Addition of New Androgen Receptor Pathway Inhibitors to Docetaxel and Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Metanalysis
Abstract
:1. Introduction
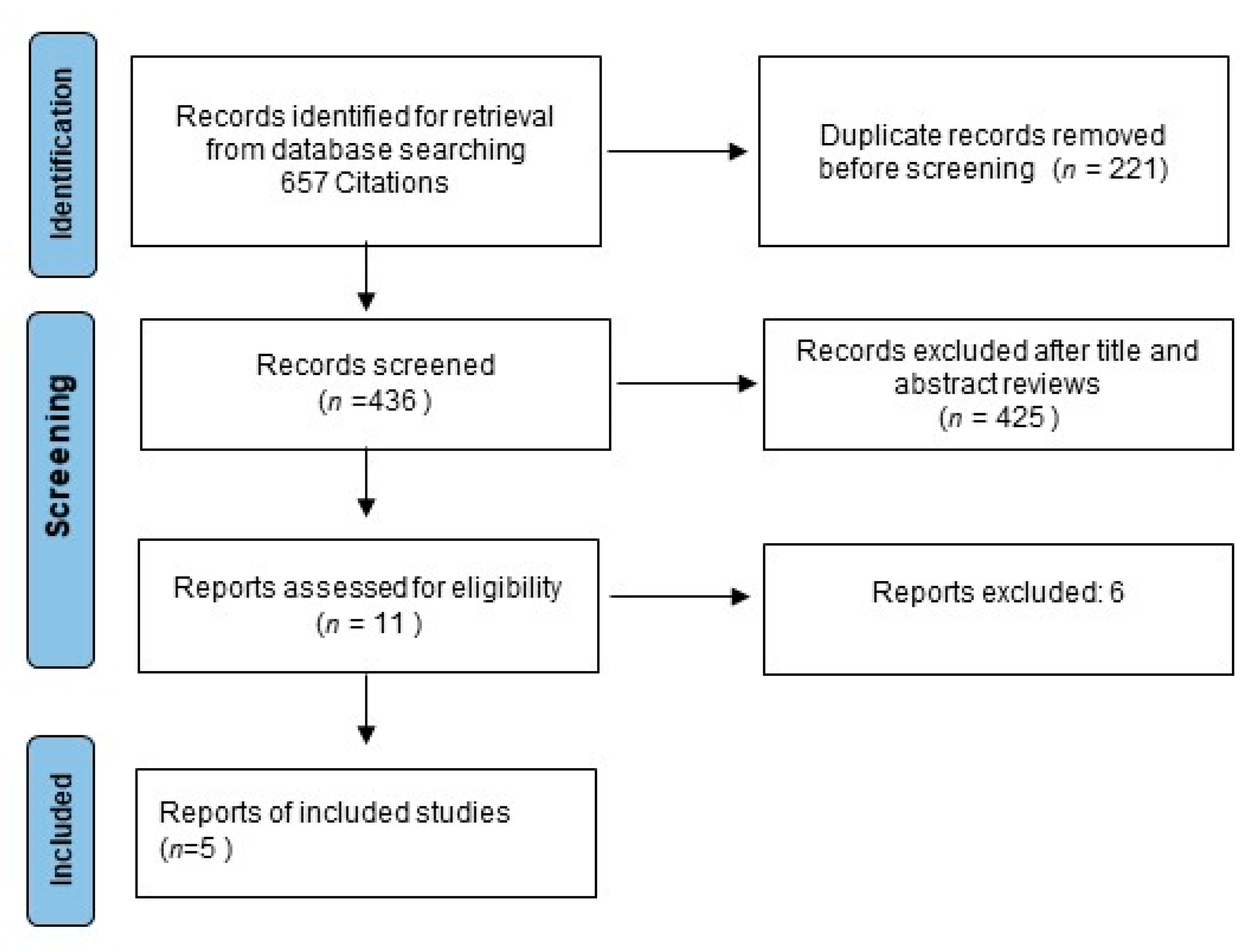
2. Methods
2.1. Study Selection and Data Extraction
2.2. Data Extraction
2.3. Statistical Methods
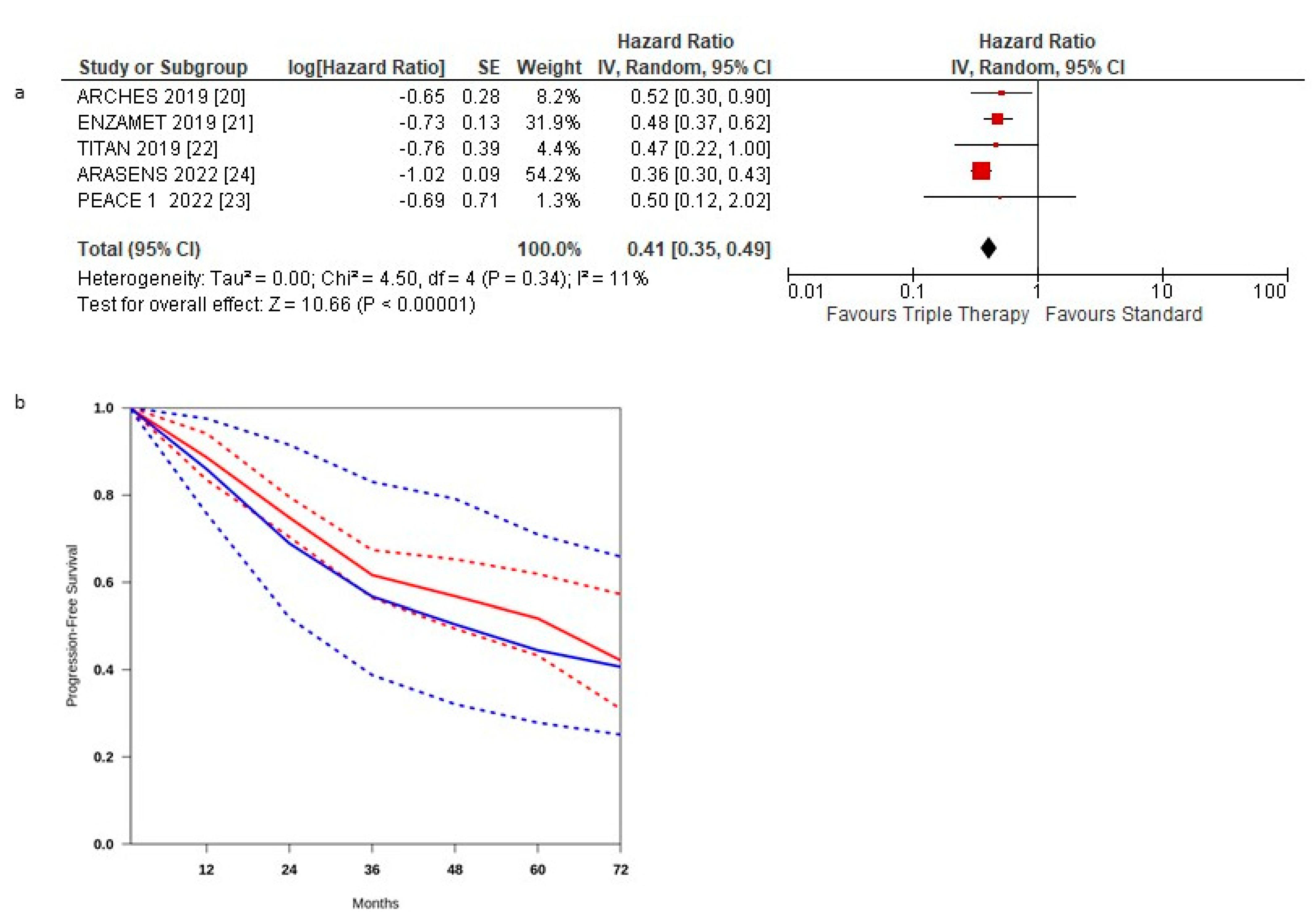
