Contralateral Neck Irradiation Can Be Omitted for Selected Lateralized Oral Cancer in Locally Advanced Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Patients
2.2. Treatments
2.3. Outcomes and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Characteristic Differences between Different Treatment Approaches
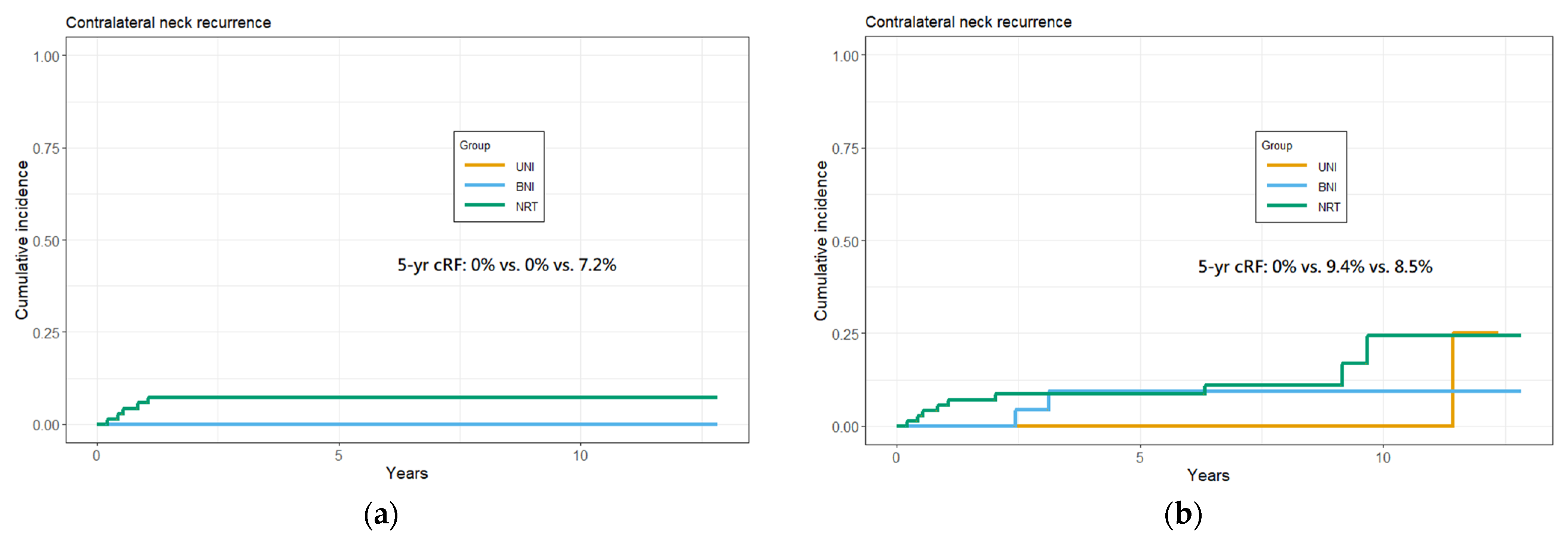
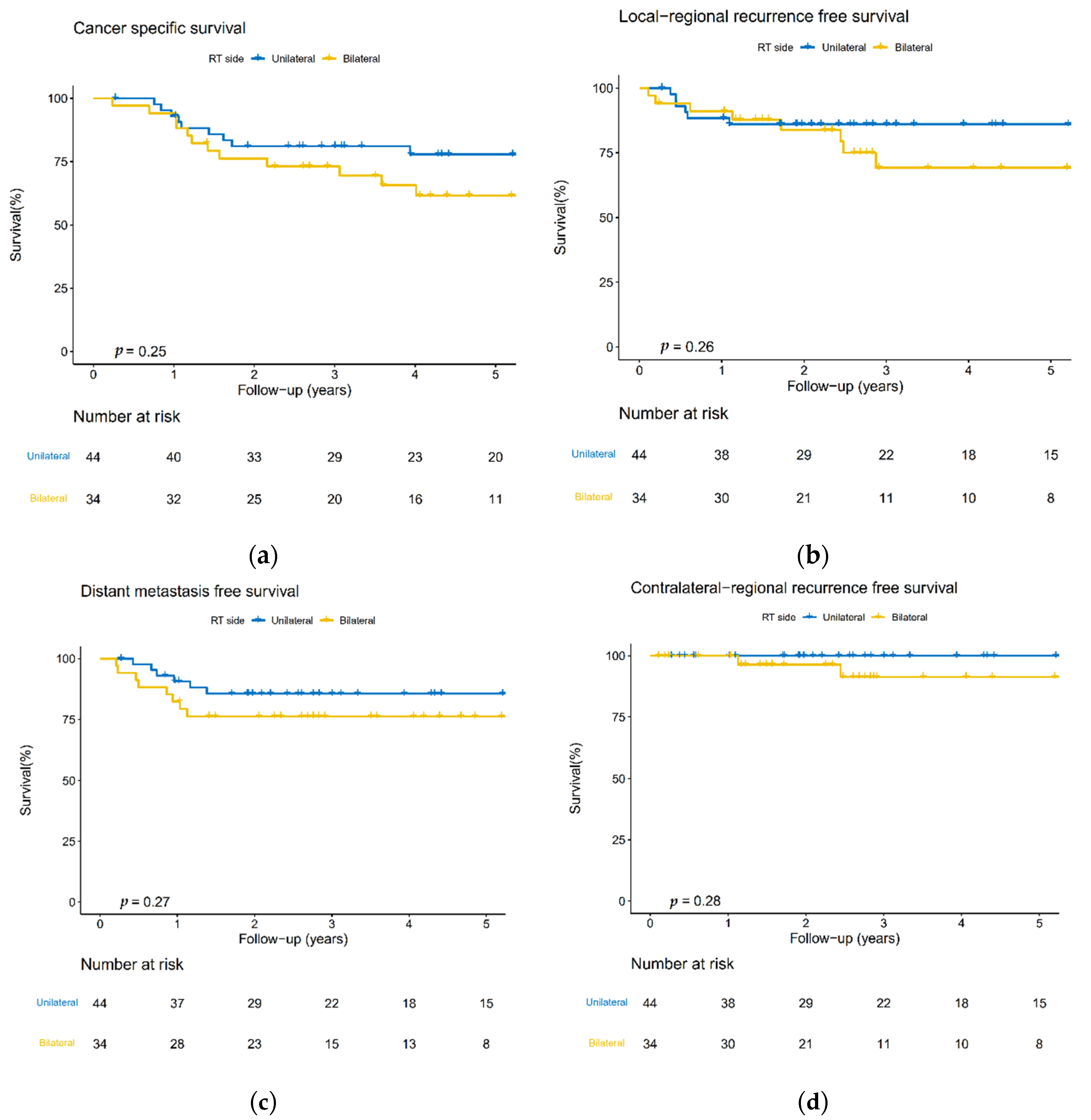
3.3. Cumulative 5-Year cRF Rate and Survival Analysis
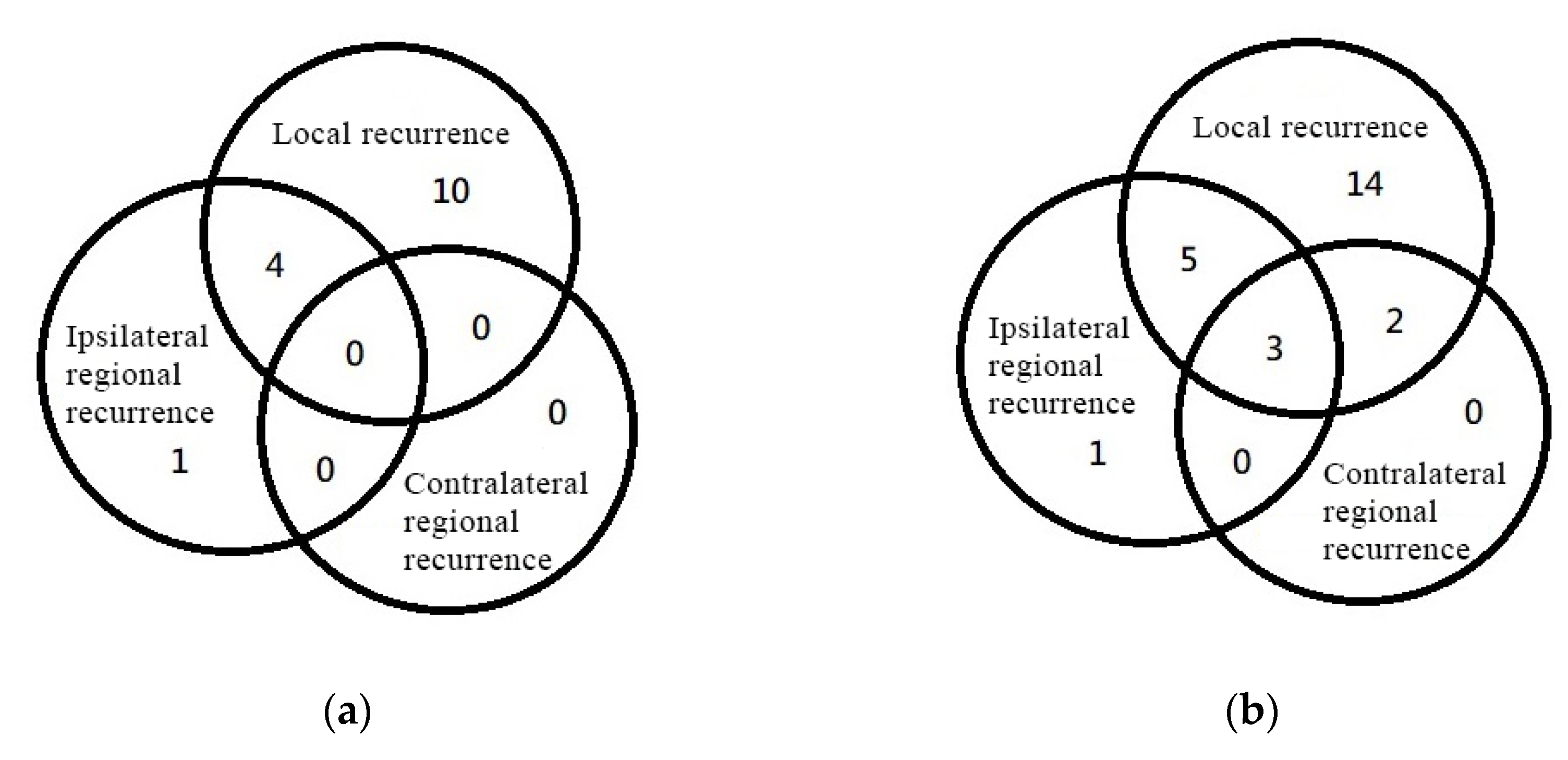
3.4. Disease-Failure Pattern
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shingaki, S.; Takada, M.; Sasai, K.; Bibi, R.; Kobayashi, T.; Nomura, T.; Saito, C. Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am. J. Surg. 2003, 185, 278–284. [Google Scholar] [CrossRef]
- Weiss, M.H.; Harrison, L.B.; Isaacs, R.S. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 699–702. [Google Scholar] [CrossRef]
- Kurita, H.; Koike, T.; Narikawa, J.N.; Sakai, H.; Nakatsuka, A.; Uehara, S.; Kobayashi, H.; Kurashina, K. Clinical predictors for contralateral neck lymph node metastasis from unilateral squamous cell carcinoma in the oral cavity. Oral Oncol. 2004, 40, 898–903. [Google Scholar] [CrossRef]
- Koo, B.S.; Lim, Y.C.; Lee, J.S.; Choi, E.C. Management of contralateral N0 neck in oral cavity squamous cell carcinoma. Head Neck 2006, 28, 896–901. [Google Scholar] [CrossRef]
- Vergeer, M.R.; Doornaert, P.A.H.; Jonkman, A.; Kaanders, J.H.A.M.; van den Ende, P.L.A.; de Jong, M.A.; Leemans, C.R.; Slotman, B.J.; Langendijk, J.A. Ipsilateral Irradiation for Oral and Oropharyngeal Carcinoma Treated with Primary Surgery and Postoperative Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.-J.; Oh, J.-I.; Choi, K.-Y.; Lee, D.-J.; Park, I.-S.; Kim, J.-H.; Rho, Y.-S. Pattern of cervical lymph node metastasis in tonsil cancer: Predictive factor analysis of contralateral and retropharyngeal lymph node metastasis. Oral Oncol. 2011, 47, 758–762. [Google Scholar] [CrossRef]
