Integrating Systematic Reviews into Supportive Care Trial Design: The Rethinking Clinical Trials (REaCT) Program
Abstract
1. Introduction
2. Materials and Methods
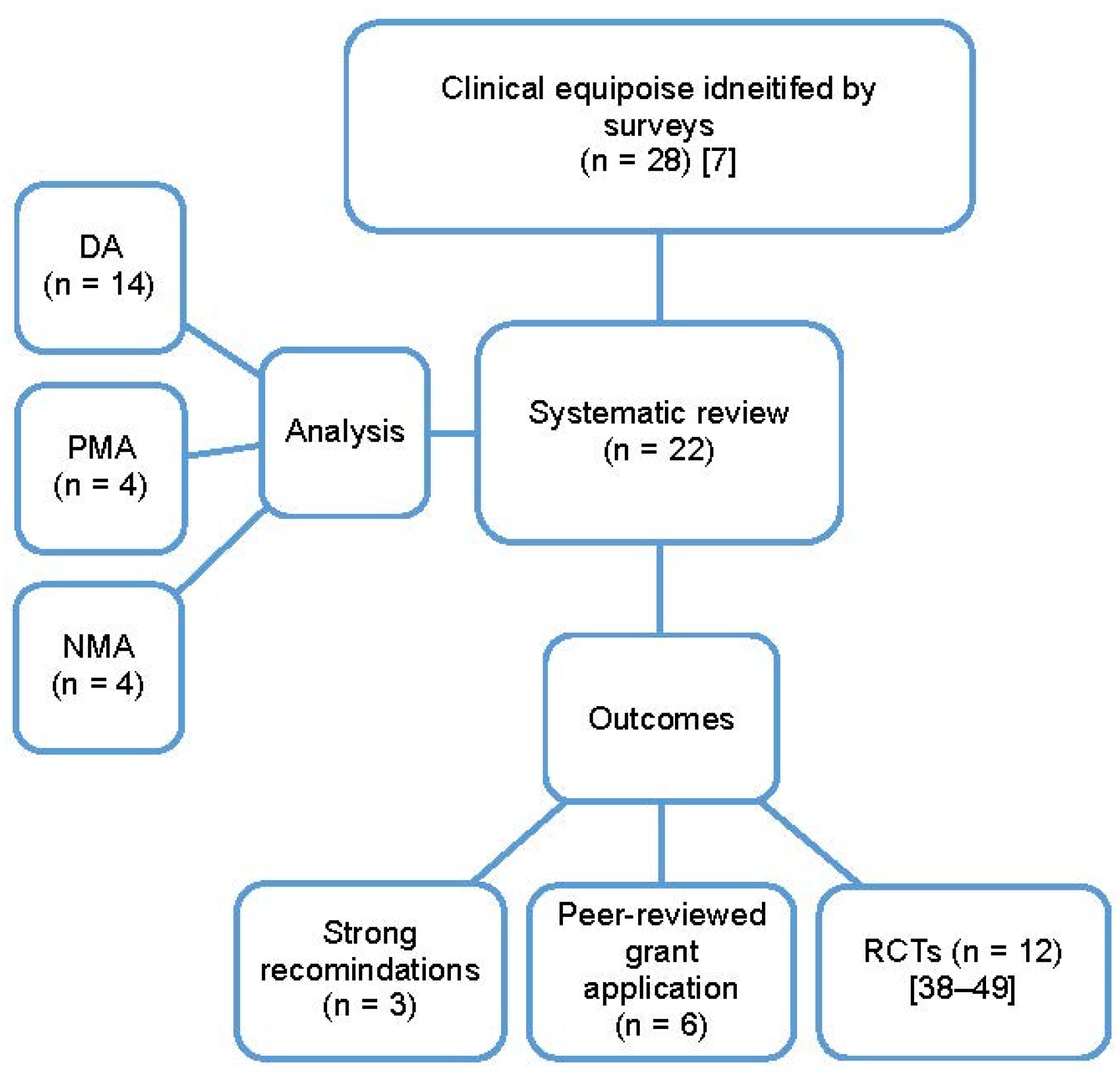
2.1. Search Strategy and Data Extraction
2.2. Review Outcomes
3. Results
3.1. Reviews Characteristics
3.2. Impact of Systemic Reviews
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilton, J.; Mazzarello, S.; Fergusson, D.; Joy, A.A.; Robinson, A.; Arnaout, A.; Hutton, B.; Vandermeer, L.; Clemons, M. Novel methodology for comparing standard-of-care interventions in patients with cancer. J. Oncol. Pract. 2016, 12, e1016–e1024. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Whitney, S.N.; Kurzrock, R. Equipoise lost: Ethics, costs, and the regulation of cancer clinical research. J. Clin. Oncol. 2010, 28, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, A.; Kuchuk, I.; Bouganim, N.; Pond, G.; Verma, S.; Segal, R.; Dent, S.; Gertler, S.; Song, X.; Kanji, F.; et al. Can the referring surgeon enhance accrual of breast cancer patients to medical and radiation oncology trials? The ENHANCE study. Curr. Oncol. 2016, 23, e276–e279. [Google Scholar] [CrossRef] [PubMed]
- Dilts, D.M.; Cheng, S.K.; Crites, J.S.; Sandler, A.B.; Doroshow, J.H. Phase III clinical trial development: A process of chutes and ladders. Clin. Cancer Res. 2010, 16, 5381–5389. [Google Scholar] [CrossRef]
- Freedman, B. Equipoise and the ethics of clinical research. N. Engl. J. Med. 1987, 317, 141–145. [Google Scholar] [CrossRef]
- Weijer, C.; Shapiro, S.H.; Cranley Glass, K. For and against: Clinical equipoise and not the uncertainty principle is the moral underpinning of the randomised controlled trial. BMJ 2000, 321, 756–758. [Google Scholar] [CrossRef]
- Saunders, D.; Liu, M.; Vandermeer, L.; Alzahrani, M.J.; Hutton, B.; Clemons, M. The rethinking clinical trials (REaCT) program. A Canadian-led pragmatic trials program: Strategies for integrating knowledge users into trial design. Curr. Oncol. 2021, 28, 3959–3977. [Google Scholar] [CrossRef]
- Basulaiman, B.; Awan, A.A.; Fergusson, D.; Vandermeer, L.; Arnaout, A.; Hilton, J.; Hutton, B.; Joy, A.A.; Robinson, A.; Califaretti, N.; et al. Creating a pragmatic trials program for breast cancer patients: Rethinking Clinical Trials (REaCT). Breast Cancer Res. Treat. 2019, 177, 93–101. [Google Scholar] [CrossRef]
- Petticrew, M.; Roberts, H. Systematic Reviews in the Social Sciences: A Practical Guide; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions, Cochrane Training. Available online: https://training.cochrane.org/handbook (accessed on 14 December 2021).
- Lu, G.; Ades, A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef]