3. Results
3.1. Progression-Free Survival
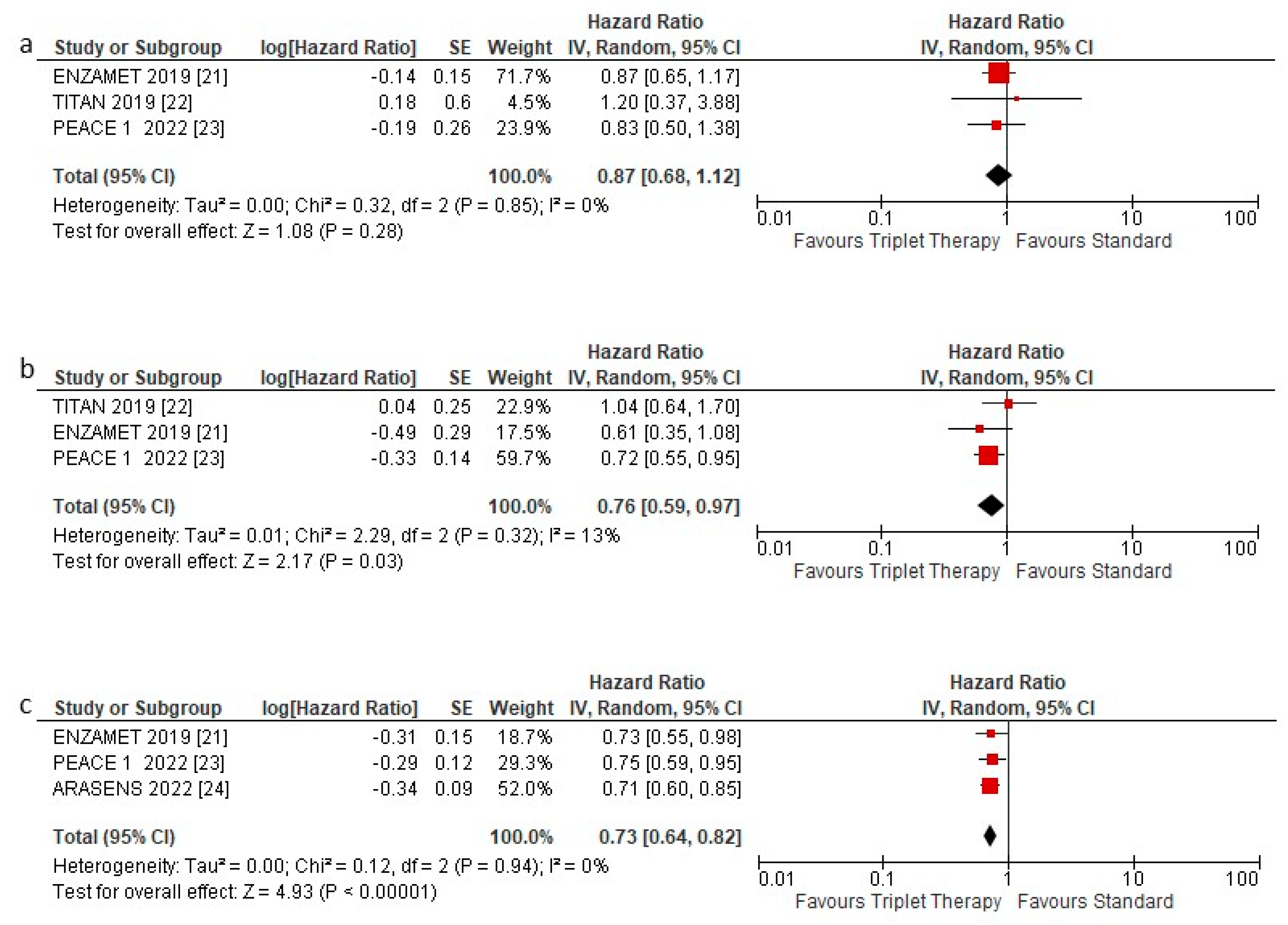
3.2. Overall Survival
3.3. Overall Survival according to Subgroups