- Capote-Moreno, A.; Naval, L.; Muñoz-Guerra, M.F.; Sastre, J.; Rodríguez-Campo, F.J. Prognostic factors influencing contralateral neck lymph node metastases in oral and oropharyngeal carcinoma. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2010, 68, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Niu, L.X.; Yuan, Y.; Peng, X.; Guo, C.B. Risk factors and treatment of contralateral neck recurrence for unilateral oral squamous cell carcinoma: A retrospective study of 1482 cases. Oral Oncol. 2014, 50, 1081–1088. [Google Scholar] [CrossRef]
- Liao, C.T.; Huang, S.F.; Chen, I.H.; Chang, J.T.; Wang, H.M.; Ng, S.H.; Hsueh, C.; Lee, L.Y.; Lin, C.H.; Cheng, A.J.; et al. Risk stratification of patients with oral cavity squamous cell carcinoma and contralateral neck recurrence following radical surgery. Ann. Surg. Oncol. 2009, 16, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Rackley, T.P.; Namelo, W.C.; Palaniappan, N.; Cole, N.; Owens, D.M.J.; Evans, M. Unilateral radiotherapy for surgically resected lateralized squamous cell carcinoma of the tonsil. Head Neck 2017, 39, 17–23. [Google Scholar] [CrossRef]
- Liu, H.Y.; Tam, L.; Woody, N.M.; Caudell, J.; Reddy, C.A.; Ghanem, A.; Schymick, M.; Joshi, N.; Geiger, J.; Lamarre, E.; et al. Failure rate in the untreated contralateral node negative neck of small lateralized oral cavity cancers: A multi-institutional collaborative study. Oral Oncol. 2021, 115, 105190. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.M.; Temming, S.; Kocher, M.; Kunze, S.; Semrau, R. Low risk of contralateral lymph node recurrence in lateralized head and neck carcinoma after postoperative ipsilateral radiotherapy. Strahlenther. Und Onkol. Organ Der Dtsch. Rontgenges. 2020, 196, 474–484. [Google Scholar] [CrossRef]
