Safety Profile of Niraparib as Maintenance Therapy for Ovarian Cancer: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Searching
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Assessment Risk of Bias
2.6. Data Analysis
2.7. Heterogeneity Analysis
3. Results
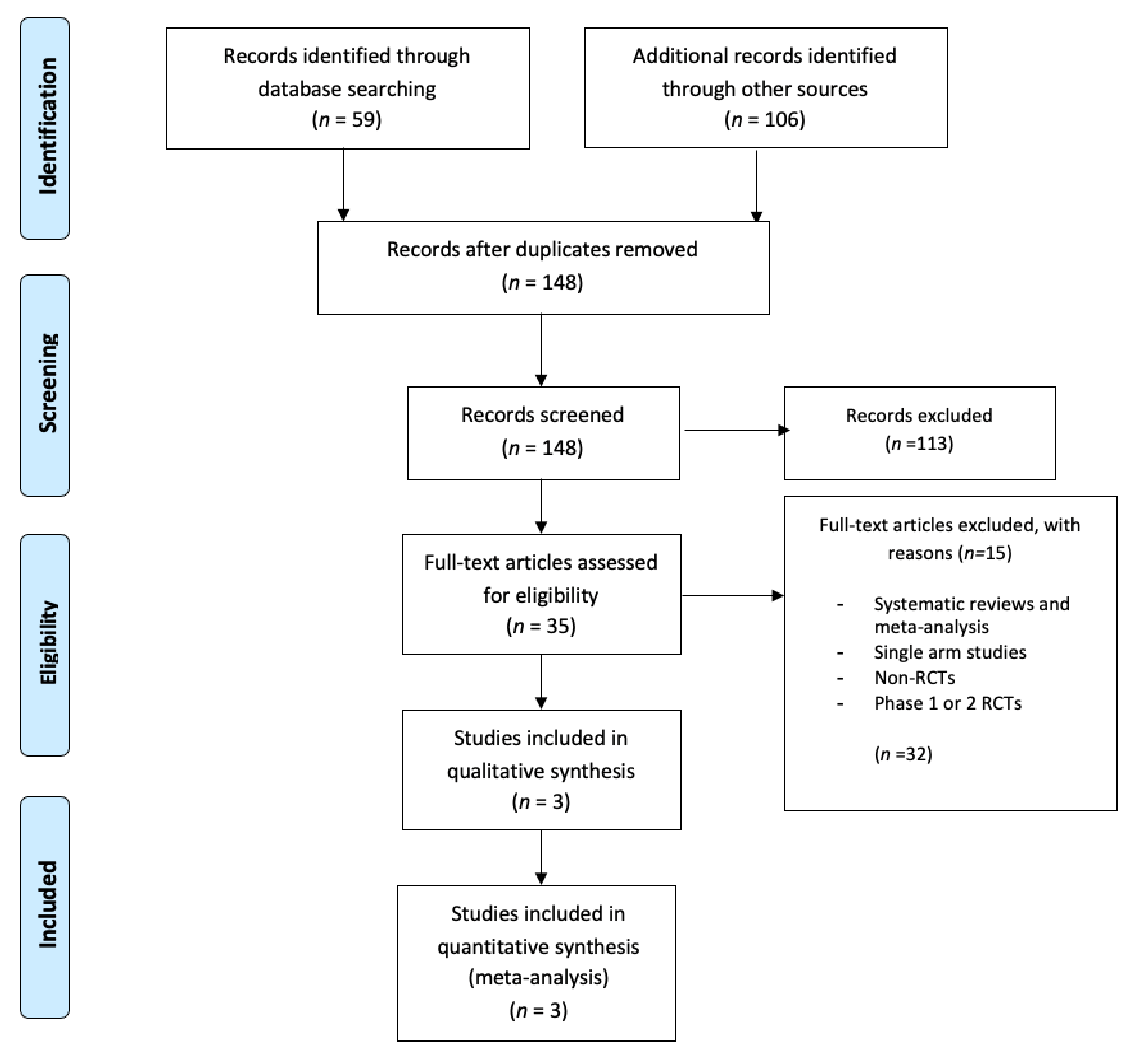
3.1. Study Selection
3.2. Dosage Modification Analysis
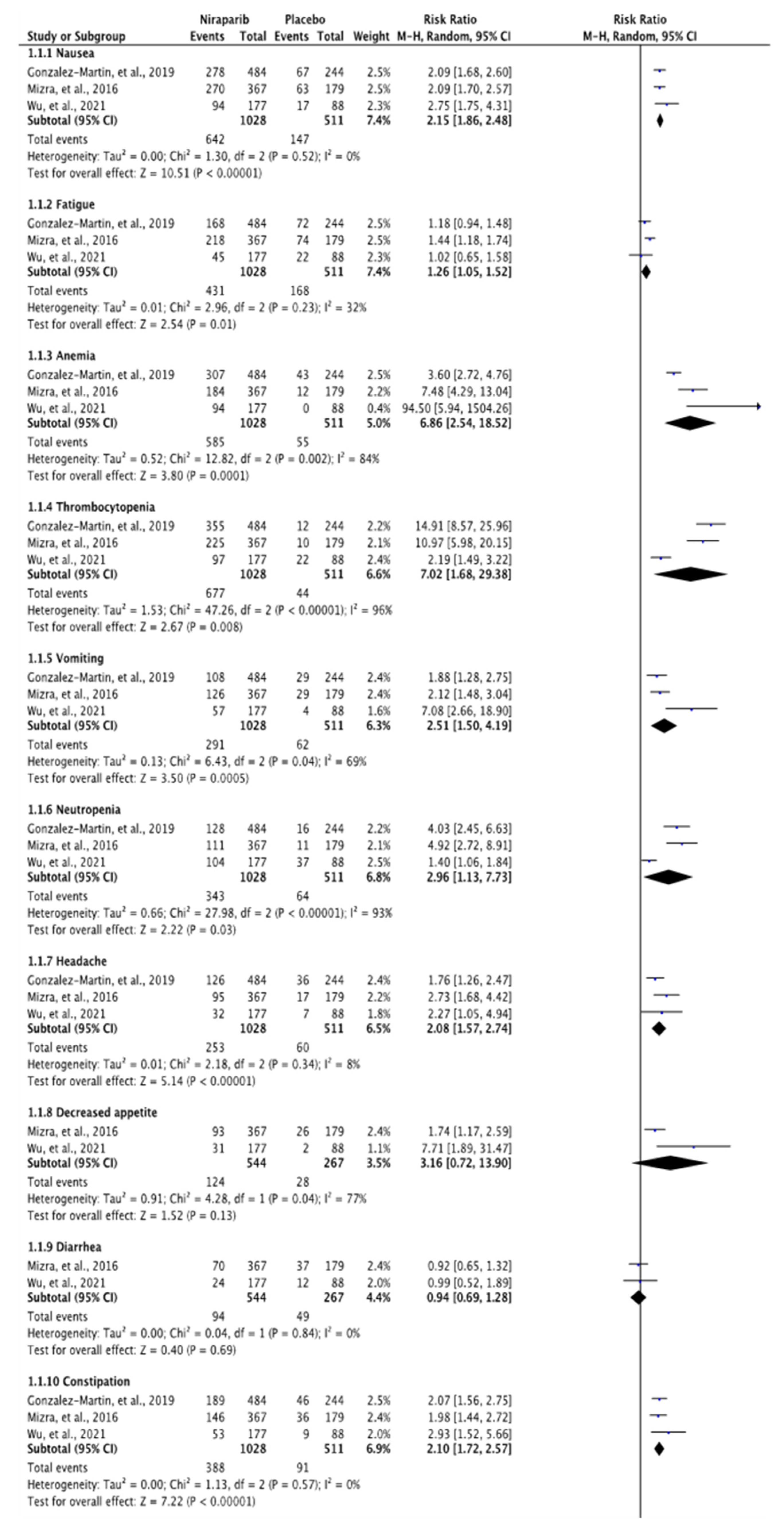
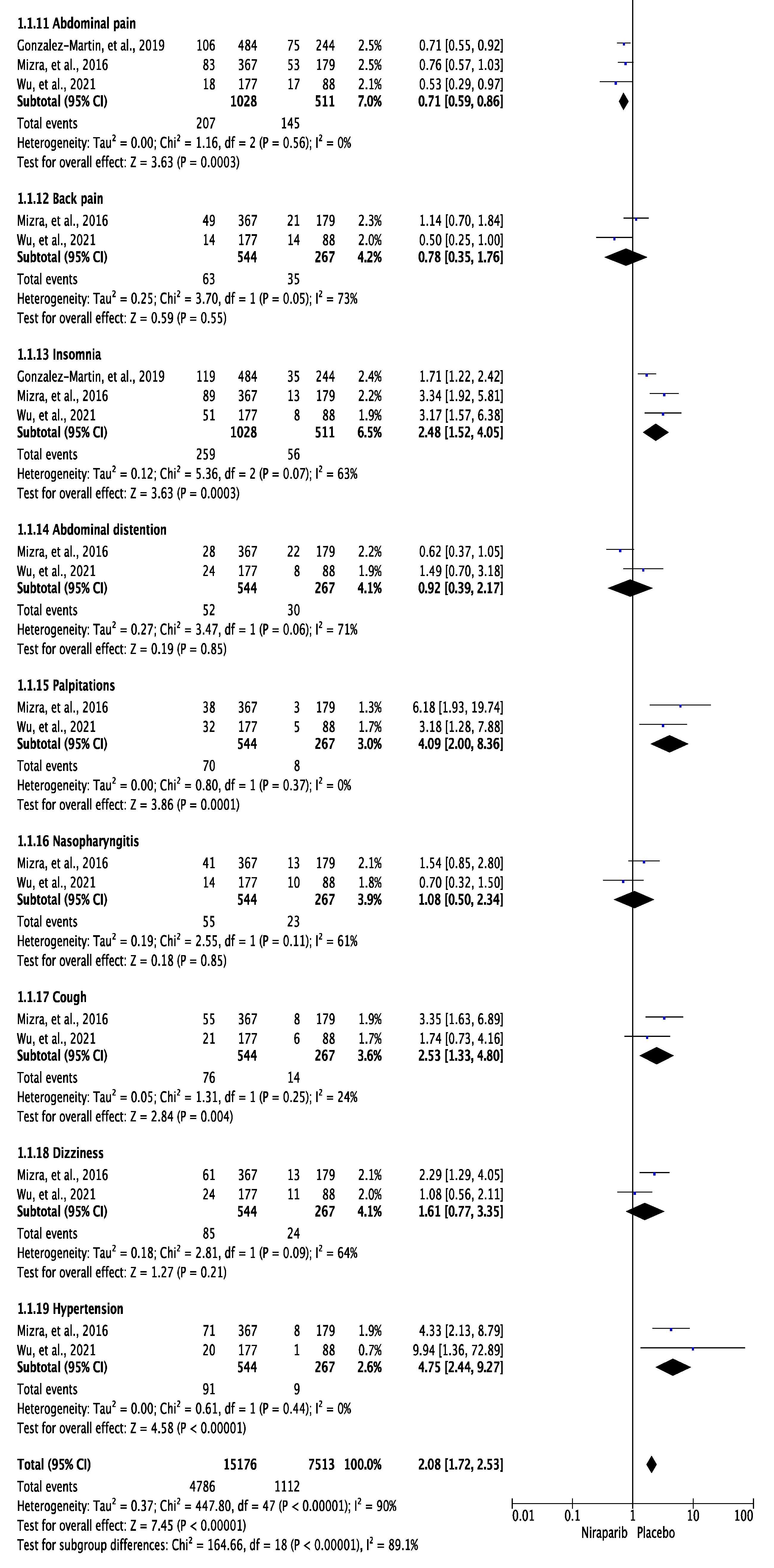
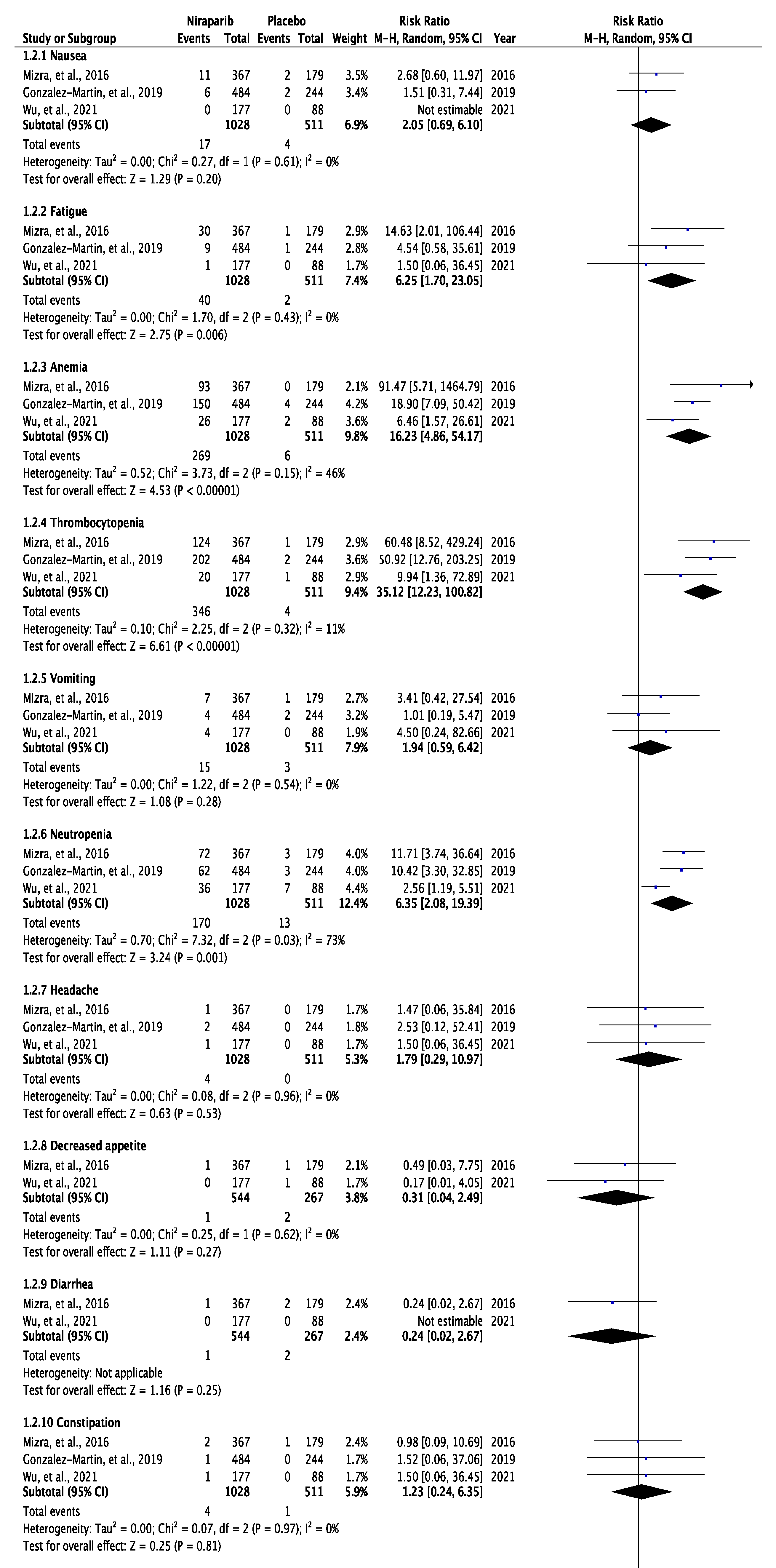
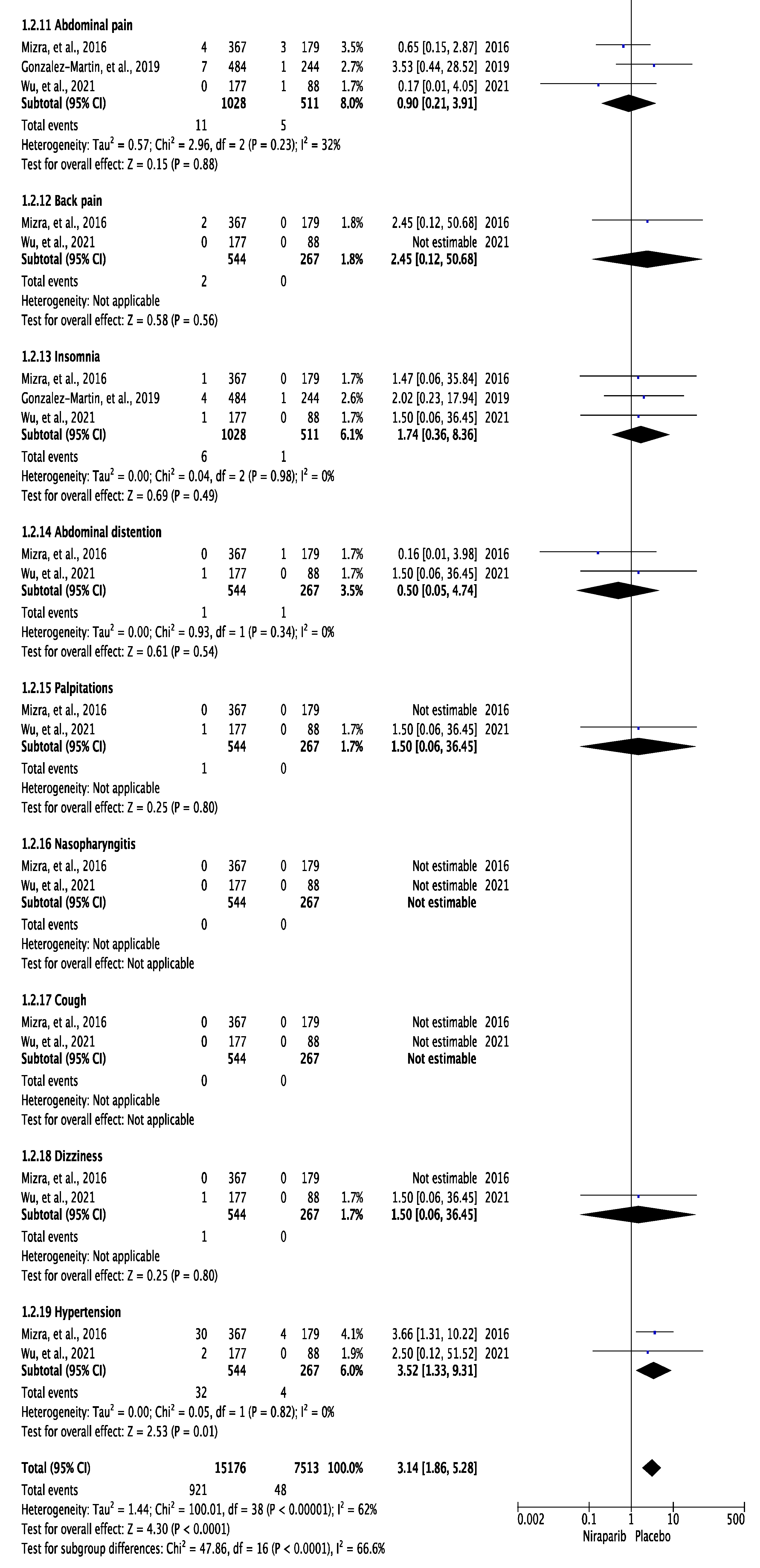