- Caldwell, D.M.; Ades, A.E.; Higgins, J.P. Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ 2005, 331, 897–900. [Google Scholar] [CrossRef]
- Li, T.; Puhan, M.A.; Vedula, S.S.; Singh, S.; Dickersin, K.; Ad Hoc Network Meta-analysis Methods Meeting Working Group. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 2011, 9, 79. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Hutton, B.; Addison, C.L.; Campbell, K.; Fergusson, D.; Mazarello, S.; Clemons, M. A systematic review of dosing frequency with bone-targeted agents for patients with bone metastases from breast cancer. J. Bone Oncol. 2013, 2, 123–131. [Google Scholar] [CrossRef][Green Version]
- Hutton, B.; Clemons, M.; Mazzarello, S.; Kuchuk, I.; Skidmore, B.; Ng, T. Identifying an optimal antiemetic regimen for patients receiving anthracycline and cyclophosphamide-based chemotherapy for breast cancer—An inspection of the evidence base informing clinical decision-making. Cancer Treat. Rev. 2015, 41, 951–959. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Mazzarello, S.; Shorr, R.; Vandermeer, L.; Jacobs, C.; Hilton, J.; Hutton, B.; Clemons, M. Should de-escalation of bone-targeting agents be standard of care for patients with bone metastases from breast cancer? A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 2205–2213. [Google Scholar] [CrossRef]
- Jacobs, C.; Hutton, B.; Ng, T.; Shorr, R.; Clemons, M. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist 2015, 20, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Mazzarello, S.; Hutton, B.; Ibrahim, M.F.K.; Jacobs, C.; Shorr, R.; Smith, S.; Ng, T.; Clemons, M. Management of urogenital atrophy in breast cancer patients: A systematic review of available evidence from randomized trials. Breast Cancer Res. Treat. 2015, 152, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dudani, S.; Mazzarello, S.; Hilton, J.; Hutton, B.; Vandermeer, L.; Fernandes, R.; Ibrahim, M.F.; Smith, S.; Majeed, H.; Al-Baimani, K.; et al. Optimal management of leptomeningeal carcinomatosis in breast cancer patients—A systematic review. Clin. Breast Cancer 2016, 16, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Mazzarello, S.; Majeed, H.; Smith, S.; Shorr, R.; Hutton, B.; Ibrahim, M.F.; Jacobs, C.; Ong, M.; Clemons, M. Treatment of taxane acute pain syndrome (TAPS) in cancer patients receiving taxane-based chemotherapy—A systematic review. Support. Care Cancer 2016, 24, 1583–1594. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Hutton, B.; Shorr, R.; Majeed, H.; Ibrahim, M.F.; Jacobs, C.; Ong, M.; Clemons, M. Taxane acute pain syndrome (TAPS) in patients receiving taxane-based chemotherapy for breast cancer—A systematic review. Support. Care Cancer 2016, 24, 3633–3650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Dennis, K.; Steinmetz, A.; Clemons, M.; Asmis, T.R.; Goodwin, R.A.; Vickers, M.M. Management of epidermal growth factor receptor inhibitor-induced hypomagnesemia: A systematic review. Clin. Colorectal Cancer 2016, 15, e117–e123. