Precision Oncology in Canada: Converting Vision to Reality with Lessons from International Programs
Abstract
:1. Introduction
1.1. Precision Oncology: The Vision and Reality
1.2. The Canadian Situation
1.3. HTA Assessment Challenges Specific to Next-Generation Sequencing
1.4. The Way Forward
1.5. Goal of the Paper
2. Materials and Methods
Search Strategy and Selection Criteria
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente, A.M.; Ballensiefen, W.; Jonsson, J.I. How personalised medicine will transform healthcare by 2030: The ICPerMed vision. J. Transl. Med. 2020, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, G.S.; Phillips, K.A. Precision Medicine: From Science to Value. Health Aff. 2018, 37, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Canada’s Drug and Health Technology Agency. Real-World Evidence for Decision-Making. 2022. Available online: https://www.cadth.ca/real-world-evidence-decision-making (accessed on 18 September 2021).
- Blasimme, A.; Fadda, M.; Schneider, M.; Vayena, E. Data Sharing for Precision Medicine: Policy Lessons and Future Directions. Health Aff. 2018, 37, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Ciliberto, G.; Conte, P.; Curigliano, G.; Seijo, L.; Montuenga, L.M.; Garassino, M.; Penault-Llorca, F.; Galli, F.; Ray-Coquard, I.; et al. Bringing Onco-Innovation to Europe’s Healthcare Systems: The Potential of Biomarker Testing, Real World Evidence, Tumour Agnostic Therapies to Empower Personalised Medicine. Cancers 2021, 13, 583. [Google Scholar] [CrossRef]
- Cobain, E.F.; Wu, Y.M.; Vats, P.; Chugh, R.; Worden, F.; Smith, D.C.; Schuetze, S.M.; Zalupski, M.M.; Sahai, V.; Alva, A.; et al. Assessment of Clinical Benefit of Integrative Genomic Profiling in Advanced Solid Tumors. JAMA Oncol. 2021, 7, 525–533. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Rhodes, D.; Wick, M.; Okuno, S.; Kunze, K.L.; Golafshar, M.; Uson, P.L.; Mountjoy, L.; et al. Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients with Hereditary Cancer Syndrome. JAMA Oncol. 2021, 7, 230–237. [Google Scholar] [CrossRef]
- Joly, Y. Approval of new pharmacogenomic tests: Is the Canadian regulatory process adequate? CJLT 2010, 8, 215–241. [Google Scholar]
- Schuler, M. The quest for efficient trial designs in precision oncology. Lancet Oncol. 2020, 21, 1539–1541. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Turner, J.H. Ethics of Pharma Clinical Trials in the Era of Precision Oncology. Cancer Biother. Radiopharm. 2021, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Canada, I.M. Canadian Public Reimbursement Timelines; Innovative Medicines Canada: Ottawa, ON, Canada, 2022. [Google Scholar]
- Hoskyn, S.L. Innovative Medicines Canada. Explaining Public Reimbursement Delays for New Medicines for Canadian Patients; Innovative Medicines Canada: Ottawa, ON, Canada, 2020. [Google Scholar]
- Organisation for Economic Co-Operation and Development (OECD). Policy Issues for the Development and Use of Biomarkers in Health. 2011. Available online: https://www.oecd.org/health/biotech/49023036.pdf (accessed on 1 March 2021).
- Yip, S.; Christofides, A.; Banerji, S.; Downes, M.R.; Izevbaye, I.; Lo, B.; MacMillan, A.; McCuaig, J.; Stockley, T.; Yousef, G.M.; et al. A Canadian guideline on the use of next-generation sequencing in oncology. Curr. Oncol. 2019, 26, e241–e254. [Google Scholar] [CrossRef] [PubMed]
- Novartis Pharmaceuticals Canada Inc. Gleevec (Imatinib Mesylate) Product Monograph, October 8, 2019; Novartis Pharmaceuticals Canada Inc.: Dorval, QC, Canada, 2019. [Google Scholar]
- Hoffmann-La Roche Limited. Tarceva (Erlotinib Hydrochloride Tablets) Product Monograph, September 4, 2018; Hoffmann-La Roche Limited: Basel, Switzerland, 2018. [Google Scholar]
- Takeda Canada Inc. Alunbrig (Brigatinib Tablets) Product Monograph, March 2, 2018; Takeda Canada Inc.: Toronto, ON, Canada, 2018. [Google Scholar]
- Pfizer Canada ULC. Xalkori (Crizotinib Capsules) Product Monograph, February 3, 2021; Pfizer Canada ULC: Kirkland, QC, Canada, 2021. [Google Scholar]
- Génome Québec. Personalized Medicine. 2021. Available online: https://www.genomequebec.com/en/health/personalized-medicine/ (accessed on 9 March 2021).