- O’Steen, L.; Amdur, R.J.; Morris, C.G.; Hitchcock, K.E.; Mendenhall, W.M. Challenging the Requirement to Treat the Contralateral Neck in Cases With >4 mm Tumor Thickness in Patients Receiving Postoperative Radiation Therapy for Squamous Cell Carcinoma of the Oral Tongue or Floor of Mouth. Am. J. Clin. Oncol. 2019, 42, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Nobis, C.-P.; Otto, S.; Grigorieva, T.; Alnaqbi, M.; Troeltzsch, M.; Schöpe, J.; Wagenpfeil, S.; Ehrenfeld, M.; Wolff, K.-D.; Kesting, M.R. Elective neck dissection in unilateral carcinomas of the tongue: Unilateral versus bilateral approach. J. Cranio Maxillofac. Surg. 2017, 45, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.A.; Spencer, C.; DeWees, T.; Haughey, B.; Henke, L.E.; Chin, R.I.; Paniello, R.; Rich, J.; Jackson, R.; Oppelt, P.; et al. Eliminating Postoperative Radiation to the Pathologically Node-Negative Neck: Long-Term Results of a Prospective Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972, 29, 1446–1449. [Google Scholar] [CrossRef]
- Fletcher, G.H. Elective irradiation of subclinical disease in cancers of the head and neck. Cancer 1972, 29, 1450–1454. [Google Scholar] [CrossRef]
- Greene, F.L.; Page, D.L.; Fleming, I.D.; Fritz, A.; Balch, C.M.; Haller, D.G.; Fleming, I.D.; Morrow, M.; Fritz, A.G. (Eds.) AJCC Cancer Staging Manual, 6th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Waldram, R.; Taylor, A.E.; Whittam, S.; Iyizoba-Ebozue, Z.; Murray, L.; Frood, R.; Cardale, K.; Dyker, K.E.; Murray, P.; Ramasamy, S.; et al. Evaluation of Locoregional Recurrence Patterns Following Adjuvant (Chemo)Radiotherapy for Oral Cavity Carcinoma. Clin. Oncol. R. Coll. Radiol. 2020, 32, 228–237. [Google Scholar] [CrossRef]
