Evaluation of Surgical Approaches and Use of Adjuvant Radiotherapy with Respect to Oncologic Outcomes in the Management of Clinically Early-Stage Cervical Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canadian Cancer Statistics: A 2022 Special Report on Cancer Prevalence; Canadian Cancer Society: Toronto, ON, Canada, 2002.
- Malzoni, M.; Tinelli, R.; Cosentino, F.; Fusco, A.; Malzoni, C. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: Our experience. Ann. Surg. Oncol. 2009, 16, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Magrina, J.F.; Kho, R.M.; Weaver, A.L.; Montero, R.P.; Magtibay, P.M. Robotic radical hysterectomy: Comparison with laparoscopy and laparotomy. Gynecol. Oncol. 2008, 109, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Aarts, J.W.M.; Nieboer, T.E.; Johnson, N.; Tavender, E.; Garry, R.; Mol, B.W.J.; Kluivers, K.B. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst. Rev. 2015, 2015, 1–178. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; del Carmen, M.G.; Yang, J.; Seagle, B.-L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A gynecologic oncology group study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef]
- Peters, W.A.; Liu, P.Y.; Barrett, R.J.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- Rotman, M.; Sedlis, A.; Piedmonte, M.R.; Bundy, B.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 169–176. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Kim, M.H.; Nam, B.H.; Lee, T.S.; Song, E.S.; Park, C.Y.; Kim, J.W.; Kim, Y.B.; Ryu, H.S.; Park, S.Y.; et al. Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: A Korean Gynecologic Oncology Group study. Br. J. Cancer 2014, 110, 278–285. [Google Scholar] [CrossRef]
- Lin, F.; Pan, L.; Li, L.; Li, D.; Mo, L. Effects of a simulated CO2 pneumoperitoneum environment on the proliferation, apoptosis, and metastasis of cervical cancer cells in vitro. Med. Sci. Monit. 2014, 20, 2497–2503. [Google Scholar]
- Volz, J.; Köster, S.; Spacek, Z.; Paweletz, N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer 1999, 86, 770–774. [Google Scholar] [CrossRef]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Arévalo-Serrano, J.; Cǎpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; Chakalova, G.; et al. SUCCOR study: An international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Landoni, F.; Colombo, A.; Milani, R.; Placa, F.; Zanagnolo, V.; Mangioni, C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB–IIA cervical cancer: 20-year update. J. Gynecol. Oncol. 