- Canadian Agency for Drugs and Technologies in Health (CADTH). Pharmacoeconomic Report Entrectinib (Rozlytrek). Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10206EntrectinibROS1NSCLC_inEGR_NOREDACT-ABBREV_Post08Jan2021_final.pdf (accessed on 1 April 2021).
- Remon, J.; Dienstmann, R. Precision oncology: Separating the wheat from the chaff. ESMO Open 2018, 3, e000446. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C.; Soria, J.C.; Bardelli, A.; Biankin, A.; Caldas, C.; Chandarlapaty, S.; de Koning, L.; Dive, C.; Feunteun, J.; Leung, S.Y.; et al. Consensus on precision medicine for metastatic cancers: A report from the MAP conference. Ann. Oncol. 2016, 27, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Perdrizet, K.; Stockley, T.; Law, J.H.; Shabir, M.; Zhang, T.; Le, L.W.; Lau, A.; Tsao, M.S.; Pal, P.; Cabanero, M.; et al. Non-small cell lung cancer next generation sequencing using the Oncomine Comprehensive Assay v3: Integrating expanded genomic sequencing into the Canadian publicly funded health care model. Poster presented by: The University of Toronto and the University Health Network. J. Clin. Oncol. 2019, 37, 2620. [Google Scholar]
- Perdrizet, K.; Stockley, T.; Tsao, C.K.; Morganstein, J.; Kamel-Reid, S.; Ranich, L.; Shepherd, F.A.; Liu, G.; Bradbury, P.A.; Hwang, D.; et al. Upfront next generation sequencing in NSCLC: A publicly funded perspective. Poster presented by: The University of Toronto and the University Health Network. J. Clin. Oncol. 2018, 36, 12062. [Google Scholar] [CrossRef]
- Colomer, R.; Mondejar, R.; Romero-Laorden, N.; Alfranca, A.; Sanchez-Madrid, F.; Quintela-Fandino, M. When should we order a next generation sequencing test in a patient with cancer? EClinicalMedicine 2020, 25, 100487. [Google Scholar] [CrossRef]
- Legras, A.; Barritault, M.; Tallet, A.; Fabre, E.; Guyard, A.; Rance, B.; Digan, W.; Pecuchet, N.; Giroux-Leprieur, E.; Julie, C.; et al. Validity of Targeted Next-Generation Sequencing in Routine Care for Identifying Clinically Relevant Molecular Profiles in Non-Small-Cell Lung Cancer: Results of a 2-Year Experience on 1343 Samples. J. Mol. Diagn. 2018, 20, 550–564. [Google Scholar] [CrossRef]
- Stockley, T.L.; Oza, A.M.; Berman, H.K.; Leighl, N.B.; Knox, J.J.; Shepherd, F.A.; Chen, E.X.; Krzyzanowska, M.K.; Dhani, N.; Joshua, A.M.; et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: The Princess Margaret IMPACT/COMPACT trial. Genome Med. 2016, 8, 109. [Google Scholar] [CrossRef]
- Roberts, M.C.; Kennedy, A.E.; Chambers, D.A.; Khoury, M.J. The current state of implementation science in genomic medicine: Opportunities for improvement. Genet. Med. 2017, 19, 858–863. [Google Scholar] [CrossRef]
- Weymann, D.; Dragojlovic, N.; Pollard, S.; Regier, D.A. Allocating healthcare resources to genomic testing in Canada: Latest evidence and current challenges. J. Community Genet. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Elements of Real World Data/Evidence Quality throughout the Prescription Drug Product Life Cycle. Available online: https://www.canada.ca/en/services/health/publications/drugs-health-products/real-world-data-evidence-drug-lifecycle-report.html (accessed on 7 June 2022).
- Health Canada. Optimizing the Use of Real World Evidence to Inform Regulatory Decision-Making. 2019. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/optimizing-real-world-evidence-regulatory-decisions.html (accessed on 28 February 2021).