- Shah, S.; Har-El, G.; Rosenfeld, R.M. Short-term and long-term quality of life after neck dissection. Head Neck 2001, 23, 954–961. [Google Scholar] [CrossRef]
- Schilling, C.; Stoeckli, S.J.; Haerle, S.K.; Broglie, M.A.; Huber, G.F.; Sorensen, J.A.; Bakholdt, V.; Krogdahl, A.; von Buchwald, C.; Bilde, A.; et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur. J. Cancer 2015, 51, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; Verheij, M.; van den Brekel, M.W.M. Elective unilateral nodal irradiation in head and neck squamous cell carcinoma: A paradigm shift. Eur. J. Cancer 2017, 82, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.A.; Dünne, A.A.; Myers, J.N. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck 2003, 25, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Den Toom, I.J.; Boeve, K.; van Weert, S.; Bloemena, E.; Brouwers, A.H.; Hoekstra, O.S.; de Keizer, B.; van der Vegt, B.; Willems, S.M.; Leemans, C.R.; et al. High rate of unexpected lymphatic drainage patterns and a high accuracy of the sentinel lymph node biopsy in oral cancer after previous neck treatment. Oral Oncol. 2019, 94, 68–72. [Google Scholar] [CrossRef]
- Mahieu, R.; den Toom, I.J.; Boeve, K.; Lobeek, D.; Bloemena, E.; Donswijk, M.L.; de Keizer, B.; Klop, W.M.C.; Leemans, C.R.; Willems, S.M.; et al. Contralateral Regional Recurrence in Lateralized or Paramedian Early-Stage Oral Cancer Undergoing Sentinel Lymph Node Biopsy—Comparison to a Historic Elective Neck Dissection Cohort. Front. Oncol. 2021, 11, 644306. [Google Scholar] [CrossRef]
| Covariate | Total | UNI Group | BNI Group | p-Value | No Adjuvant RT Group | p-Value |
|---|---|---|---|---|---|---|
| No. of cases | 149 | 44 | 34 | 71 | ||
| Sex (%) | ||||||