2017, 28, e34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Ni, L.Q.; Wang, S.S.; Lv, Q.L.; Chen, W.J.; Ying, S.P. Outcome and prognostic factors in cervical cancer patients treated with surgery and concurrent chemoradiotherapy: A retrospective study. World J. Surg. Oncol. 2018, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, H.H.B.; Smolders, R.G.V.; Beltman, J.J.; Lambrechts, S.; Trum, H.W.; Yigit, R.; Zusterzeel, P.L.M.; Zweemer, R.P.; Mom, C.H.; Bekkers, R.L.M.; et al. Survival of patients with early-stage cervical cancer after abdominal or laparoscopic radical hysterectomy: A nationwide cohort study and literature review. Eur. J. Cancer 2020, 133, 14–21. [Google Scholar] [CrossRef]
- Paik, E.S.; Lim, M.C.; Kim, M.H.; Kim, Y.H.; Song, E.S.; Seong, S.J.; Suh, D.H.; Lee, J.M.; Lee, C.; Choi, C.H. Comparison of laparoscopic and abdominal radical hysterectomy in early stage cervical cancer patients without adjuvant treatment: Ancillary analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028). Gynecol. Oncol. 2019, 154, 547–553. [Google Scholar] [CrossRef]
- Uppal, S.; Gehrig, P.A.; Peng, K.; Bixel, K.L.; Matsuo, K.; Vetter, M.H.; Davidson, B.A.; Cisa, M.P.; Lees, B.F.; Brunette, L.L.; et al. Recurrence rates in patients with cervical cancer treated with abdominal versus minimally invasive radical hysterectomy: A multi-institutional retrospective review study. J. Clin. Oncol. 2020, 38, 1030–1040. [Google Scholar] [CrossRef]
- Jensen, P.T.; Schnack, T.H.; Frøding, L.P.; Bjørn, S.F.; Lajer, H.; Markauskas, A.; Jochumsen, K.M.; Fuglsang, K.; Dinesen, J.; Søgaard, C.H.; et al. Survival after a nationwide adoption of robotic minimally invasive surgery for early-stage cervical cancer—A population-based study. Eur. J. Cancer 2020, 128, 47–56. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, L.; Xu, H.; Zhang, Y.; Liang, Z. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. BMC Cancer 2015, 15, 928–938. [Google Scholar] [CrossRef]
- Cao, T.; Feng, Y.; Huang, Q.; Wan, T.; Liu, J. Prognostic and Safety Roles in Laparoscopic Versus Abdominal Radical Hysterectomy in Cervical Cancer: A Meta-analysis. J. Laparoendosc. Adv. Surg. Tech. 2015, 25, 990–998. [Google Scholar] [CrossRef]
- Frumovitz, M.; Dos Reis, R.; Sun, C.C.; Milam, M.R.; Bevers, M.W.; Brown, J.; Slomovitz, B.M.; Ramirez, P.T. Comparison of total laparoscopic and abdominal radical hysterectomy for patients with early-stage cervical cancer. Obstet. Gynecol. 2007, 110, 96–102. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer (Version I.2018); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2017. [Google Scholar]