- U.S. Food and Drug Administration. Use of Public Human Genetic Variant Databases to Support Clinical Validity for Genetic and Genomic-Based In Vitro Diagnostics: Guidance for Stakeholders and Food and Drug Administration Staff. 2018. Available online: https://www.fda.gov/media/99200/download (accessed on 26 February 2021).
- Impact Medicom. Determining Priority Access to Comprehensive Genomic Profiling for Canadian Patients with Cancer. 2020. Available online: https://www.impactmedicom.com/publications/reports#h.bas714sn3vxo (accessed on 9 March 2021).
- Weber, S.; Spiegl, B.; Perakis, S.O.; Ulz, C.M.; Abuja, P.M.; Kashofer, K.; van der Leest, P.; Azpurua, M.A.; Tamminga, M.; Brudzewsky, D.; et al. Technical Evaluation of Commercial Mutation Analysis Platforms and Reference Materials for Liquid Biopsy Profiling. Cancers 2020, 12, 1588. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Guidelines Index. Available online: https://www.ich.org/page/search-index-ich-guidelines (accessed on 16 March 2021).
- Zhang, R.; Li, Q.; Fu, J.; Jin, Z.; Su, J.; Zhang, J.; Chen, C.; Geng, Z.; Zhang, D. Comprehensive analysis of genomic mutation signature and tumor mutation burden for prognosis of intrahepatic cholangiocarcinoma. BMC Cancer 2021, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Precision Medicine Vision Statement: A Product of the World Economic Forum Global Precision Medicine Council. World Economic Forum. 2020. Available online: https://www.weforum.org/reports/precision-medicine-vision-statement-a-product-of-the-world-economic-forum-global-precision-medicine-council (accessed on 2 March 2021).
- Dana, G. Why Precision Medicine Won’t Transform Healthcare—But Governance Could. World Economic Forum. 2020. Available online: https://www.weforum.org/agenda/2020/05/why-precision-medicine-won-t-transform-healthcare-but-governance-could/ (accessed on 1 March 2021).
- Vayena, E. Value from health data: European opportunity to catalyse progress in digital health. Lancet 2021, 397, 652–653. [Google Scholar] [CrossRef]
- National Institutes of Health. All of Us Research Program. Available online: https://allofus.nih.gov/ (accessed on 27 February 2021).
- Sungwon, J.; Youngjune, B.; Yeonsong, C.; Yeonsu, J.; Seunghoon, K.; Jaeyoung, J.; Jang, J.; Blazyte, A.; Kim, C.; Kim, Y.; et al. Korean Genome Project: 1094 Korean personal genomes with clinical information. Sci. Adv. 2020, 6, eaaz7835. [Google Scholar]
- Ginsburg, G.S. A Global Collaborative to Advance Genomic Medicine. Am. J. Hum. Genet. 2019, 104, 407–409. [Google Scholar] [CrossRef]
- McDermott, F.D.; Newton, K.; Beggs, A.D.; Clark, S.K. Implications for the colorectal surgeon following the 100,000 Genomes Project. Colorectal Dis. 2021, 23, 1049–1058. [Google Scholar] [CrossRef]
- Turnbull, C. Introducing Whole-Genome Sequencing into Routine Cancer Care: The Genomics England 100,000 Genomes Project. Ann. Oncol. 2018, 29, 784–787. [Google Scholar] [CrossRef]
- Global Alliance for Genomics and Health. Available online: https://www.ga4gh.org/ (accessed on 3 March 2021).
- Australian Genomics Health Alliance. Available online: https://www.australiangenomics.org.au/ (accessed on 27 February 2021).
- Hofman, P.; Rouleau, E.; Sabourin, J.C.; Denis, M.; Deleuze, J.F.; Barlesi, F.; Laurent-Puig, P. Predictive molecular pathology in non-small cell lung cancer in France: The past, the present and the perspectives. Cancer Cytopathol. 2020, 128, 601–610. [Google Scholar] [CrossRef]
- Group FGMWS; Auzanneau, C.; Bacq, D.; Bellera, C.; Blons, H.; Boland, A.; Boucheix, M.; Bourdon, A.; Chollet, E.; Chomienne, C.; et al. Feasibility of high-throughput sequencing in clinical routine cancer care: Lessons from the cancer pilot project of the France Genomic Medicine 2025 plan. ESMO Open 2020, 5, e000744. [Google Scholar] [CrossRef]
- Unicancer. Available online: http://www.unicancer.fr/en/unicancer-group/organisation (accessed on 9 March 2021).
