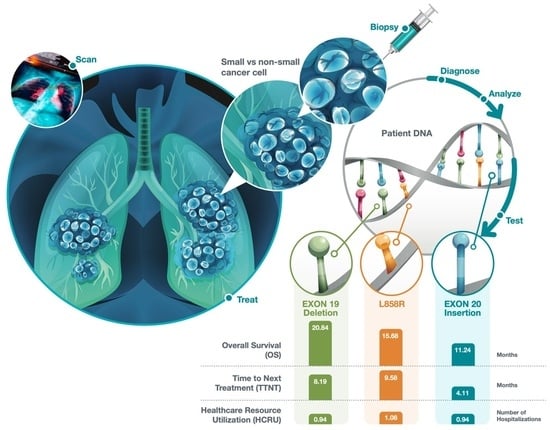
Prevalence, Treatment Patterns, and Outcomes of Individuals with EGFR Positive Metastatic Non-Small Cell Lung Cancer in a Canadian Real-World Setting: A Comparison of Exon 19 Deletion, L858R, and Exon 20 Insertion EGFR Mutation Carriers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
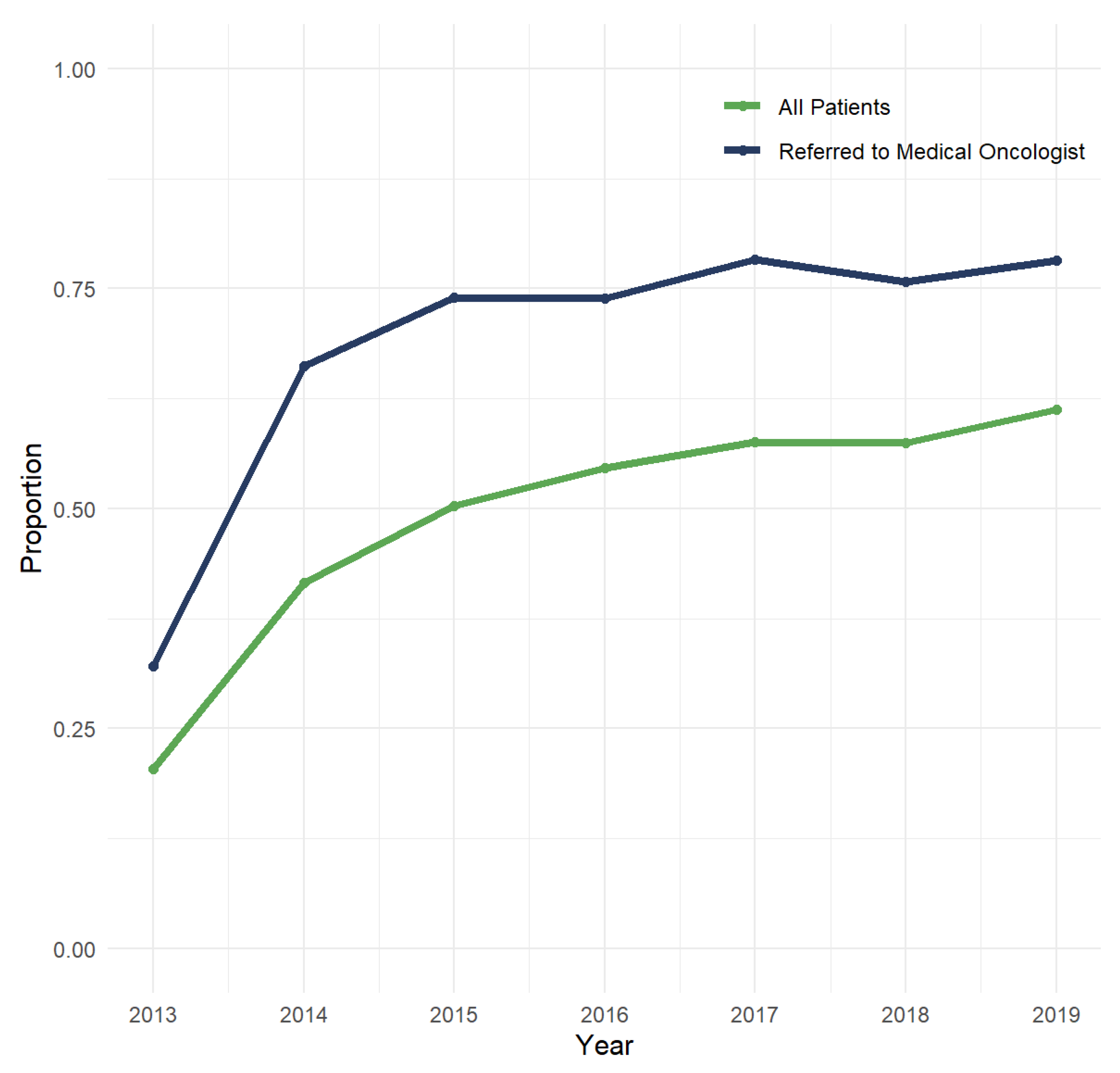
3.2. EGFR Testing Patterns and Prevalence of EGFR Mutations
3.3. Baseline Characteristics
3.4. Treatment Patterns
3.5. Time-to-Event Outcomes
3.6. Healthcare Resource Utilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.M.; Demers, A.A. Projected estimates of cancer in Canada in 2022. CMAJ Can. Med. Assoc. J. J. De L’association Med. Can. 2022, 194, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart, 3rd ed.; Travis, W.D., Brambilla, E., Müller-Hermelink, H.K., Harris, C.C., Eds.; IARC Publications: Geneva, Switzerland, 2004; Volume 10. [Google Scholar]
- Da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR mutations and lung cancer. Annu. Rev. Pathol. 2011, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.P.; Treece, A.L.; Lindeman, N.I.; Vasalos, P.; Shan, M.; Jennings, L.J.; Rimm, D.L. Worldwide frequency of commonly detected EGFR mutations. Arch. Pathol. Lab. Med. 2018, 142, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Banerji, S.; Blais, N.; Chu, Q.; Juergens, R.; Leighl, N.B.; Liu, G.; Cheema, P. Canadian Consensus: A New Systemic Treatment Algorithm for Advanced EGFR-Mutated Non-Small-Cell Lung Cancer. Curr. Oncol. 2020, 27, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, J.R. The Epidermal Growth Factor Receptor and Its Inhibition in Cancer Therapy. Pharmacol. Ther. 1999, 82, 241–250. [Google Scholar] [CrossRef]
- Riess, J.W.; Gandara, D.R.; Frampton, G.M.; Madison, R.; Peled, N.; Bufill, J.A.; Dy, G.K.; Ou, S.-H.I.; Stephens, P.J.; McPherson, J.D.; et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J. Thorac. Oncol. 2018, 13, 1560–1568. [Google Scholar] [CrossRef]
- Russo, A.; Franchina, T.; Ricciardi, G.; Battaglia, A.; Picciotto, M.; Adamo, V. Heterogeneous Responses to Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs) in Patients with Uncommon EGFR Mutations: New Insights and Future Perspectives in this Complex Clinical Scenario. Int. J. Mol. Sci. 2019, 20, 1431. [Google Scholar] [CrossRef]