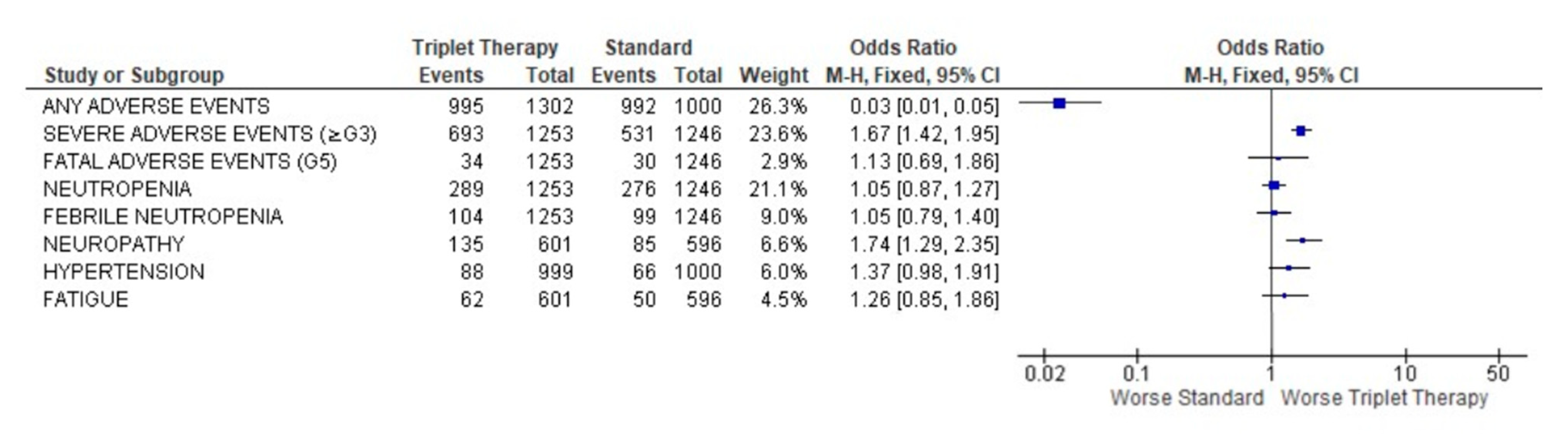
3.4. Safety Profile of Triplet Therapy
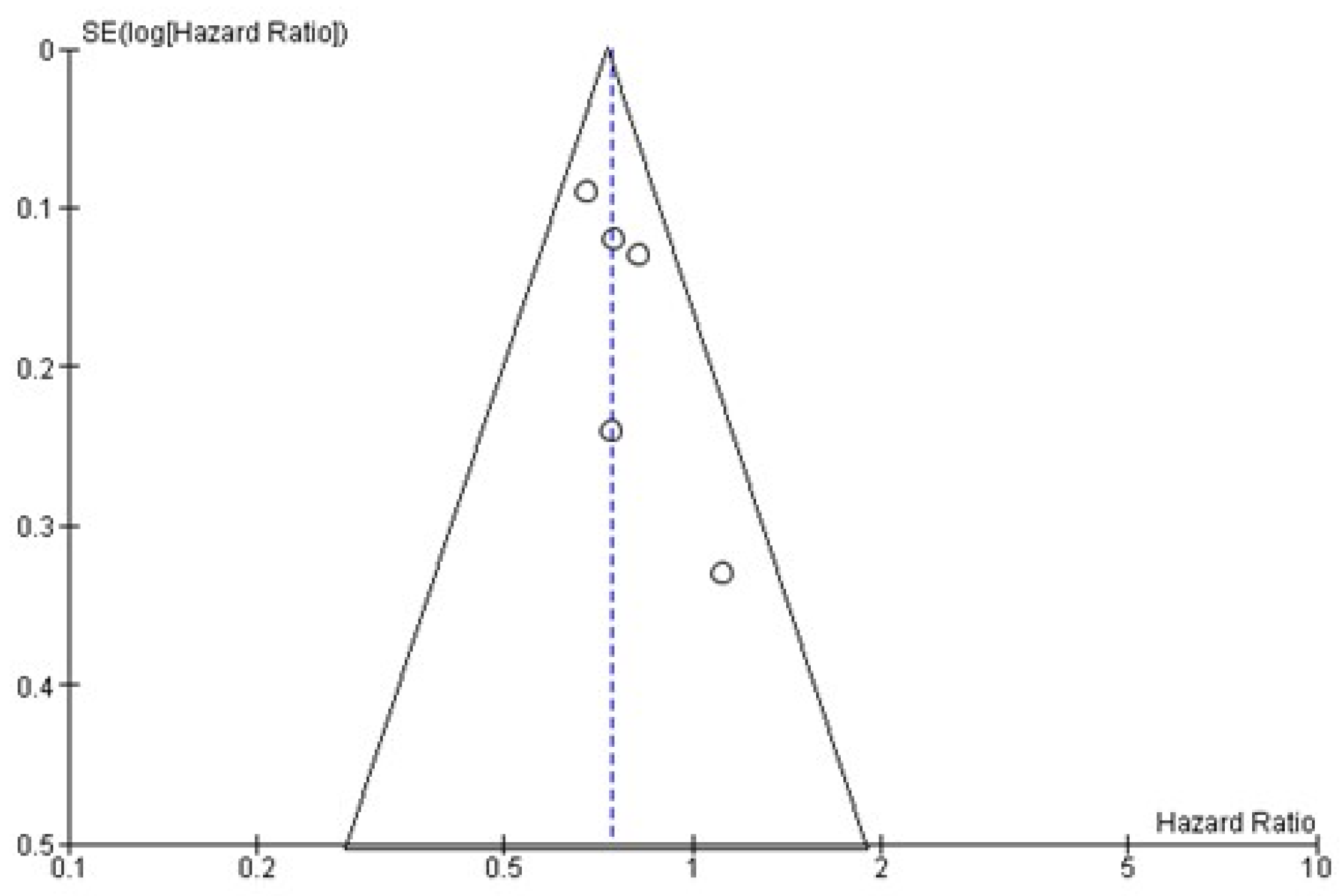
4. Publication Bias
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Cancer Society. Cancer Facts & Figures 2022. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html (accessed on 3 August 2022).