- Doebele, R.C.; Perez, L.; Trinh, H.; Martinec, M.; Martina, R.; Riehl, T.; Krebs, M.G.; Meropol, N.J.; Wong, W.B.; Crane, G. Comparative efficacy analysis between entrectinib trial and crizotinib real-world ROS1 fusion-positive (ROS1+) NSCLC Patients. J. Thoracic Oncol. 2019, 14, S392. [Google Scholar] [CrossRef]
- Canadian Personalized Healthcare Innovation Network. Available online: http://www.cphin.ca/ (accessed on 26 February 2021).
- Osterman, T.J.; Terry, M.; Miller, S.R. Improving cancer data interoperability: The promise of the minimal common oncology data elements (mCODE) initiative. JCO Clin. Cancer Inform. 2020, 4, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Medical Informatics Initiative Germany. About the Initiative. Available online: https://www.medizininformatik-initiative.de/en/about-initiative (accessed on 9 March 2021).
- Cancer-ID. Innovative Medicines Initiative. Innovation in Medicine. Available online: https://www.cancer-id.eu/the-project/ (accessed on 1 March 2021).
| Name | Country | Purpose | Description | Links | Benefits |
|---|---|---|---|---|---|
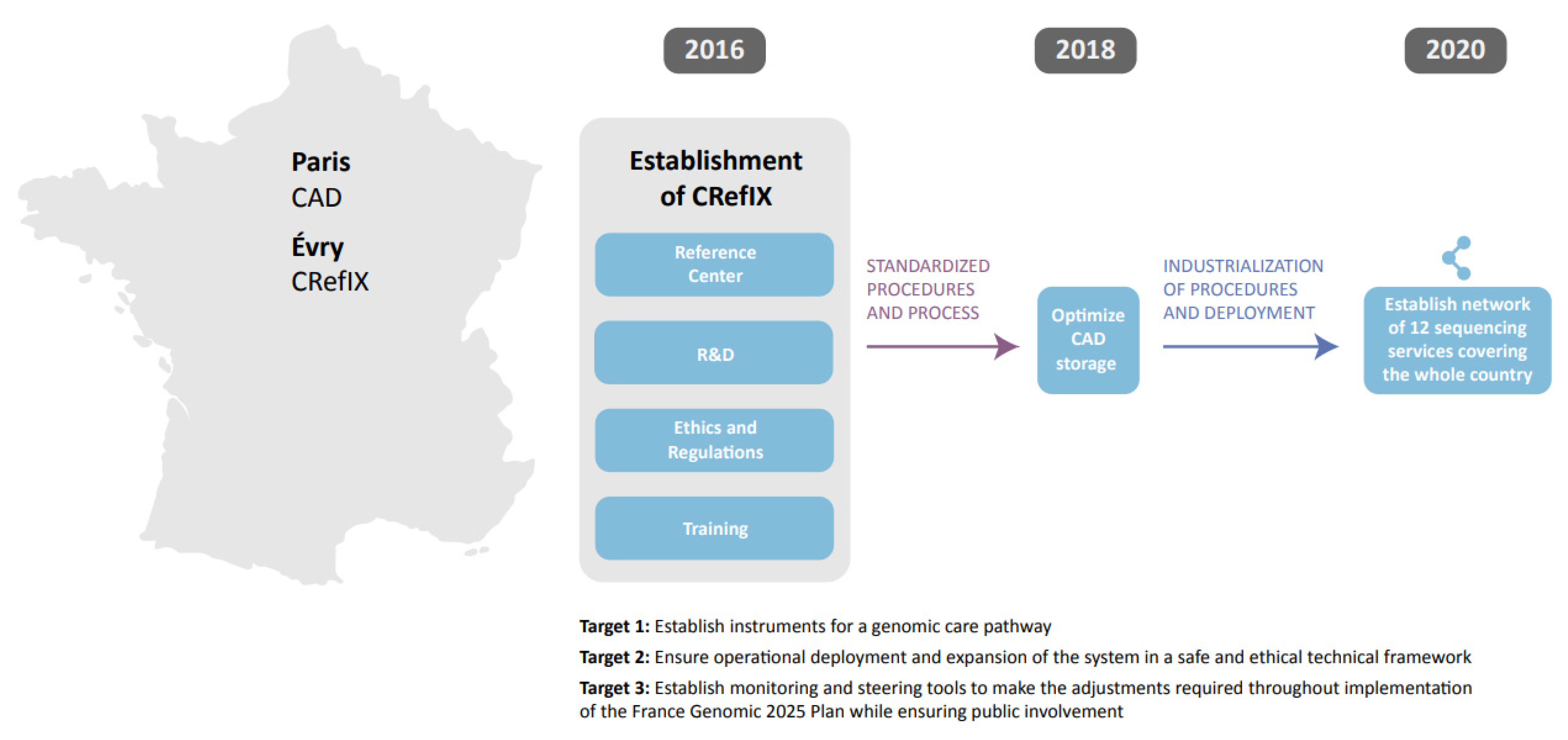