- Passaro, A.; Mok, T.; Peters, S.; Popat, S.; Ahn, M.-J.; de Marinis, F. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J. Thorac. Oncol. 2021, 16, 764–773. [Google Scholar] [CrossRef]
- Xu, J.; Jin, B.; Chu, T.; Dong, X.; Yang, H.; Zhang, Y.; Wu, D.; Lou, Y.; Zhang, X.; Wang, H.; et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in China. Lung Cancer 2016, 96, 87–92. [Google Scholar] [CrossRef]
- Vyse, S.; Huang, P.H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Li, H.; Ma, S.; He, Z.; Yang, S.; Hao, L.; Zhou, H.; Zhang, Z.; Han, J.; Wang, L.; et al. EGFR exon 20 insertion mutations in advanced non-small-cell lung cancer: Current status and perspectives. Biomark. Res. 2022, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.E.; Cheung, W.Y.; Syed, I.A.; Moldaver, D.; Shanahan, M.K.; Bebb, D.G.; Sit, C.; Brenner, D.R.; Boyne, D.J. Real-World Treatment Patterns, Clinical Outcomes, and Health Care Resource Utilization in Extensive-Stage Small Cell Lung Cancer in Canada. Curr. Oncol. 2021, 28, 3091–3103. [Google Scholar] [CrossRef] [PubMed]
- Thi, A.M.; Tin Tin, S.; McKeage, M.; Elwood, J.M. Utilisation and Determinants of Epidermal Growth Factor Receptor Mutation Testing in Patients with Non-small Cell Lung Cancer in Routine Clinical Practice: A Global Systematic Review. Target Oncol. 2020, 15, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Tin Tin, S.; McKeage, M.J.; Khwaounjoo, P.; Thi, A.M.; Elwood, J.M. Incomplete uptake of EGFR mutation testing and its impact on estimation of mutation prevalence in patients with non-squamous NSCLC: A population-based study in New Zealand. Cancer Epidemiol. 2018, 57, 24–32. [Google Scholar] [CrossRef]
- Forsythe, M.L.; Alwithenani, A.; Bethune, D.; Castonguay, M.; Drucker, A.; Flowerdew, G.; French, D.; Fris, J.; Greer, W.; Henteleff, H.; et al. Molecular profiling of non-small cell lung cancer. PLoS ONE 2020, 15, e0236580. [Google Scholar] [CrossRef] [PubMed]
