutritional and Immune Status and Clinicopathologic Factors in Patients with Pancreatic Cancer: A Comprehensive Analysis. Cancers 2021, 13, 5041. [Google Scholar] [CrossRef]
- Huang, H.; Wang, C.; Ji, F.; Han, Z.; Xu, H.; Cao, M. Nomogram Based on Albumin and Neutrophil-to-Lymphocyte Ratio for Predicting Postoperative Complications after Pancreaticoduodenectomy. Gland Surg. 2021, 10, 877–891. [Google Scholar] [CrossRef]
- Fan, Z.; Luo, G.; Gong, Y.; Xu, H.; Qian, Y.; Deng, S.; Huang, Q.; Yang, C.; Cheng, H.; Jin, K.; et al. Prognostic Value of the C-Reactive Protein/Lymphocyte Ratio in Pancreatic Cancer. Ann. Surg. Oncol. 2020, 27, 4017–4025. [Google Scholar] [CrossRef]
- Emanuel, A.; Krampitz, J.; Rosenberger, F.; Kind, S.; Rötzer, I. Nutritional Interventions in Pancreatic Cancer: A Systematic Review. Cancers 2022, 14, 2212. [Google Scholar] [CrossRef]
- Yoshida, N.; Baba, H. The C-Reactive Protein/Albumin Ratio May Predict the Long-Term Outcome in Patients with Malignant Pleural Mesothelioma. Ann. Surg. Oncol. 2018, 25, 1471–1472. [Google Scholar] [CrossRef]
- Matsubara, T.; Takamori, S.; Haratake, N.; Fujishita, T.; Toyozawa, R.; Ito, K.; Shimokawa, M.; Yamaguchi, M.; Seto, T.; Okamoto, T. Identification of the Best Prognostic Marker among Immunonutritional Parameters Using Serum C-Reactive Protein and Albumin in Non-Small Cell Lung Cancer. Ann. Surg. Oncol. 2021, 28, 3046–3054. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, Y.; Gao, Z. Pretreatment C-Reactive Protein/Albumin Ratio for Predicting Overall Survival in Pancreatic Cancer: A Meta-Analysis. Medicine 2020, 99, e20595. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yagyu, Y.; Uchinaka, E.I.; Morimoto, M.; Hanaki, T.; Tokuyasu, N.; Honjo, S.; Fujiwara, Y. Predictive Significance of C-Reactive Protein-to-Albumin Ratio for Postoperative Pancreatic Fistula after Pancreaticoduodenectomy. Anticancer Res. 2019, 39, 6283–6290. [Google Scholar] [CrossRef]
| Preoperative Variables | POPF Group | Non-POPF Group | p-Value |
|---|---|---|---|
| N = 38) | (N = 165) | ||
| Age (years) | 73 (46–85) | 71 (34–88) | 0.435 |
| Sex | |||
| Male (%) | 25 (65.8%) | 94 (57.0%) | 0.32 |
| BMI | 23.2 (16.5–29.4) | 21.7 (14.0–32.1) | 0.042 |
| ASA classification | 0.393 | ||
| 1 or 2 | 36 (94.7%) | 151 (91.5%) | |
| 3 | 2 (5.3%) | 14 (8.5%) | |
| Diabetes Mellitus (%) | 14 (36.8%) | 52 (32.1%) | 0.567 |
| Pancreatic cancer (%) | 10 (26.3%) | 85 (51.5%) | |
| Ampullary cancer (%) | 6 (15.8%) | 12 (7.3%) | |
| Distal bile duct cancer (%) | 15 (39.5%) | 25 (15.2%) | |
| Duodenal cancer (%) | 2 (5.3%) | 2 (1.2%) | |
| IPMN (%) | 3 (7.9%) | 23 (13.9%) | |
| Others (%) | 2 (5.3%) | 18 (10.9%) | |
| Preoperative biliary drainage (%) | 29 (76.3%) | 128 (77.6%) | 0.833 |
| HbA1c (%) | 5.8 (3.8–9.8) | 5.8 (4.3–11.9) | 0.594 |
| WBC (×103/μL) | 5.2 (3.0–11.7) | 5.1 (2.6–11.8) | 0.945 |
| Hb (g/dL) | 11.9 (9.0–16.2) | 12.3 (8.6–16.6) | 0.279 |
| Plt (×10⁴/μL) | 20.1 (10.1–38.5) | 22.2 (8.0–49.7) | 0.159 |