| Genomic Medicine France 2025 | France | To prepare for the integration of genomic medicine into the care pathway and management of common diseases with genomic medicine available to all affected patients in the country by 2025. | Development of a 10-year national plan, with a total investment of 670 million euros of which 230 million is from industry and the rest from the French government. | https://solidarites-sante.gouv.fr/IMG/pdf/genomic_medicine_france_2025.pdf (accessed on 21 July 2021) | The savings for the French healthcare system are potentially large and expected to be accompanied by social and economic benefits resulting from a significant improvement in healthy life expectancy. |
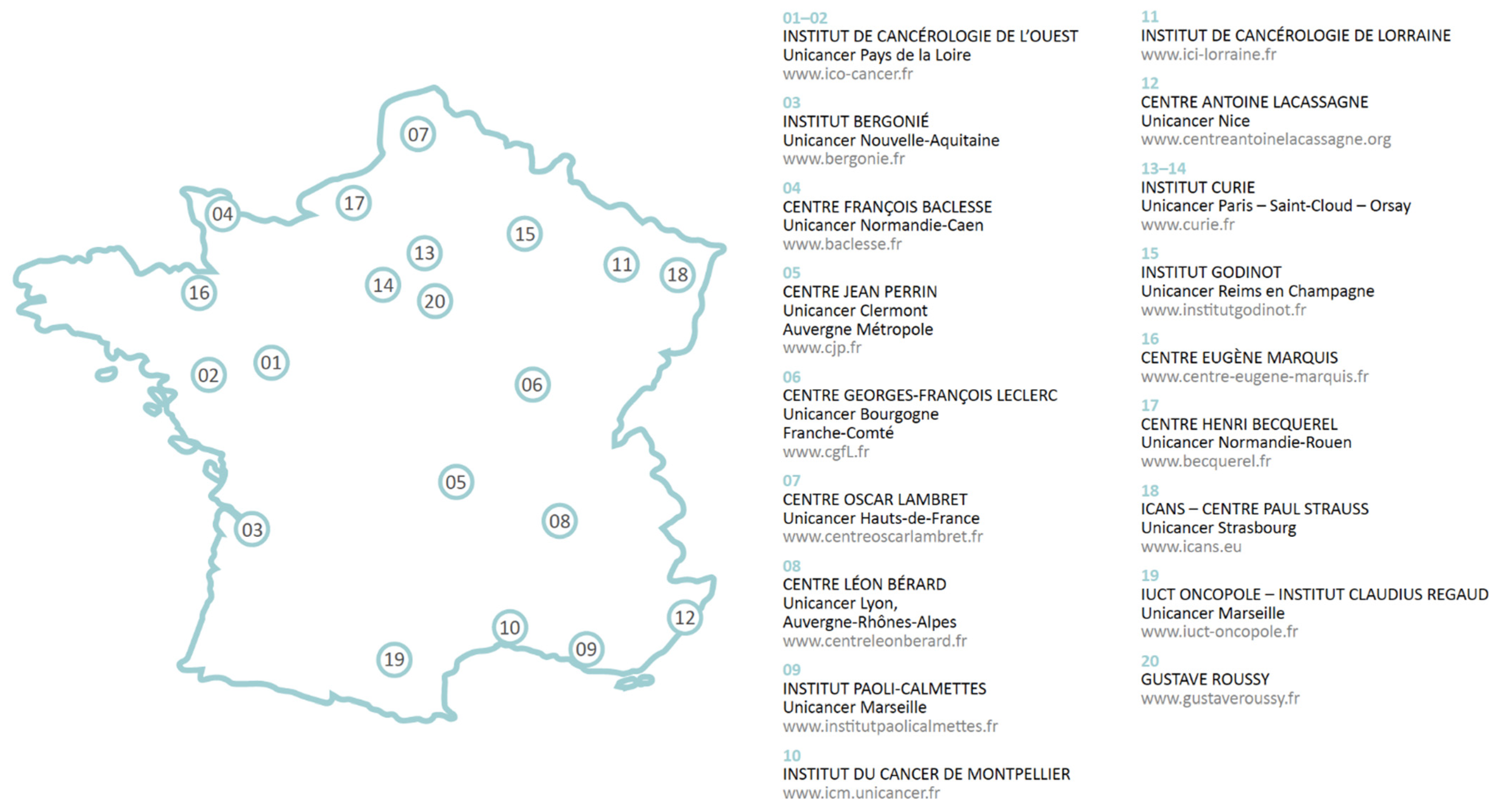
| UNICANCER | France | To build on the historical community of the French Comprehensive Cancer Centers (FCCCs) to facilitate cooperation and creation of synergies between different teams within the network. | UNICANCER groups together with the 20 FCCCs, which are private, non-profit hospitals located throughout the French territory. FCCCs contribute to the public hospital service in accordance with statutory fees, with no additional charge to patients. | http://www.unicancer.fr/ (accessed on 14 June 2021) | UNICANCER