- Bazhenova, L.; Minchom, A.; Viteri, S.; Bauml, J.M.; Ou, S.-H.I.; Gadgeel, S.M.; Trigo, J.M.; Backenroth, D.; Li, T.; Londhe, A.; et al. Comparative clinical outcomes for patients with advanced NSCLC harboring EGFR exon 20 insertion mutations and common EGFR mutations. Lung Cancer 2021, 162, 154–161. [Google Scholar] [CrossRef]
- Burnett, H.; Emich, H.; Carroll, C.; Stapleton, N.; Mahadevia, P.; Li, T. Epidemiological and clinical burden of EGFR Exon 20 insertion in advanced non-small cell lung cancer: A systematic literature review. PLoS ONE 2021, 16, e0247620. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Sequist, L.V.; Geater, S.L.; Tsai, C.-M.; Mok, T.S.K.; Schuler, M.; Yamamoto, N.; Yu, C.-J.; Ou, S.-H.I.; Zhou, C.; et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015, 16, 830–838. [Google Scholar] [CrossRef]
- Kate, S.; Chougule, A.; Joshi, A.; Noronha, V.; Patil, V.; Dusane, R.; Solanki, L.; Tiwrekar, P.; Trivedi, V.; Prabhash, K. Outcome of uncommon EGFR mutation positive newly diagnosed advanced non-small cell lung cancer patients: A single center retrospective analysis. Lung Cancer 2019, 10, 1. [Google Scholar] [CrossRef]
- Cardona, A.F.; Rojas, L.; Zatarain-Barrón, Z.L.; Freitas, H.C.; Granados, S.T.; Castillo, O.; Oblitas, G.; Corrales, L.; Castro, C.D.; Ruiz-Patiño, A.; et al. EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1. 2-CLICaP). Lung Cancer 2018, 125, 265–272. [Google Scholar] [CrossRef]
- Fang, W.; Huang, Y.; Hong, S.; Zhang, Z.; Wang, M.; Gan, J.; Wang, W.; Guo, H.; Wang, K.; Zhang, L. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer 2019, 19, 595. [Google Scholar] [CrossRef] [PubMed]
- DerSarkissian, M.; Li, S.; Galaznik, A.; Bhak, R.; Bocharova, I.; Kulalert, T.; Lin, H.M.; Huang, H.; Duh, M.S. Real-world treatment patterns and clinical outcomes in non-small cell lung cancer patients with EGFR exon 20 insertion mutations. J. Natl. Compr. Cancer Netw. 2019, 17, HSR19-084. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, W.; Yang, Y.; Zhang, L. P1. 01-110 three specific HER2 mutations predict favorable outcomes in advanced lung cancer patients treated with afatinib. J. Thorac. Oncol. 2018, 13, S506–S507. [Google Scholar]