- Huggins, C.; Hodges, C.V. Studies on Prostatic Cancer: I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Motoyama, D.; Ito, T.; Sugiyama, T.; Otsuka, A. Prognostic Significance of Time to Castration Resistance in Patients with Metastatic Castration-sensitive Prostate Cancer. Anticancer Res. 2019, 39, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; De Bono, J.S.; Molina, A.; Logothetis, C.J.; De Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; De Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [Green Version]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [Green Version]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Carducci, M.; Eisenberger, M.; Kohli, M.; Vogelzang, N.J.; Hussain, M. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [Green Version]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; De Bono, J.S.; Gale, J.; Hetherington, J.; Hoskin, P.J.; Jones, R.J.; Laing, R.; et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur. Urol. 2015, 67, 1028–1038. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.; Soto, A.J.; Merseburger, A.S.; Özgüroglu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Koschel, S.; Thangasamy, I.A.; Teh, J.; Alghazo, O.; Butcher, G.; Howard, H.; Kapoor, J.; Lawrentschuk, N.; Siva, S.; et al. Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur. Urol. 2019, 77, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sydes, M.; Spears, M.; Mason, M.; Clarke, N.; Dearnaley, D.; de Bono, J.; Attard, G.; Chowdhury, S.; Cross, W.; Gillessen, S.; et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: Directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann. Oncol. 2018, 29, 1235–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Combescure, C.; Foucher, Y.; Jackson, D. Meta-analysis of single-arm survival studies: A distribution-free approach for estimating summary survival curves with random effects. Stat. Med. 2014, 33, 2521–2537. [Google Scholar] [CrossRef]
- Earle, C.C.; Wells, G.A. An Assessment of Methods to Combine Published Survival Curves. Med. Decis. Mak. 2000, 20, 104–111. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cabibbo, G.; Enea, M.; Attanasio, M.; Bruix, J.; Craxi, A.; Cammà, C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2009, 51, 1274–1283. [Google Scholar] [CrossRef]
- Rush, H.L.; Murphy, L.; Morgans, A.K.; Clarke, N.W.; Cook, A.D.; Attard, G.; Macnair, A.; Dearnaley, D.P.; Parker, C.C.; Russell, J.M.; et al. Quality of Life in Men with Prostate Cancer Randomly Allocated to Receive Docetaxel or Abiraterone in the STAMPEDE Trial. J. Clin. Oncol. 2022, 40, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Boher, J.M.; Chen, Y.H.; Liu, G.; Fizazi, K.; Carducci, M.A.; Oudard, S.; Joly, F.; Jarrard, D.M.; Soulie, M.; et al. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur. Urol. 2018, 73, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- Zong, Y.; Goldstein, A. Adaptation or selection—Mechanisms of castration-resistant prostate cancer. Nat. Rev. Urol. 2012, 10, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Caffo, O.; Buttigliero, C.; Cavaliere, C.; D’Aniello, C.; Di Maio, M.; Kinspergher, S.; Maines, F.; Rizzo, M.; Rossetti, S.; et al. Therapeutic options for first-line metastatic castration-resistant prostate cancer: Suggestions for clinical practise in the CHAARTED and LATITUDE era. Cancer Treat. Rev. 2019, 74, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Bai, S.; Zhang, B.Y. Impact of taxanes on androgen receptor signaling. Asian J. Androl. 2019, 21, 249–252. [Google Scholar] [CrossRef]
- Eisenberger, M.A.; Blumenstein, B.A.; Crawford, E.D.; Miller, G.; McLeod, D.G.; Loehrer, P.J.; Wilding, G.; Sears, K.; Culkin, D.J.; Thompson, I.M.; et al. Bilateral Orchiectomy with or without Flutamide for Metastatic Prostate Cancer. N. Engl. J. Med. 1998, 339, 1036–1042. [Google Scholar] [CrossRef] [Green Version]