helps FCCCs access latest innovations for the benefit of their patients. It also advocates for FCCCs with public authorities and focusses on promoting their organization model in cancer care. |
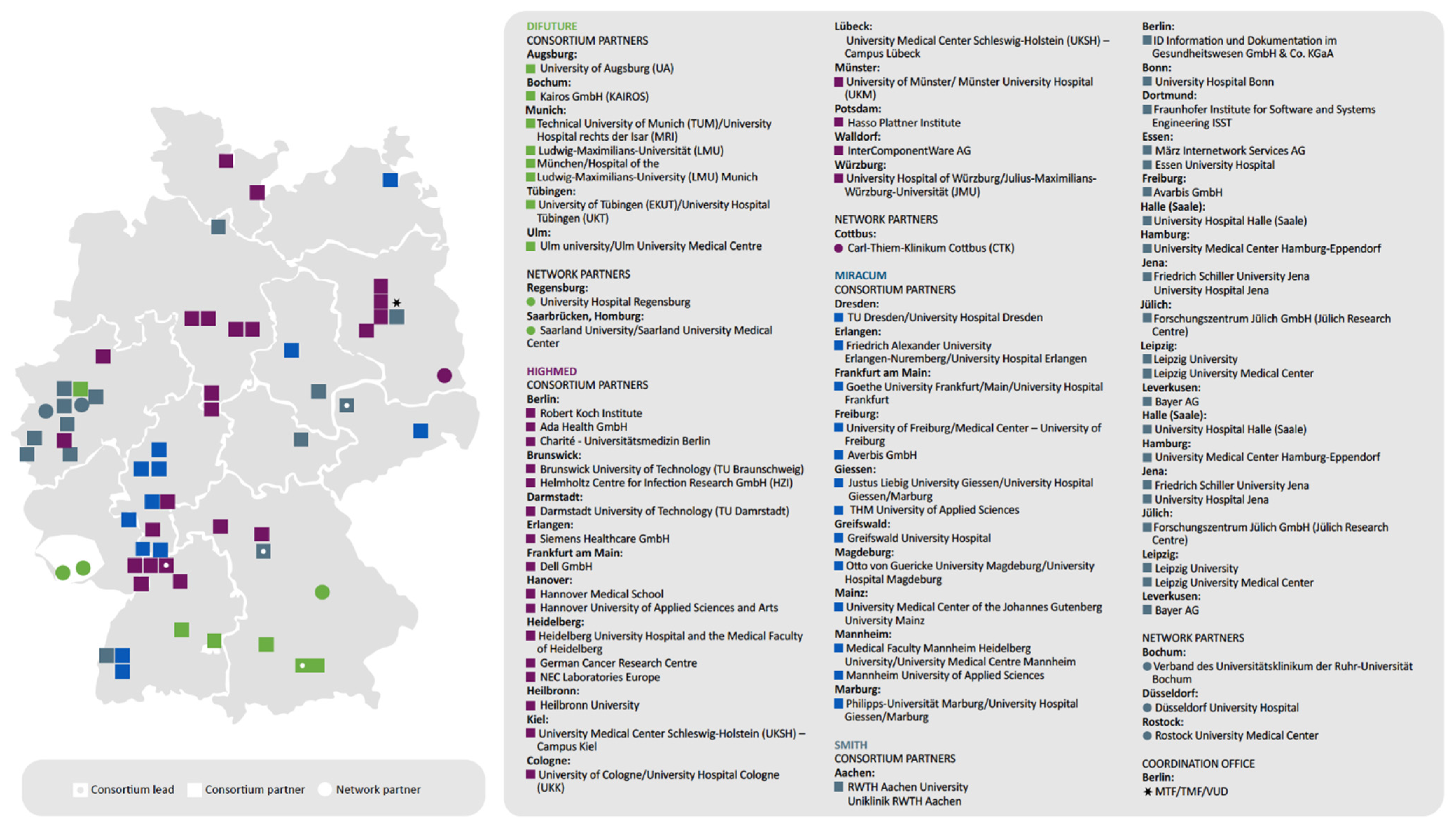
| German Medical Informatics Initiative | Germany | To create a framework in which all of Germany’s university hospitals join forces with research institutions, businesses, health insurers, and patient advocacy groups to harness research findings for the direct benefit of patients. | The German Federal Ministry of Education and Research is investing a total of 180 million euros in the Medical Informatics Initiative (MII) through 2022. | https://www.medizininformatik-initiative.de/en (accessed on 18 July 2021) | The MII initiative enables collaboration between medical and IT professionals with researchers and scientists across multiple disciplines in German university hospitals. The shared goal to digitally capture hospital healthcare data and make it available for research is intended to help provide patients with better medical treatments. |
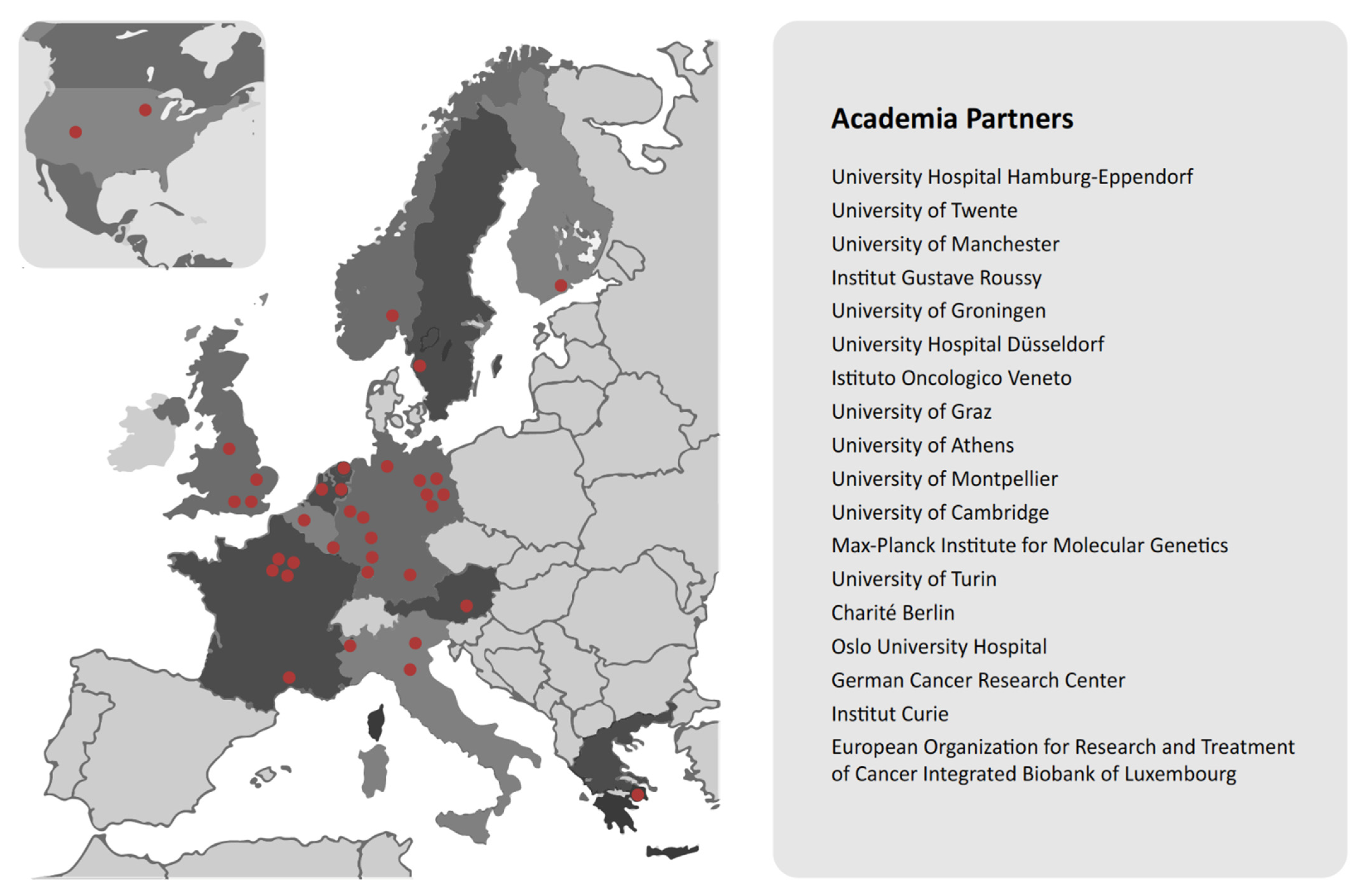