| Female | 4 (2.7) | 1 (2.3) | 1 (2.9) | 1.000 | 2 (2.8) | 0.979 |
| Male | 145 (97.3) | 43 (97.7) | 33 (97.1) | 69 (97.2) | ||
| Diagnosed age (median [IQR]) | 52.0 [47.0, 59.0] | 56.0 [47.8, 62.0] | 50.0 [45.3, 55.8] | 0.018 | 52.0 [47.0, 61.0] | 0.075 |
| Cancer site (%) | ||||||
| Buccal + cheek | 95 (63.8) | 31 (70.5) | 25 (73.5) | 0.744 | 39 (54.9) | 0.243 |
| Retromolar | 8 (5.4) | 3 (6.8) | 1 (2.9) | 4 (5.6) | ||
| Gum | 46 (30.9) | 10 (22.7) | 8 (23.5) | 28 (39.4) | ||
| AJCC pT (%) | ||||||
| 1 | 10 (6.7) | 3 (6.8) | 3 (8.8) | 0.645 | 4 (5.6) | 0.336 |
| 2 | 21 (14.1) | 6 (13.6) | 7 (20.6) | 8 (11.3) | ||
| 3 | 32 (21.5) | 10 (22.7) | 4 (11.8) | 18 (25.4) | ||
| 4A | 78 (52.3) | 22 (50.0) | 16 (47.1) | 40 (56.3) | ||
| 4B | 8 (5.4) | 3 (6.8) | 4 (11.8) | 1 (1.4) | ||
| AJCC pN (%) | ||||||
| 0 | 97 (65.1) | 27 (61.4) | 15 (44.1) | 0.171 | 55 (77.5) | 0.001 |
| 1 | 27 (18.1) | 5 (11.4) | 9 (26.5) | 13 (18.3) | ||
| 2B | 25 (16.8) | 12 (27.3) | 10 (29.4) | 3 (4.2) | ||
| AJCC pStage (%) | ||||||
| 3 | 46 (30.9) | 9 (20.5) | 10 (29.4) | 0.425 | 27 (38.0) | 0.079 |
| 4A | 95 (63.8) | 32 (72.7) | 20 (58.8) | 43 (60.6) | ||
| 4B | 8 (5.4) | 3 (6.8) | 4 (11.8) | 1 (1.4) | ||
| ND side (%) | ||||||
| Bilateral | 18 (12.1) | 5 (11.4) | 7 (20.6) | 0.534 | 6 (8.5) | 0.247 |
| Left | 69 (46.3) | 23 (52.3) | 16 (47.1) | 30 (42.3) | ||
| Right | 62 (41.6) | 16 (36.4) | 11 (32.4) | 35 (49.3) | ||
| Number of node examed (median [IQR]) | 27.0 [16.0, 35.0] | 29.0 [23.8, 36.3] | 32.5 [22.0, 44.8] | 0.452 | 20.0 [13.5, 33.0] | 0.001 |
| Histology grade (%) | ||||||
| 1 | 74 (49.7) | 18 (40.9) | 21 (61.8) | 0.090 | 35 (49.3) | 0.323 |
| 2 | 68 (45.6) | 23 (52.3) | 13 (38.2) | 32 (45.1) | ||
| 3 | 7 (4.7) | 3 (6.8) | 0 (0.0) | 4 (5.6) | ||