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Mazzarello, S.; Hutton, B.; Shorr, R.; Ibrahim, M.F.K.; Jacobs, C.; Ong, M.; Clemons, M. A systematic review of the incidence and risk factors for taxane acute pain syndrome in patients receiving taxane-based chemotherapy for prostate cancer. Clin. Genitourin. Cancer 2017, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Mazzarello, S.; Stober, C.; Vandermeer, L.; Dudani, S.; Ibrahim, M.F.; Majeed, H.; Perdrizet, K.; Shorr, R.; Hutton, B.; et al. Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer: A systematic review. Breast Cancer Res. Treat. 2017, 161, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Mazzarello, S.; Stober, C.; Ibrahim, M.F.K.; Dudani, S.; Perdrizet, K.; Majeed, H.; Vandermeer, L.; Shorr, R.; Hutton, B.; et al. Primary febrile neutropenia prophylaxis for patients who receive FEC-D chemotherapy for breast cancer: A systematic review. J. Glob. Oncol. 2018, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.F.; Mazzarello, S.; Caudrelier, J.M.; Lima, M.A.G.; Hutton, B.; Sienkiewicz, M.; Stober, C.; Fernandes, R.; Ibrahim, M.F.K.; Vandermeer, L.; et al. Optimal sequence of adjuvant endocrine and radiation therapy in early-stage breast cancer—A systematic review. Cancer Treat. Rev. 2018, 69, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Souied, O.; Bota, A.B.; Levasseur, N.; Stober, C.; Hilton, J.; Kamel, D.; Hutton, B.; Vandermeer, L.; Mazzarello, S.; et al. Optimal vascular access strategies for patients receiving chemotherapy for early-stage breast cancer: A systematic review. Breast Cancer Res. Treat. 2018, 171, 607–620. [Google Scholar] [CrossRef]
- Awan, A.A.; Hutton, B.; Hilton, J.; Mazzarello, S.; Van Poznak, C.; Vandermeer, L.; Bota, B.; Stober, C.; Sienkiewicz, M.; Fergusson, D.; et al. De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 507–517. [Google Scholar] [CrossRef]
- Pratt, M.; Stevens, A.; Thuku, M.; Butler, C.; Skidmore, B.; Wieland, L.S.; Clemons, M.; Kanji, S.; Hutton, B. Benefits and harms of medical cannabis: A scoping review of systematic reviews. Syst. Rev. 2019, 8, 320. [Google Scholar] [CrossRef]
- Hutton, B.; Hersi, M.; Cheng, W.; Pratt, M.; Barbeau, P.; Mazzarello, S.; Ahmadzai, N.; Skidmore, B.; Morgan, S.C.; Bordeleau, L.; et al. Comparing interventions for management of hot flashes in patients with breast and prostate cancer: A systematic review with meta-analyses. Oncol. Nurs. Forum 2020, 47, E86–E106. [Google Scholar]