- Rodriguez, J.; Viveros-Carreño, D.; Pareja, R. Adjuvant treatment after radical surgery for cervical cancer with intermediate risk factors: Is it time for an update? Int. J. Gynecol. Cancer 2022, 32, 1219–1226. [Google Scholar] [CrossRef]
- Cibula, D.; Bor, M.; Kocian, R.; Feltl, D.; Argalacsova, S.; Dvorak, P.; Fischerová, D.; Dundr, P.; Jarkovsky, J.; Höschlová, E.; et al. CERVANTES: An international randomized trial of radical surgery followed by adjuvant (chemo) radiation versus no further treatment in patients with early risk cervical cancer (CEEGOG-CX 05; ENGOT-CX16). Int. J. Gynecol. Cancer 2022, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Falconer, H.; Palsdottir, K.; Stalberg, K.; Dahm-Kähler, P.; Ottander, U.; Lundin, E.S.; Wijk, L.; Kimmig, R.; Jensen, P.T.; Eriksson, A.G.Z.; et al. Robot-assisted approach to cervical cancer (RACC): An international multi-center, open-label randomized controlled trial. Int. J. Gynecol. Cancer 2019, 29, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Bixel, K.L.; Leitao, M.M.; Chase, D.M.; Quick, A.; Lim, P.C.; Eskander, R.N.; Gotlieb, W.H.; LoCoco, S.; Martino, M.A.; McCormick, C.; et al. ROCC/GOG-3043: A randomized non-inferiority trial of robotic versus open radical hysterectomy for early-stage cervical cancer. J. Clin. Oncol. 2022, 40, TPS5605. [Google Scholar] [CrossRef]

| A | ||||
|---|---|---|---|---|
| Total (174) | MIS (28) | Open (146) | p-Value | |
| Age (years, median, range) | 43 (22–79) | 41 (24–57) | 43 (22–79) | 0.13 |
| F/U (years, median, range) | 4.1 (0–14.8) | 3.4 (0–7.9) | 4.3 (0.1–14.8) | 0.17 |
| Histopathology | 0.016 | |||
| Adenocarcinoma | 70 (40.2%) | 18 (64.3%) | 52 (35.6%) | |
| Squamous cell carcinoma | 94 (54.0%) | 10 (35.7%) | 84 (57.5%) | |
| Other | 10 (5.7%) | 0 (0%) | 10 (6.8%) | |
| Final stage | 0.003 | |||
| 1A1/2 | 23 (13.2%) | 7 (25%) | 16 (11%) | |
| 1B1 | 100 (57.5%) | 20 (71.4%) | 80 (54.8%) | |
| 1B2 | 22 (12.6%) | 0 (0%) | 22 (15.1%) | |
| >1B2 | 29 (16.7%) | 1 (3.6%) | 28 (19.2%) | |
| Sedlis positive | 60 (34.5%) | 3 (10.7%) | 57 (39.0%) | 0.004 |
| Tumor size (mm, median, range) | 22.3 (no residual–79) | 12 (2.7–33) | 24 (no residual–79) | 0.005 |
| LVSI positive | 66 (37.9%) | 6 (21.4%) | 60 (41.1%) | 0.05 |
| Deep stromal invasion | 72 (41.4%) | 5 (17.9%) | 67 (45.9%) | 0.006 |
| 4-factor model positive | 77 (44.3%) | 6 (21.4%) | 71 (48.6%) | 0.008 |
| Peters criteria positive | 31 (17.8%) | 2 (7.1%) | 29 (19.9%) | 0.17 |
| LN removed (n, median, range) | 12 (0–32) | 11.5 (5–24) | 12 (0–32) | 0.42 |