- Malapelle, U.; Pilotto, S.; Reale, M.L.; Passiglia, F.; Pisapia, P.; Pepe, F.; Belluomini, L.; Galetta, D.; Cortinovis, D.; Tiseo, M.; et al. Epidermal growth factor receptor exon 20 insertion variants in non-small cell lung cancer patients. Crit. Rev. Oncol Hematol. 2022, 169, 103536. [Google Scholar] [CrossRef]

| Variable | Overall n = 398 | Exon 19 n = 221 | Exon 20 n = 18 | L858R n = 159 | p-Value |
|---|---|---|---|---|---|
| Age at Diagnosis (mean (SD)) | 66.7 (13.6) | 65.3 (13.9) | 63.9 (15.2) | 68.9 (12.6) | 0.029 |
| Diagnosed 2016–2019 (%) | 252 (63.3) | 144 (65.2) | 12 (66.7) | 96 (60.4) | 0.606 |
| Male (%) | 143 (35.9) | 89 (40.3) | 8 (44.4) | 46 (28.9) | 0.056 |
| 1+ Charlson Comorbidities (%) | 167 (42.0) | 94 (42.5) | 6 (33.3) | 67 (42.1) | 0.748 |
| Adenocarcinoma (%) | 370 (93.0) | 208 (94.1) | 18 (100.0) | 144 (90.6) | 0.201 |
| No. of Metastatic Sites at Diagnosis (%) | 0.777 | ||||
| 1 | 152 (38.2) | 82 (37.1) | 6 (33.3) | 64 (40.3) | |
| 2 | 110 (27.6) | 63 (28.5) | 6 (33.3) | 41 (25.8) | |
| 3+ | 133 (33.4) | 73 (33.0) | 6 (33.3) | 54 (34.0) | |
| Missing | 3 (0.8) | 3 (1.4) | 0 (0.0) | 0 (0.0) | |
| Brain Metastasis at Diagnosis (%) | 106 (26.6) | 57 (25.8) | 3 (16.7) | 46 (28.9) | 0.490 |
| Time to First EGFR Test, Days (median [IQR]) | 18.0 [14.0, 26.0] | 18.0 [14.0, 25.0] | 16.0 [14.0, 26.3] | 19.0 [14.0, 26.0] | 0.772 |
| Variable | N | Median (Months) | 1-Year (%) | 2-Year (%) |
|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | ||
| Overall Survival from Diagnosis | ||||
| Exon 19 | 221 | 20.84 (18.25–24.39) | 69.1 (63.3–75.5) | 43.6 (37.5–50.8) |
| L858R | 159 | 15.68 (14.07–21.90) | 62.0 (54.9–70.1) | 37.6 (30.8–46.1) |
| Exon 20 | 18 | 11.24 (9.47–24.43) | 44.4 (26.5–74.5) | 22.2 (9.4–52.7) |
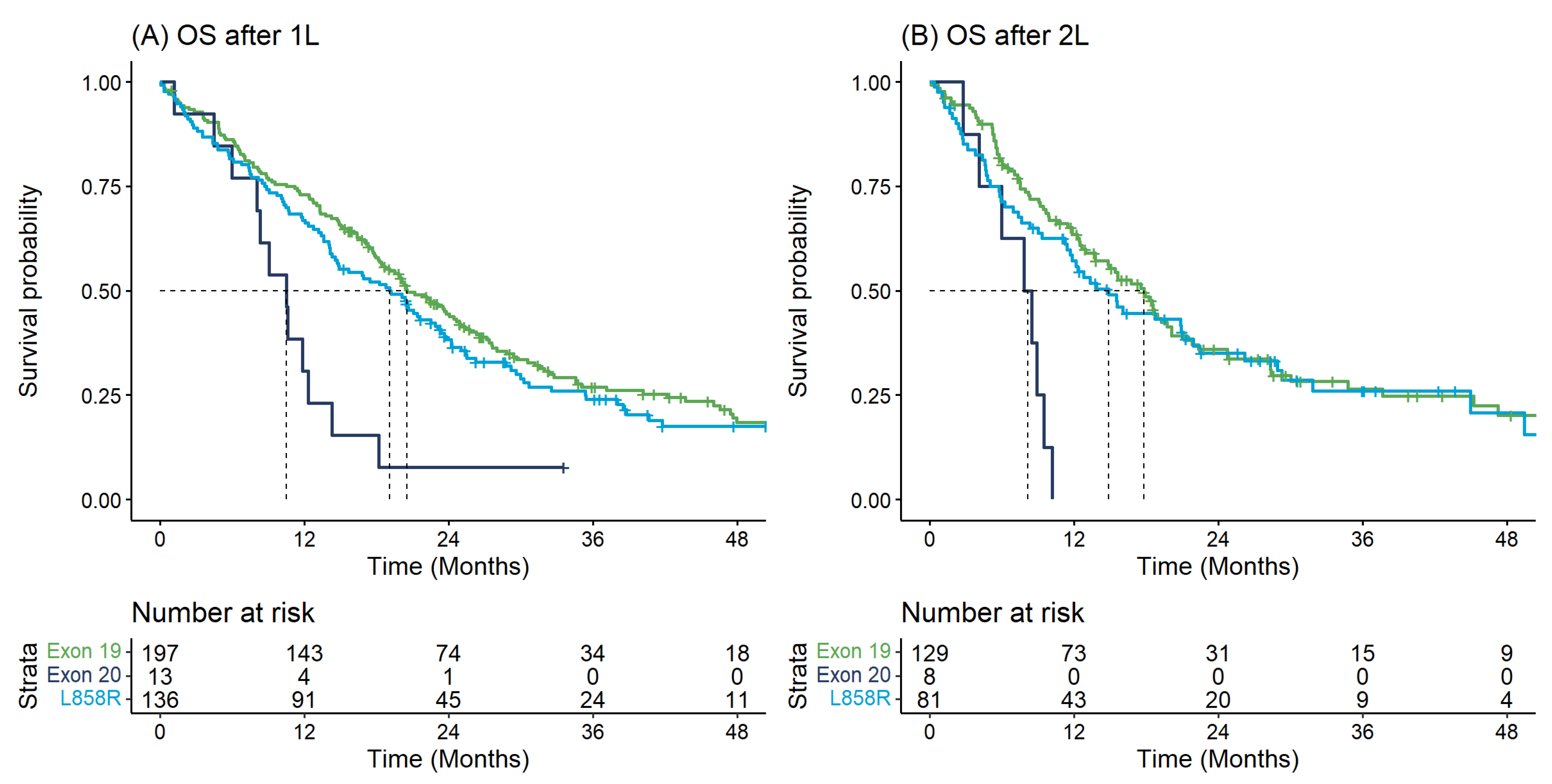
| Overall Survival from 1L | ||||
| Exon 19 | 197 | 20.55 (18.41–24.89) | 73.0 (67.0–79.5) | 44.4 (37.8–52.1) |
| L858R | 136 | 19.10 (14.53–23.11) | 66.9 (59.5–75.3) | 38.2 (30.8–47.5) |
| Exon 20 | 13 | 10.52 (8.02–NA) | 30.8 (13.6–69.5) | 7.7 (1.2–50.6) |
| Overall Survival from 2L | ||||
| Exon 19 | 129 | 17.79 (13.74–20.09) | 63.5 (55.5–72.6) | 35.9 (27.8–46.3) |
| L858R | 81 | 14.89 (11.41–21.90) | 57.2 (47.2–69.2) | 35.1 (25.6–48.2) |
| Exon 20 | 8 | 8.14 (5.98–NA) | 0.0 (NA–NA) | 0.0 (NA–NA) |
| Overall Survival from 3L | ||||