| CRP (mg/dL) | 1.54 (0.02–9.55) | 0.09 (0.01–3.33) | <0.001 |
| Total bilirubin (mg/dL) | 1.11(0.3–4.0) | 1.26 (0.2–10.0) | 0.610 |
| Albumin (g/dL) | 3.4 (2.4–4.7) | 3.8 (2.2–4.7) | 0.001 |
| CAR | 0.48 (0.01–2.81) | 0.02 (0.002–1.18) | <0.001 |
| Intra- and Postoperative | POPF Group | Non-POPF Group | p-Value |
|---|---|---|---|
| Variables | (N = 38) | (N = 165) | |
| Operation time (min) | 602 (361–1003) | 538 (318–1045) | 0.003 |
| Estimated blood loss (mL) | 1133 (155–3450) | 756 (50–6375) | 0.001 |
| Blood transfusion (%) | 14 (36.8) | 43 (26.1) | 0.182 |
| Blumgart method (%) | 65.8% | 78.8% | 0.089 |
| Portal vein resection (%) | 3 (7.9) | 37 (22.4) | 0.042 |
| Soft pancreas (%) | 31 (81.6) | 105 (63.6) | 0.033 |
| POCs excluding POPF-related POCs | |||
| CD grade over III (%) | 3 (7.9) | 16 (9.7) | 0.121 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Parameters | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value |
| BMI ≥ 21.6 | 2.700 (1.233–5.913) | 0.013 | 2.269 (0.757–6.801) | 0.143 |
| Estimated blood loss ≥ 790 (g) | 4.391 (1.900–10.150) | 0.001 | 2.980 (0.942–9.430) | 0.063 |
| Operation time ≧ 534 (min) | 6.364 (2.368–17.108) | <0.001 | 4.715 (1.307–17.011) | 0.018 |
| Portal vein resection (%) | 0.297 (0.086–1.019) | 0.054 | ||
| Soft pancreas texture (%) | 1.794 (0.796–4.043) | 0.159 | ||
| CAR ≥ 0.09 | 32.928 (12.396–87.464) | <0.001 | 34.511 (11.748–101.381) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funamizu, N.; Utsunomiya, T.; Honjo, M.; Ito, C.; Shine, M.; Uraoka, M.; Nagaoka, T.; Tamura, K.; Sakamoto, K.; Ogawa, K.; et al. Preoperative C-Reactive Protein-to-Albumin Ratio Predicts Postoperative Pancreatic Fistula following Pancreatoduodenectomy: A Single-Center, Retrospective Study. Curr. Oncol. 2022, 29, 9867-9874. https://doi.org/10.3390/curroncol29120775
Funamizu N, Utsunomiya T, Honjo M, Ito C, Shine M, Uraoka M, Nagaoka T, Tamura K, Sakamoto K, Ogawa K, et al. Preoperative C-Reactive Protein-to-Albumin Ratio Predicts Postoperative Pancreatic Fistula following Pancreatoduodenectomy: A Single-Center, Retrospective Study. Current Oncology. 2022; 29(12):9867-9874. https://doi.org/10.3390/curroncol29120775
Chicago/Turabian StyleFunamizu, Naotake, Takeshi Utsunomiya, Masahiko Honjo, Chihiro Ito, Mikiya Shine, Mio Uraoka, Tomoyuki Nagaoka, Kei Tamura, Katsunori Sakamoto, Kohei Ogawa, and et al. 2022. "Preoperative C-Reactive Protein-to-Albumin Ratio Predicts Postoperative Pancreatic Fistula following Pancreatoduodenectomy: A Single-Center, Retrospective Study" Current Oncology 29, no. 12: 9867-9874. https://doi.org/10.3390/curroncol29120775
APA StyleFunamizu, N., Utsunomiya, T., Honjo, M., Ito, C., Shine, M., Uraoka, M., Nagaoka, T., Tamura, K., Sakamoto, K., Ogawa, K., & Takada, Y. (2022). Preoperative C-Reactive Protein-to-Albumin Ratio Predicts Postoperative Pancreatic Fistula following Pancreatoduodenectomy: A Single-Center, Retrospective Study. Current Oncology, 29(12), 9867-9874. https://doi.org/10.3390/curroncol29120775