| LN positive | 26 (14.9%) | 1 (3.6%) | 25 (17.1%) | 0.082 |
| Margin positive | 6 (3.5%) | 0 (0%) | 6 (4.1%) | 0.59 |
| Parametria positive | 7 (4.0%) | 0 (0%) | 7 (4.8%) | 0.60 |
| Adjuvant RT | 81 (46.6%) | 5 (17.9%) | 76 (52.1%) | <0.001 |
| Adjuvant ChemoRT | 62 (35.6%) | 2 (7.1%) | 60 (41.1%) | <0.001 |
| Interval between surgery and RT (days, median, range) | 63.5 | 61 | 64 | 0.59 |
| B | ||||
| Total (174) | Adjuvant RT (81) | No RT (93) | p-Value | |
| Age (years, median, range) | 43 (22–79) | 43 (24–79) | 42 (22–77) | 0.38 |
| F/U (years, median, range) | 4.1 (0–14.8) | 5.2 (0.2–14.8) | 3.3 (0–13.8) | <0.0001 |
| Histopathology | 0.061 | |||
| Adenocarcinoma | 70 (40.2%) | 28 (34.6%) | 42 (45.2%) | |
| Squamous cell carcinoma | 94 (54.0%) | 45 (55.6%) | 49 (52.7%) | |
| Other | 10 (6.9%) | 8 (9.9%) | 2 (2.2%) | |
| Final stage | <0.0001 | |||
| 1A | 23 (13.2%) | 0 (0%) | 23 (24.7%) | |
| 1B1 | 100 (57.5%) | 32 (39.5%) | 68 (73.1%) | |
| 1B2 | 22 (12.6%) | 21 (25.9%) | 1 (1.1%) | |
| >1B2 | 29 (16.7%) | 28 (34.6%) | 1 (1.1%) | |
| Sedlis positive | 60 (34.5%) | 56 (69.1%) | 4 (4.3%) | <0.0001 |
| Tumor size (mm, median, range) | 22.3 (no residual–79) | 36 (6.2–79) | 12 (no residual–55) | <0.0001 |
| LVSI positive | 66 (37.9%) | 54 (66.7%) | 12 (12.9%) | <0.0001 |
| Deep stromal invasion | 72 (41.4%) | 63 (77.8%) | 9 (9.7%) | <0.0001 |
| 4-factor model positive | 77 (44.3%) | 68 (84.0%) | 9 (9.7%) | <0.0001 |
| Peters criteria positive | 31 (17.8%) | 29 (35.8%) | 2 (2.2%) | <0.0001 |
| LN removed (number, median, range) | 12(0–32) | 13 (3–32) | 11 (0–27) | 0.38 |
| LN positive | 26 (14.9%) | 25 (30.9%) | 1 (1.1%) | <0.0001 |
| Margin positive | 6 (3.5%) | 4 (4.9%) | 2 (2.2%) | 0.42 |
| Parametria positive | 7 (4.8%) | 6 (7.4%) | 1 (1.1%) | 0.051 |
| C | ||||
| Open (146) | Adjuvant RT (76) | No RT (70) | p-Value | |
| Age (years, median, range) | 43 (22–79) | 43 (24–79) | 43 (22–77) | 0.78 |
| F/U (years, median, range) | 4.3 (0.1–14.8) | 5.2 (0.2–14.8) | 3.3 (0.1–13.8) | <0.0001 |
| Histopathology | 0.187 | |||
| Adenocarcinoma | 52 (35.6%) | 26 (34.2%) | 26 (37.1%) | |
| Squamous cell carcinoma | 84 (57.5%) | 42 (55.3%) | 42 (60%) | |
| Other | 10 (6.8%) | 8 (10.5%) | 2 (2.9%) | |
| Final stage | <0.0001 | |||
| 1A | 16 (11.0%) | 0 | 16 | |
| 1B1 | 80 (54.8%) | 28 | 52 | |
| 1B2 | 22 (15.1%) | 21 | 1 | |
| >1B2 | 28 (19.2%) | 27 | 1 | |
| Sedlis positive | 57 (39.0%) | 53 (69.7%) | 4 (5.7%) | <0.0001 |
| Tumor size (mm, median, range) | 24 (no residual–79) | 38 (6.2–79) | 12 (no residual–55) | <0.0001 |