- Bradbury, M.; Hutton, B.; Beltran-Bless, A.A.; Alzahrani, M.; Lariviere, T.; Fernandes, R.; Ibrahim, M.F.; Cole, K.; Hilton, J.; Vandermeer, L.; et al. Time to update evidence-based guideline recommendations about concurrent tamoxifen and antidepressant use? A systematic review. Clin. Breast Cancer 2022, 22, e362–e373. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.; Tu, M.M.; Ibrahim, M.F.K.; Basulaiman, B.; McGee, S.F.; Srikanthan, A.; Fernandes, R.; Vandermeer, L.; Stober, C.; Sienkiewicz, M.; et al. Long-term impact of bone-modifying agents for the treatment of bone metastases: A systematic review. Support. Care Cancer 2021, 29, 925–943. [Google Scholar] [CrossRef] [PubMed]
- LeVasseur, N.; Cheng, W.; Mazzarello, S.; Clemons, M.; Vandermeer, L.; Jones, L.; Joy, A.A.; Barbeau, P.; Wolfe, D.; Ahmadzai, N.; et al. Optimising weight-loss interventions in cancer patients—A systematic review and network meta-analysis. PLoS ONE. 2021, 16, e0245794. [Google Scholar] [CrossRef] [PubMed]
- Savard, M.F.; Clemons, M.; Hutton, B.; Jemaan Alzahrani, M.; Caudrelier, J.M.; Vandermeer, L.; Liu, M.; Saunders, D.; Sienkiewicz, M.; Stober, C.; et al. De-escalating adjuvant therapies in older patients with lower risk estrogen receptor-positive breast cancer treated with breast-conserving surgery: A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 99, 102254. [Google Scholar] [CrossRef] [PubMed]
- Surujballi, J.; Shah, H.; Hutton, B.; Alzahrani, M.; Beltran-Bless, A.A.; Shorr, R.; Larocque, G.; McGee, S.; Cole, K.; Ibrahim, M.F.K.; et al. The COVID-19 pandemic: An opportunity to rethink and harmonise the frequency of follow-up visits for patients with early stage breast cancer. Cancer Treat. Rev. 2021, 97, 102188. [Google Scholar] [CrossRef] [PubMed]
- Bourque, J.M.; McGee, S. Evaluating Optimal Timing of Endocrine Therapy and Radiation Therapy in Early-Stage Breast Cancer (REaCT-RETT), NCT03948568, ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03948568 (accessed on 12 November 2021).
- Savard, M.F. Evaluating Harms and Benefits of Endocrine Therapy in Patients ≥70 Years of Age with Lower Risk Breast Cancer, NCT04921137, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04921137 (accessed on 12 November 2021).
- Clemons, M. Individualised Versus Standard Care for Breast Cancer Patients at High-Risk for Chemotherapy-Induced Nausea and Vomiting the ILIAD Study, NCT02861859, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02861859 (accessed on 15 December 2021).
- Clemons, M. Granulocyte-Colony Stimulating Factors or Antibiotics for Primary Prophylaxis for Febrile Neutropenia, NCT02816112, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02816112 (accessed on 12 November 2021).