| Tumor size (median [IQR]) | 40.0 [27.0, 50.0] | 40.0 [25.8, 50.0] | 37.0 [30.0, 45.0] | 0.840 | 40.0 [28.5, 50.0] | 0.800 |
| PNI (%) | ||||||
| Negative | 104 (69.8) | 27 (61.4) | 22 (64.7) | 0.947 | 55 (77.5) | 0.144 |
| Positive | 45 (30.2) | 17 (38.6) | 12 (35.3) | 16 (22.5) | ||
| LVI (%) | ||||||
| Negative | 121 (81.2) | 37 (84.1) | 23 (67.6) | 0.150 | 61 (85.9) | 0.068 |
| Positive | 28 (18.8) | 7 (15.9) | 11 (32.4) | 10 (14.1) | ||
| Surgical margin (%) | ||||||
| Free | 122 (81.9) | 30 (68.2) | 27 (79.4) | 0.271 | 65 (91.5) | 0.016 |
| Close | 13 (8.7) | 8 (18.2) | 2 (5.9) | 3 (4.2) | ||
| Positive | 14 (9.4) | 6 (13.6) | 5 (14.7) | 3 (4.2) | ||
| Adjuvant treatment (%) | ||||||
| Negative | 71 (47.7) | NA | NA | 71 (100.0) | ||
| RT alone | 42 (28.2) | 24 (54.5) | 18 (52.9) | NA | ||
| CCRT | 36 (24.2) | 20 (45.5) | 16 (47.1) | NA | ||
| Concurrent CT regimen (%) | ||||||
| Cisplatin | 11 (30.6) | 6 (30.0) | 5 (31.3) | 0.640 | NA | |
| PF | 19 (52.8) | 11 (55.0) | 8 (50.0) | NA | ||
| PF+ Erbitux | 1 (2.8) | 0 (0.0) | 1 (6.3) | NA | ||
| Unknown | 5 (13.9) | 3 (15.0) | 2 (12.5) | NA | ||
| Smoking (%) | ||||||
| Negative | 24 (17.1) | 7 (15.9) | 3 (9.7) | 0.662 | 14 (21.5) | 0.342 |
| Positive | 116 (82.9) | 37 (84.1) | 28 (90.3) | 51 (78.5) | ||
| Betel nut chewing (%) | ||||||
| Negative | 31 (22.1) | 8 (18.2) | 4 (12.9) | 0.770 | 19 (29.2) | 0.147 |
| Positive | 109 (77.9) | 36 (81.8) | 27 (87.1) | 46 (70.8) | ||
| Alcohol drinking (%) | ||||||
| Negative | 52 (36.9) | 18 (40.9) | 8 (25.8) | 0.268 | 26 (39.4) | 0.347 |
| Positive | 59 (63.1) | 26 (59.1) | 23 (74.2) | 40 (60.6) |
| Univariate Analysis | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| pT Stage (T3–4 vs. T1–2) | 0.367 (0.06–2.20) | 0.272 |
| pN number (positive vs. negative) | 3.117 (0.52–18.67) | 0.213 |
| PNI (presence vs. absence) | 1.753 (0.29–10.51) | 0.539 |