| Exon 19 | 81 | 13.22 (10.55–22.16) | 54.3 (44.0–67.0) | 35.6 (25.5–49.5) |
| L858R | 41 | 18.71 (10.16–NA) | 55.7 (41.8–74.2) | 39.4 (25.1–61.9) |
| Exon 20 | <5 | |||
| TTNT from 1L | ||||
| Exon 19 | 197 | 8.19 (7.36–9.30) | 34.2 (28.2–41.5) | 9.6 (6.2–14.9) |
| L858R | 136 | 9.58 (8.22–10.95) | 34.6 (27.4–43.6) | 8.1 (4.5–14.5) |
| Exon 20 | 13 | 4.11 (2.30–NA) | 7.7 (1.2–50.6) | 7.7 (1.2–50.6) |
| TTNT from 2L | ||||
| Exon 19 | 129 | 7.13 (6.28–8.22) | 22.0 (15.7–30.7) | 6.6 (3.2–13.7) |
| L858R | 81 | 6.44 (5.33–8.98) | 23.3 (15.6–34.8) | 9.1 (4.4–19.0) |
| Exon 20 | 8 | 5.05 (3.42–NA) | 0.0 (NA–NA) | 0.0 (NA–NA) |
| TTNT from 3L | ||||
| Exon 19 | 81 | 5.75 (5.10–7.76) | 15.7 (9.2–26.9) | 4.5 (1.3–15.9) |
| L858R | 41 | 6.51 (5.75–8.88) | 24.9 (14.5–42.9) | 9.3 (3.3–26.6) |
| Exon 20 | <5 |
| EGFR Strata | Construct | Outcome | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|---|---|
| Exon 19 | N | N | 221 | 152 | 85 | 39 | 23 |
| Hospital | Hospitalizations | 0.94 (1.13) | 0.74 (1.03) | 0.49 (0.93) | 0.38 (0.71) | 0.57 (1.04) | |
| Days Hospitalized | 11.52 (23.71) | 6.75 (13.50) | 4.09 (8.53) | 9.69 (29.60) | 5.65 (14.66) | ||
| Ambulatory | All Encounters | 9.76 (7.21) | 8.25 (23.98) | 4.67 (5.65) | 4.10 (3.64) | 4.96 (5.57) | |
| Emergency | 1.65 (1.86) | 1.35 (1.83) | 1.15 (2.48) | 0.82 (1.10) | 1.00 (1.51) | ||
| Non-Emergency | 8.12 (6.79) | 6.90 (23.94) | 3.52 (4.41) | 3.28 (3.38) | 3.96 (4.63) | ||
| Cancer Physician | All Visits | 10.27 (6.65) | 8.93 (7.93) | 7.46 (7.26) | 7.33 (7.09) | 8.30 (9.08) | |
| Medical Oncologist | 7.79 (5.54) | 7.23 (6.69) | 6.32 (6.74) | 6.00 (6.32) | 7.35 (7.98) | ||
| Radiation Oncologist | 2.01 (3.04) | 1.13 (2.14) | 0.76 (1.55) | Suppressed | Suppressed | ||
| Other Cancer Physician | 0.47 (1.52) | 0.57 (1.62) | 0.38 (1.36) | <10 | <10 | ||
| Non-Cancer Practitioner | Claims | 53.56 (46.72) | 35.15 (36.54) | 26.80 (28.67) | 27.18 (42.82) | 28.61 (47.02) | |
| Costs | 4117.06 (3521.70) | 2413.29 (2511.54) | 1958.29 (2223.42) | 1625.30 (2081.08) | 2570.54 (4278.09) | ||
| Encounters | 28.82 (23.86) | 18.12 (15.62) | 14.84 (13.04) | 14.54 (18.00) | 14.91 (16.98) | ||
| Radiation Therapy | Days | 6.41 (9.49) | 3.37 (6.88) | 2.28 (5.45) | 2.87 (6.80) | 1.70 (5.49) | |