- Clemons, M.; Fergusson, D.; Simos, D.; Mates, M.; Robinson, A.; Califaretti, N.; Zibdawi, L.; Bahl, M.; Raphael, J.; Ibrahim, M.F.K.; et al. A multicentre, randomised trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy-induced febrile neutropenia in early stage breast cancer. Ann. Oncol. 2020, 31, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Stober, C.; Fergusson, D.; Kehoe, A.; Bedard, D.; MacDonald, F.; Brunet, M.C.; Saunders, D.; Mazzarello, S.; Vandermeer, L.; et al. A multicentre, randomized pilot trial comparing vascular access strategies for early stage breast cancer patients receiving non-trastuzumab containing chemotherapy. Breast Cancer Res. Treat. 2019, 178, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Liu, M.; Stober, C.; Pond, G.; Jemaan Alzahrani, M.; Ong, M.; Ernst, S.; Booth, C.; Mates, M.; Abraham Joy, A.; et al. Two-year results of a randomised trial comparing 4- versus 12-weekly bone-targeted agent use in patients with bone metastases from breast or castration-resistant prostate cancer. J. Bone Oncol. 2021, 30, 100388. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Ong, M.; Stober, C.; Ernst, S.; Booth, C.; Canil, C.; Mates, M.; Robinson, A.; Blanchette, P.; Joy, A.A.; et al. A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer 2021, 142, 132–140. [Google Scholar] [CrossRef]
- Clemons, M. Comparing a Single-Dose vs. Twice Yearly Zoledronate in Patients with Early Stage Breast Cancer (REaCT-ZOL), NCT03664687, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03664687 (accessed on 12 November 2021).
- Ng, T. Comparing Continuation or De-Escalation of Bone Modifying Agents (BMA) in Patients Treated for Over 2 Years for Bone Metastases from Either Breast or Castration-resistant Prostate Cancer, NCT04549207, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04549207 (accessed on 12 November 2021).
- Clemons, M.; Simos, D.; Sienkiewicz, M.; Ng, T.; Zibdawi, L.; Basulaiman, B.; Awan, A.; Fergusson, D.; Vandermeer, L.; Saunders, D.; et al. A prospective multi-centre, randomized study comparing the addition of tapering dexamethasone to other standard of care therapies for taxane-associated pain syndrome (TAPS) in breast cancer patients. Support. Care Cancer 2021, 29, 5787–5795. [Google Scholar] [CrossRef]
- Vickers, M. Feasibility of Using an Integrated Consent Model to Compare Two Standard of Care Regimens for the Management of Hypomagnesemia from Anti-Cancer Therapies, NCT02690012, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02690012 (accessed on 12 November 2021).
- Clemons, M.; Dranitsaris, G.; Sienkiewicz, M.; Sehdev, S.; Ng, T.; Robinson, A.; Mates, M.; Hsu, T.; McGee, S.; Freedman, O.; et al. A randomized trial of individualized versus standard of care antiemetic therapy for breast cancer patients at high risk for chemotherapy-induced nausea and vomiting. Breast 2020, 54, 278–285. [Google Scholar] [CrossRef] [PubMed]
- REaCT: REthinking Clinical Trials. Available online: https://react.ohri.ca/ (accessed on 1 August 2021).