| LVI (presence vs. absence) | 1.190 (0.13–10.65) | 0.876 |
| Margin (close vs. negative) | 3.369 (0.35–32.40) | 0.293 |
| Margin (positive vs. negative) | 3.212 (0.33–30.96) | 0.313 |
| Study | n | Primary Site | Lateralized Primary | ND | pT Status | pN Status | Adjuvant Tx | RT Neck Side | Rate of cRF | Predictors of cRF |
|---|---|---|---|---|---|---|---|---|---|---|
| Present study | 149 | BUC (63.8%) GUM (30.9%) RMT (5.4%) | Y | Uni (88%) Bil (12%) | T1 (6.7%) T2 (14.1%) T3 (21.5%) T4 (57.7%) | N+ (34.9%) N0 (65.1%) | NRT (47.7%) RT (28.2%) CRT (24.2%) | 56.4% UNI 43.6% BNI | 5 year rate 3.6% Crude rate 3.4% | None (100% SLRF) |
| Liu (2021) [11] | 176 | OT (82%) FOM (18%) | Y | Uni (100%) | T1 (68%) T2 (32%) | N+ (12%) N0 (81%) UNK (7%) | NRT (83%) RT (17%) | 100% PTB ± UNI 0% BNI | 2 year rate 3.6% 5 year rate 4.3% Crude rate 5% | DOI > 10 mm (22% SLRF; 22% LFTR; 56% iCLF) |
| Waldram (2020) [20] | 101 | OT (52%) FOM (17%) BUC (7%) GUM (15%) RMT (10%) | NR | Uni (69%) Bil (27%) | T1 (11%) T2 (40%) T3 (9%) T4 (40%) | N+ (63%) N0 (37%) | RT (75%) CRT (25%) | 43% UNI 53% BNI | Crude rate 5% | NR |
| Contreras (2019) [15] | 72 | Oral cavity (20%) Oropharynx (51%) Hypopharynx (6%) Larynx (22%) | No, 71% involved/cross midline | Uni (8%) Bil (92%) | T1-T2 (49%) T3-T4 (51%) | N2-N3 (58%) N0-N1 (42%) | RT (53%) CRT (47%) | 24% PTB 76% UNI 0% BNI | 5 year contralateral neck control 94.5% Crude rate 2.8% | NR (50% SLRF; 50% LFTR) |
| Wirtz (2019) [12] | 197 | Oral cavity (38%) Oropharynx (53%) Hypopharynx (10%) | Y | Uni (27%) Bil (73%) | T0 (1%) T1 (36%) T2 (41%) T3 (10%) T4 (11%) | N+ (80.7%) N0 (19.3%) | RT (70%) CRT (30%) | 100% UNI 0% BNI | 5 year contralateral neck control 94.6% Crude rate 6.1% | None (42% SLRF) |