- Crawford, E.D.; Eisenberger, M.A.; McLeod, D.G.; Spaulding, J.T.; Benson, R.; Dorr, F.A.; Blumenstein, B.A.; Davis, M.A.; Goodman, P.J. A Controlled Trial of Leuprolide with and without Flutamide in Prostatic Carcinoma. N. Engl. J. Med. 1989, 321, 419–424. [Google Scholar] [CrossRef]
- Hussain, M.; Tangen, C.M.; Berry, D.L.; Higano, C.S.; Crawford, E.D.; Liu, G.; Wilding, G.; Prescott, S.; Sundaram, S.K.; Small, E.J.; et al. Intermittent versus Continuous Androgen Deprivation in Prostate Cancer. N. Engl. J. Med. 2013, 368, 1314–1325. [Google Scholar] [CrossRef]
- Naqvi, S.A.A.; Bin Riaz, Z.; Riaz, A.; Islam, M.; Siddiqi, R.; Ikram, W.; Jafar, M.A.; Singh, P.; Ravi, P.K.; Bin Riaz, I.; et al. Triplet therapy in metastatic castration-sensitive prostate cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2022, 40, 136. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttigliero, C.; Tucci, M.; Bertaglia, V.; Vignani, F.; Bironzo, P.; Di Maio, M.; Scagliotti, G.V. Understanding and overcoming the mechanisms of primary and acquired resistance to abiraterone and enzalutamide in castration resistant prostate cancer. Cancer Treat. Rev. 2015, 41, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.W.; Soto, Á.J.; Merseburger, A.S.; Ozguroglu, M.; Uemura, H.; et al. Time to second progression (PFS2) in patients (pts) from TITAN with metastatic castration-sensitive prostate cancer (mCSPC) by first subsequent therapy (hormonal vs. taxane). J. Clin. Oncol. 2020, 38, 82. [Google Scholar] [CrossRef]

| Type of Study | Experimental Arm | Sample Size | Inclusion Criteria | Median F-Up Months | Mean Age | Gleason Score ≥ 8 (%) | ECOG 0 (%) | High Volume Disease (%) | Bone Metastasis (%) | Visceral Metastasis (%) | De Novo Metastasis (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARCHES [20] | Post hoc analysis RCT | ARPI + Docetaxel + hormosoppression | 576 | mHSPC | 14.4 * | 70 * | 66 * | 77.5 * | 63.2 * | 84.4 * | 4.9 * | 72 * |
| ENZAMET [21] | Post hoc analysis RCT | ARPI + Docetaxel + hormosoppression | 503 | mHSPC | 34 * | 69.2 * | 58.3 * | 72 * | 52.3 * | 80.7 * | 12 * | 58 * |
| TITAN [22] | Post hoc analysis RCT | ARPI + Docetaxel + hormosoppression | 113 | mHSPC EXCLUDED if visceral met is the only site of metastasis | 22.9 * | 69 * | 67.4 * | 64.3 * | 62.7 * | 100 * | 13.6 * | 85 * |
| PEACE 1 [23] | Randomized trial | ARPI + Docetaxel + hormosoppression | 710 | mHSPC | 42 | 66 | 76.9 | 69.8 | 64.2 | 79.7 | 12.4 | 100 |
| ARASENS [24] | Randomized trial | ARPI + Docetaxel + hormosoppression | 1305 | mHSPC | nr | 67 | 78.2 | 71.1 | nr | 79.5 | 17.5 | 86.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorica, F.; Buttigliero, C.; Grigolato, D.; Muraro, M.; Turco, F.; Munoz, F.; Tucci, M. Addition of New Androgen Receptor Pathway Inhibitors to Docetaxel and Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Metanalysis. Curr. Oncol. 2022, 29, 9511-9524. https://doi.org/10.3390/curroncol29120747
Fiorica F, Buttigliero C, Grigolato D, Muraro M, Turco F, Munoz F, Tucci M. Addition of New Androgen Receptor Pathway Inhibitors to Docetaxel and Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Metanalysis. Current Oncology. 2022; 29(12):9511-9524. https://doi.org/10.3390/curroncol29120747
Chicago/Turabian StyleFiorica, Francesco, Consuelo Buttigliero, Daniela Grigolato, Marco Muraro, Fabio Turco, Fernando Munoz, and Marcello Tucci. 2022. "Addition of New Androgen Receptor Pathway Inhibitors to Docetaxel and Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Metanalysis" Current Oncology 29, no. 12: 9511-9524. https://doi.org/10.3390/curroncol29120747
APA StyleFiorica, F., Buttigliero, C., Grigolato, D., Muraro, M., Turco, F., Munoz, F., & Tucci, M. (2022). Addition of New Androgen Receptor Pathway Inhibitors to Docetaxel and Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Metanalysis. Current Oncology, 29(12), 9511-9524. https://doi.org/10.3390/curroncol29120747