| CANCER-ID | European Consortium | The CANCER-ID project aims to develop new, less invasive ways of capturing cancer cells and genetic material from tumours using blood samples, and analyzing them for clues as to what treatment is needed and how to apply them. | CANCER-ID is funded by the Innovative Medicines Initiative (IMI), which currently involves 36 partners from 13 countries. Contributions to the project of currently 8.2 million euros by industrial partners are complemented by funding from the IMI Joint Undertaking, resulting in a total budget of 16.7 million euros. | https://www.cancer-id.eu/the-project/ (accessed on 14 July 2021) | CANCER-ID brings together experts from academic and clinical research, innovative small- to medium-sized enterprises, diagnostics companies, and the pharmaceutical industry to help establish the clinical utility of liquid biopsies in cancer care. |
| Canadian Challenge | |||
|---|---|---|---|
| Access to Publicly Funded Next-Generation Sequencing Testing | Access to Publicly Funded Molecularly Matched Therapies | Harmonized Real-World Data Collection and Interoperable Real-World Evidence | |
| Genomic Medicine France 2025 | X | X | X |
| UNICANCER | X | X | |
| German Medical Informatics Initiative | X | ||
| CANCER-ID | X | X | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Cheung, W.Y.; Feilotter, H.; Manthorne, J.; Stockley, T.; Yeung, M.; Renouf, D.J. Precision Oncology in Canada: Converting Vision to Reality with Lessons from International Programs. Curr. Oncol. 2022, 29, 7257-7271. https://doi.org/10.3390/curroncol29100572
Liu G, Cheung WY, Feilotter H, Manthorne J, Stockley T, Yeung M, Renouf DJ. Precision Oncology in Canada: Converting Vision to Reality with Lessons from International Programs. Current Oncology. 2022; 29(10):7257-7271. https://doi.org/10.3390/curroncol29100572
Chicago/Turabian StyleLiu, Geoffrey, Winson Y. Cheung, Harriet Feilotter, Jackie Manthorne, Tracy Stockley, ManTek Yeung, and Daniel J. Renouf. 2022. "Precision Oncology in Canada: Converting Vision to Reality with Lessons from International Programs" Current Oncology 29, no. 10: 7257-7271. https://doi.org/10.3390/curroncol29100572
APA StyleLiu, G., Cheung, W. Y., Feilotter, H., Manthorne, J., Stockley, T., Yeung, M., & Renouf, D. J. (2022). Precision Oncology in Canada: Converting Vision to Reality with Lessons from International Programs. Current Oncology, 29(10), 7257-7271. https://doi.org/10.3390/curroncol29100572