| O’steen (2019) [13] | 32 | OT (72%) FOM (28%) | Y | Uni (78%) Bil (22%) | T1 (47%) T2 (41%) T3 (9%) T4 (3%) | N+ (19%) N0 (81%) | RT (62%) CRT (38%) | 41% PTB 59% UNI 0% BNI | Crude rate 0% | NR |
| Nobis (2017) [14] | 150 | OT (100%) | Y | Uni (70%) Bil (30%) | T1 (71%) T2 (29%) | N+ (23%) N0 (77%) | NRT (75%) RT (19%) CRT (6%) | NR | Crude rate 2.7% | NR |
| Vergeer (2010) [5] | 123 | Oral cavity (85%) -OT (25%) -FOM (8%) -BUC (19%) -GUM (48%) Oropharynx (15%) | No, 7% close/ cross midline | Uni (83%) Bil (0%) | T1 (22%) T2 (35%) T3 (11%) T4 (33%) | N+ (41%) N0 (59%) | RT (100%) | 10% PTB 90% UNI 0% BNI | 5 year contralateral neck control 92% Crude rate 5.7% | Number of positive nodes (14% SLRF) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-J.; Lin, H.-Y.; Tsai, M.-H.; Pao, T.-H.; Hsu, C.-H.; Wu, Y.-H. Contralateral Neck Irradiation Can Be Omitted for Selected Lateralized Oral Cancer in Locally Advanced Stage. Curr. Oncol. 2022, 29, 6956-6967. https://doi.org/10.3390/curroncol29100547
Cheng Y-J, Lin H-Y, Tsai M-H, Pao T-H, Hsu C-H, Wu Y-H. Contralateral Neck Irradiation Can Be Omitted for Selected Lateralized Oral Cancer in Locally Advanced Stage. Current Oncology. 2022; 29(10):6956-6967. https://doi.org/10.3390/curroncol29100547
Chicago/Turabian StyleCheng, Yung-Jen, Hsin-Ying Lin, Mu-Hung Tsai, Tzu-Hui Pao, Chia-Hsiang Hsu, and Yuan-Hua Wu. 2022. "Contralateral Neck Irradiation Can Be Omitted for Selected Lateralized Oral Cancer in Locally Advanced Stage" Current Oncology 29, no. 10: 6956-6967. https://doi.org/10.3390/curroncol29100547
APA StyleCheng, Y.-J., Lin, H.-Y., Tsai, M.-H., Pao, T.-H., Hsu, C.-H., & Wu, Y.-H. (2022). Contralateral Neck Irradiation Can Be Omitted for Selected Lateralized Oral Cancer in Locally Advanced Stage. Current Oncology, 29(10), 6956-6967. https://doi.org/10.3390/curroncol29100547