| LVSI positive | 60 (41.1%) | 50 (65.8%) | 10 (14.3%) | <0.0001 |
| Deep stromal invasion | 67 (45.9%) | 60 (79.0%) | 7 (10%) | <0.0001 |
| 4-factor model positive | 71 (48.6%) | 64 (84.2%) | 7 (10%) | <0.0001 |
| Peters criteria positive | 29 (19.9%) | 27 (35.5%) | 2 (2.9%) | <0.0001 |
| LN removed (number, median, range) | 12 (0–32) | 13 (3–32) | 11 (0–27) | 0.16 |
| LN positive | 25 (17.1%) | 24 (31.6%) | 1 (1.4%) | <0.0001 |
| Margin positive | 6 (4.1%) | 4 (5.3%) | 2 (2.9%) | 0.68 |
| Parametria positive | 7 (4.8%) | 6 (7.9%) | 1 (1.4%) | 0.118 |
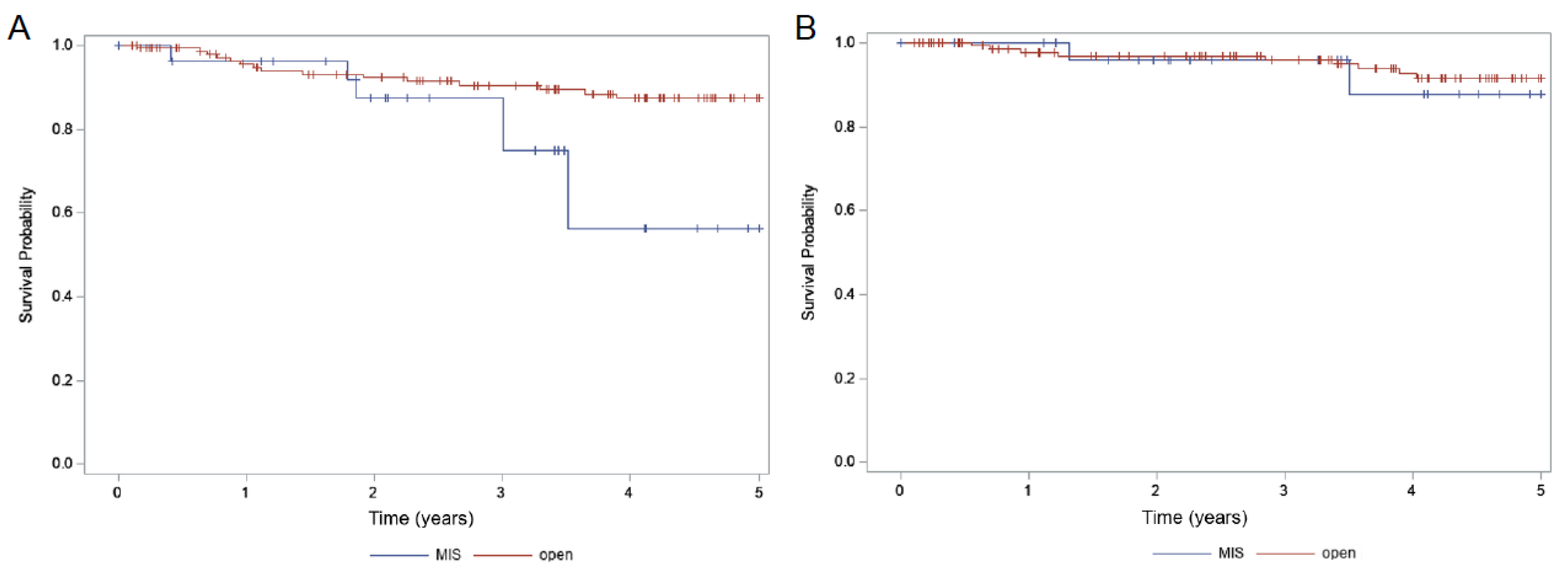
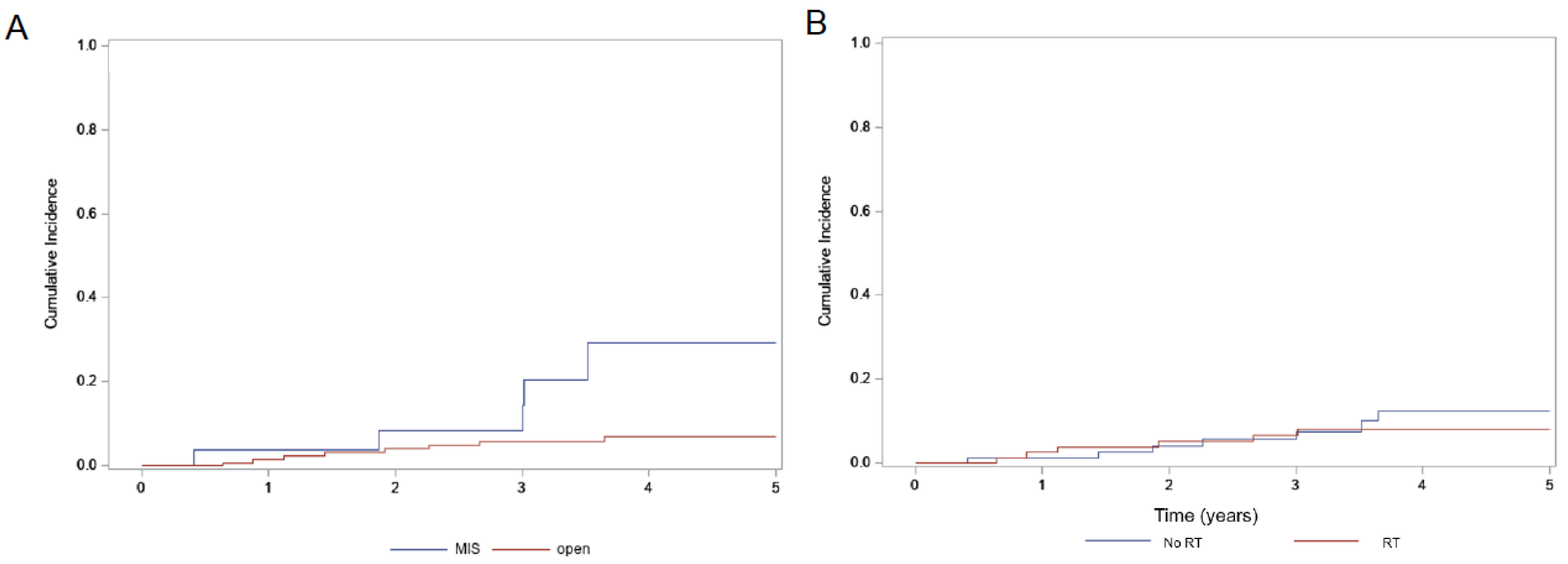
| 5-Year OS (%) | p-Value | 5-Year PFS (%) | p-Value | 5-Year Locoregional Recurrence (%) | p-Value | |
|---|---|---|---|---|---|---|
| Total (174) | 91.1% | 83.6% | 9.7% | |||
| MIS (28) | 87.9% | 0.72 | 56.1% | 0.014 | 29.4% | 0.011 |
| Open (146) | 91.5% | 87.3% | 6.8% | |||
| Adjuvant RT (81) | 88.5% | 0.25 | 82.4% | 0.54 | 8.2% | 0.59 |
| No RT (93) | 94.7% | 83.8% | 12.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgess, L.; AlDuwaisan, W.; Zhang, T.; Lupe, K.; Fung-Kee-Fung, M.; Faught, W.; Le, T.; Samant, R. Evaluation of Surgical Approaches and Use of Adjuvant Radiotherapy with Respect to Oncologic Outcomes in the Management of Clinically Early-Stage Cervical Carcinoma. Curr. Oncol. 2022, 29, 9525-9534. https://doi.org/10.3390/curroncol29120748
Burgess L, AlDuwaisan W, Zhang T, Lupe K, Fung-Kee-Fung M, Faught W, Le T, Samant R. Evaluation of Surgical Approaches and Use of Adjuvant Radiotherapy with Respect to Oncologic Outcomes in the Management of Clinically Early-Stage Cervical Carcinoma. Current Oncology. 2022; 29(12):9525-9534. https://doi.org/10.3390/curroncol29120748
Chicago/Turabian StyleBurgess, Laura, Wafa AlDuwaisan, Tinghua Zhang, Krystine Lupe, Michael Fung-Kee-Fung, Wylam Faught, Tien Le, and Rajiv Samant. 2022. "Evaluation of Surgical Approaches and Use of Adjuvant Radiotherapy with Respect to Oncologic Outcomes in the Management of Clinically Early-Stage Cervical Carcinoma" Current Oncology 29, no. 12: 9525-9534. https://doi.org/10.3390/curroncol29120748
APA StyleBurgess, L., AlDuwaisan, W., Zhang, T., Lupe, K., Fung-Kee-Fung, M., Faught, W., Le, T., & Samant, R. (2022). Evaluation of Surgical Approaches and Use of Adjuvant Radiotherapy with Respect to Oncologic Outcomes in the Management of Clinically Early-Stage Cervical Carcinoma. Current Oncology, 29(12), 9525-9534. https://doi.org/10.3390/curroncol29120748