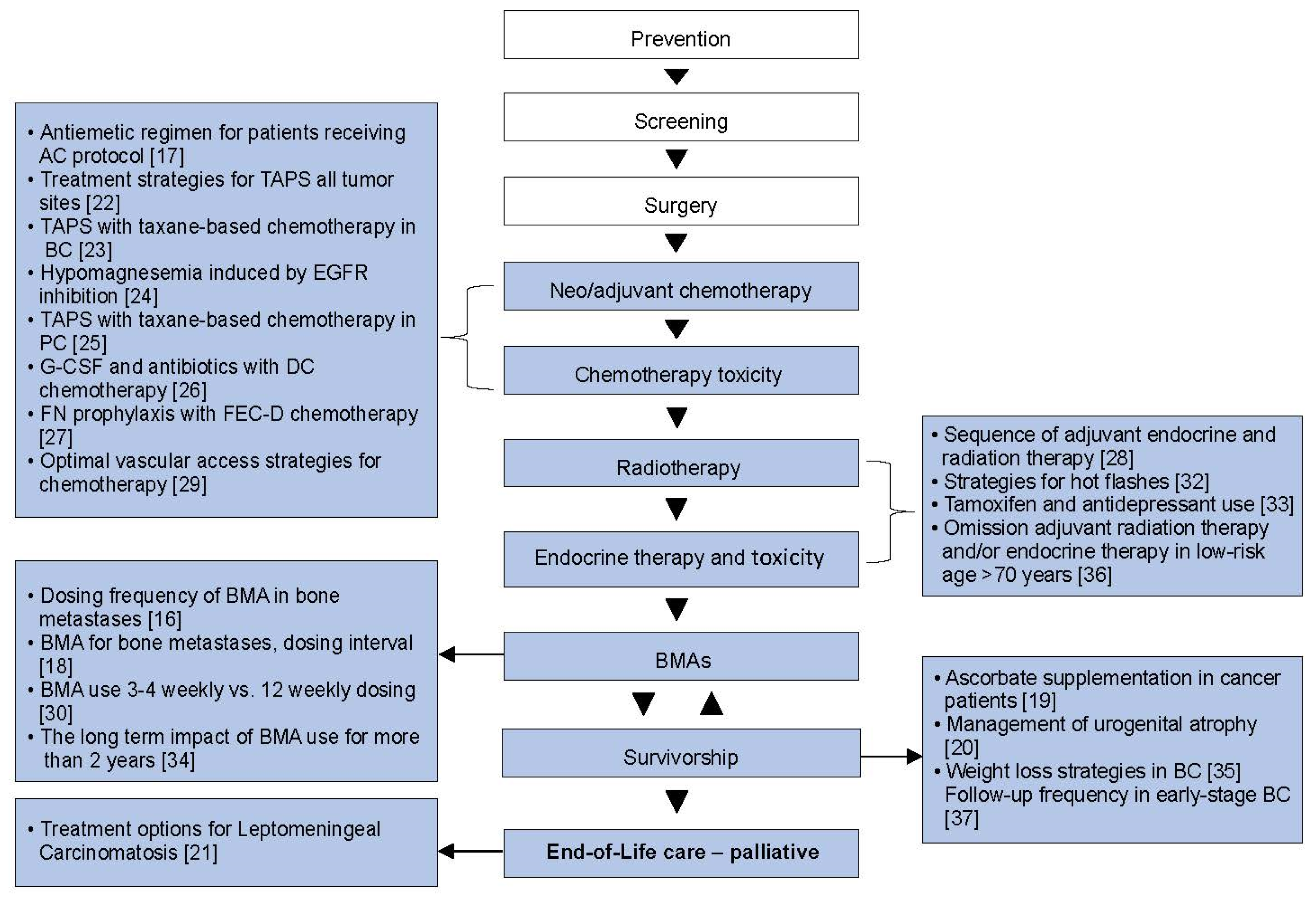
| Phase of Cancer Journey | References | Cancer Type | Topic/Question | Type of Analysis | Synopsis of Review Findings | The Systemic Review Led to |
|---|---|---|---|---|---|---|
| Adjuvant | [28] | BC | Sequence of adjuvant ET and RT in early-stage BC | DA | Concurrent treatment appears safe. Further studies are needed to evaluate treatment-related toxicities. | REaCT-RETT [38] |
| [36] | BC | Omission of adjuvant RT and/or ET in older patients treated with BCS for low-risk BC | MA | RT can be omitted in older patients with lower-risk diseases. | REaCT-70 [39] | |
| Adjuvant Supportive care | [17] | BC | Optimal antiemetic regimen for patients receiving anthracycline and cyclophosphamide-based chemotherapy for BC | NMA | High variability in the outcomes reported by individual RCTs. Identifying an optimal antiemetic regimen was not possilbe. | REaCT-ILIAD [40] |
| [26] | BC | G-CSF and antibiotics use for primary FN prophylaxis in patients receiving DC chemotherapy for BC | DA | Insufficient data to make a recommendation of one strategy over another (G-CSF vs. antibiotics) | REaCT-TC and TC2 [41] | |
| [27] | BC | Primary FN Prophylaxis for Patients who receive FEC-D chemotherapy for BC | DA | Identification of the optimal choice and timing of primary FN prophylaxis was not possible. | REaCT-G and G2 [42] | |
| [29] | BC | Optimal vascular access strategies for patients receiving chemotherapy for early-stage BC | DA | The published evidence identifying the optimal type of venous access is weak. | REaCT-VA Her2 Negative [43] | |
| Metastatic | [21] | BC | Treatment options for leptomeningeal carcinomatosis in BC patients | DA | Limited high-quality evidence exists regarding the optimal treatment of LC-BC | Recommend RCT |
| Palliative Supportive care | [16] | BC | Dosing frequency of BMA for patients with bone metastases from breast cancer | DA | The benefits of standard treatment compared to de-escalated therapy for commonly used BMA requires further study. | Recommend RCT |
| [18] | BC | 4-weekly of BMA vs. de-escalated (Q12-weekly) dosing in BC patients with bone metastases. | MA | No difference in SREs or pain with de-escalated therapy. | REaCT-BTA [44,45] | |
| [25] | PC | TAPS in PC patients who have received taxane-based chemotherapy | DA | Quantified the incidence of TAPS and contributed to explaining the potential risks of developing TAPS in PC. | Recommend RCT | |
| [30] | BC | Efficacy and harms of standard 3–4-weekly versus 12-weekly dosing of BMAs in breast cancer patients with bone metastases | MA | The literature supports de-escalation of zoledronate from the onset for patients with bone metastases from breast cancer. | REaCT-BTA [44,45] REaCT-ZOL [46] | |
| [34] | BC&PC | The long-term impact of BMA use for >2 years in BC or CRPC for the treatment of bone metastases | DA | No high-quality evidence to support the use of BMA for more than two years. | REaCT-HOLD BMA [47] | |