| Chemotherapy Cycles | Cycles | 7.43 (5.15) | 5.41 (5.32) | 4.41 (4.66) | 3.30 (3.44) | 4.55 (4.10) | |
| Exon 20 | N | N | 18 | 8 | <10 | <10 | <10 |
| Hospital | Hospitalizations | 0.94 (0.73) | 0.50 (0.76) | ||||
| Days Hospitalized | 9.89 (10.62) | 8.12 (16.27) | |||||
| Ambulatory | All Encounters | 9.56 (7.06) | 3.38 (3.96) | ||||
| Emergency | 2.50 (2.83) | 1.12 (1.36) | |||||
| Non-Emergency | 7.06 (6.17) | 2.25 (2.92) | |||||
| Cancer Physician | All Visits | 15.72 (10.97) | 3.50 (3.42) | ||||
| Medical Oncologist | 10.78 (9.39) | 2.75 (3.06) | |||||
| Radiation Oncologist | 3.00 (4.64) | 0.25 (0.46) | |||||
| Other Cancer Physician | 1.94 (3.78) | 0.50 (1.07) | |||||
| Non-Cancer Practitioner | Claims | 47.44 (44.44) | 27.00 (29.39) | ||||
| Costs | 3448.43 (2288.78) | 1562.61 (1771.47) | |||||
| Encounters | 26.00 (21.48) | 14.00 (15.76) | |||||
| Radiation Therapy | Days | 10.39 (13.86) | 1.00 (2.45) | ||||
| Chemotherapy Cycles | Cycles | 8.62 (4.17) | 1.17 (1.94) | ||||
| L858R | N | N | 159 | 98 | 55 | 27 | 12 |
| Hospital | Hospitalizations | 1.08 (1.23) | 0.85 (1.26) | 0.76 (1.25) | <10 | <10 | |
| Days Hospitalized | 13.11 (21.63) | 8.83 (15.23) | 10.71 (23.83) | 3.74 (11.74) | 12.42 (24.69) | ||
| Ambulatory | All Encounters | 9.55 (8.62) | 6.45 (6.29) | 4.13 (6.10) | 1.89 (2.81) | 4.00 (6.05) | |
| Emergency | 2.21 (3.90) | 1.47 (2.01) | 1.09 (2.03) | 0.52 (0.75) | 1.17 (1.85) | ||
| Non-Emergency | 7.35 (7.01) | 4.98 (5.57) | 3.04 (5.15) | 1.37 (2.53) | 2.83 (4.32) | ||
| Cancer Physician | All Visits | 10.88 (8.07) | 9.70 (8.81) | 7.60 (8.25) | 7.41 (6.88) | 8.83 (6.55) | |
| Medical Oncologist | 8.21 (6.81) | 8.07 (8.12) | 6.87 (7.74) | 6.41 (6.46) | 7.75 (6.06) | ||
| Radiation Oncologist | 2.16 (2.97) | 1.12 (1.97) | 0.33 (0.79) | Suppressed | Suppressed | ||
| Other Cancer Physician | 0.52 (1.53) | 0.51 (1.52) | 0.40 (1.23) | <10 | <10 | ||
| Non-Cancer Practitioner | Claims | 59.50 (54.13) | 42.64 (47.93) | 35.38 (55.42) | 21.22 (37.21) | 45.75 (73.76) | |
| Costs | 4885.42 (4806.53) | 2984.61 (3384.87) | 2433.08 (3906.33) | 1971.04 (4076.41) | 2884.29 (4868.36) | ||
| Encounters | 30.46 (24.59) | 23.53 (22.59) | 18.13 (21.75) | 10.44 (15.02) | 21.00 (30.93) | ||