| Both Adjuvant/Palliative Supportive care | [19] | All cancer types | Administration of oral or IV ascorbate in cancer patients | DA | No evidence to suggest that ascorbate in cancer patients either enhances the antitumor effects of chemotherapy or reduces its toxicity | Recommend RCT |
| [22] | All cancer types | Treatment strategies for TAPS across all tumour sites | DA | TAPS remains poorly researched. Fw studies evaluate its optimal management. | Recommend RCT | |
| [23] | BC | TAPS in BC patients who have received taxane-based chemotherapy | DA | The incidence of TAPS varies between taxanes, regimens, and disease settings. | REaCT-TAPS [48] | |
| [24] | All cancer types | Evaluating interventions on hypomagnesemia induced by EGFR inhibition | DA | There is an absence of high-quality evidence for the management of EGFRI-induced hypomagnesemia. | REaCT-Mg [49] | |
| [31] | All cancer types | Benefits and harms of cannabis-based medicines | DA | It is possible that the harms of cannabis-based medicines may outweigh the benefits | Recommend RCT | |
| Survivorship | [20] | BC | Management of urogenital atrophy in breast cancer patients | NMA | Treatment of urogenital atrophy remains a challenging issue. | Recommend RCT |
| [32] | BC&PC | Management of hot flashes in BC or PC patients | NMA | Many interventions may offer improvements for HFs versus no treatment, but no optiml therapy idenified. | Recommend RCT | |
| [33] | BC | Concurrent tamoxifen and antidepressant use | MA | The totality of evidence suggests that concurrent antidepressant and tamoxifen is likely safe. | Conclusion | |
| [35] | BC | Weight loss strategies in patients with early-BC | NMA | Diet and exercise alone or in combination are effective lifestyle interventions. | Conclusion | |
| [37] | BC | Frequency of follow-up visits for patients with early-stage BC | DA | Reduced frequency of follow-up has no adverse effects on BC outcomes. | Conclusion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshamsan, B.; Hutton, B.; Liu, M.; Vandermeer, L.; Clemons, M. Integrating Systematic Reviews into Supportive Care Trial Design: The Rethinking Clinical Trials (REaCT) Program. Curr. Oncol. 2022, 29, 9550-9559. https://doi.org/10.3390/curroncol29120750
Alshamsan B, Hutton B, Liu M, Vandermeer L, Clemons M. Integrating Systematic Reviews into Supportive Care Trial Design: The Rethinking Clinical Trials (REaCT) Program. Current Oncology. 2022; 29(12):9550-9559. https://doi.org/10.3390/curroncol29120750
Chicago/Turabian StyleAlshamsan, Bader, Brian Hutton, Michelle Liu, Lisa Vandermeer, and Mark Clemons. 2022. "Integrating Systematic Reviews into Supportive Care Trial Design: The Rethinking Clinical Trials (REaCT) Program" Current Oncology 29, no. 12: 9550-9559. https://doi.org/10.3390/curroncol29120750
APA StyleAlshamsan, B., Hutton, B., Liu, M., Vandermeer, L., & Clemons, M. (2022). Integrating Systematic Reviews into Supportive Care Trial Design: The Rethinking Clinical Trials (REaCT) Program. Current Oncology, 29(12), 9550-9559. https://doi.org/10.3390/curroncol29120750