| Radiation Therapy | Days | 7.53 (9.91) | 2.57 (5.88) | 1.11 (3.59) | 2.63 (6.51) | 2.42 (4.46) | |
| Chemotherapy Cycles | Cycles | 10.85 (31.35) | 7.04 (17.01) | 5.48 (12.50) | 3.04 (4.21) | 2.83 (3.27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Sullivan, D.E.; Jarada, T.N.; Yusuf, A.; Hu, L.; Gogna, P.; Brenner, D.R.; Abbie, E.; Rose, J.B.; Eaton, K.; Elia-Pacitti, J.; et al. Prevalence, Treatment Patterns, and Outcomes of Individuals with EGFR Positive Metastatic Non-Small Cell Lung Cancer in a Canadian Real-World Setting: A Comparison of Exon 19 Deletion, L858R, and Exon 20 Insertion EGFR Mutation Carriers. Curr. Oncol. 2022, 29, 7198-7208. https://doi.org/10.3390/curroncol29100567
O’Sullivan DE, Jarada TN, Yusuf A, Hu L, Gogna P, Brenner DR, Abbie E, Rose JB, Eaton K, Elia-Pacitti J, et al. Prevalence, Treatment Patterns, and Outcomes of Individuals with EGFR Positive Metastatic Non-Small Cell Lung Cancer in a Canadian Real-World Setting: A Comparison of Exon 19 Deletion, L858R, and Exon 20 Insertion EGFR Mutation Carriers. Current Oncology. 2022; 29(10):7198-7208. https://doi.org/10.3390/curroncol29100567
Chicago/Turabian StyleO’Sullivan, Dylan E., Tamer N. Jarada, Amman Yusuf, Leo (Xun Yang) Hu, Priyanka Gogna, Darren R. Brenner, Erica Abbie, Jennifer B. Rose, Kiefer Eaton, Julia Elia-Pacitti, and et al. 2022. "Prevalence, Treatment Patterns, and Outcomes of Individuals with EGFR Positive Metastatic Non-Small Cell Lung Cancer in a Canadian Real-World Setting: A Comparison of Exon 19 Deletion, L858R, and Exon 20 Insertion EGFR Mutation Carriers" Current Oncology 29, no. 10: 7198-7208. https://doi.org/10.3390/curroncol29100567
APA StyleO’Sullivan, D. E., Jarada, T. N., Yusuf, A., Hu, L., Gogna, P., Brenner, D. R., Abbie, E., Rose, J. B., Eaton, K., Elia-Pacitti, J., Ewara, E. M., Pabani, A., Cheung, W. Y., & Boyne, D. J. (2022). Prevalence, Treatment Patterns, and Outcomes of Individuals with EGFR Positive Metastatic Non-Small Cell Lung Cancer in a Canadian Real-World Setting: A Comparison of Exon 19 Deletion, L858R, and Exon 20 Insertion EGFR Mutation Carriers. Current Oncology, 29(10), 7198-7208. https://doi.org/10.3390/curroncol29100567