3.3. Meta-Analysis of Any Grade and Grade 3 or 4 Adverse Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Ovarian Cancer, National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/ovary.html. (accessed on 10 April 2021).
- Pignata, S.; Cecere, S.C.; Du Bois, A.; Harter, P.; Heitz, F. Treatment of recurrent ovarian cancer. Ann. Oncol. 2017, 28, viii51–viii56. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, L.; Verrico, M.; Zaccarelli, E.; Papa, A.; Colonna, M.; Strudel, M.; Vici, P.; Bianco, V.; Tomao, F. Bevacizumab in ovarian cancer: A critical review of phase III studies. Oncotarget 2016, 8, 12389–12405. [Google Scholar] [CrossRef] [Green Version]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Genta, S.; Aglietta, M.; Sapino, A.; Valabrega, G. PARP Inhibitors in Ovarian Cancer. Recent. Pat. Anticancer. Drug. Discov. 2018, 13, 392–410. [Google Scholar] [CrossRef]
- GLAXOSMITHKLINE. Zejula (Niraparib Tosylate) [208477]. U.S. Food and Drug Administration Website. 20 March. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process (accessed on 10 September 2021).
- Caruso, D.; Papa, A.; Tomao, S.; Vici, P.; Panici, P.B.; Tomao, F. Niraparib in ovarian cancer: Results to date and clinical potential. Ther. Adv. Med Oncol. 2017, 9, 579–588. [Google Scholar] [CrossRef] [Green Version]
- LaFargue, C.; Molin, G.Z.D.; Sood, A.K.; Coleman, R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhu, J.; Yin, R.; Yang, J.; Liu, J.; Wang, J.; Wu, L.; Liu, Z.; Gao, Y.; Wang, D.; et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): A randomized, double-blind, placebo-controlled phase III trial☆. Ann. Oncol. 2021, 32, 512–521. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. New Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [Green Version]
- Berek, J.S.; Matulonis, U.A.; Peen, U.; Ghatage, P.; Mahner, S.; Redondo, A.; Lesoin, A.; Colombo, N.; Vergote, I.; Rosengarten, O.; et al. Safety and dose modification for patients receiving niraparib. Ann. Oncol. 2018, 29, 1784–1792. [Google Scholar] [CrossRef]
- Fabbro, M.; Moore, K.N.; Dørum, A.; Tinker, A.V.; Mahner, S.; Bover, I.; Banerjee, S.; Tognon, G.; Goffin, F.; Shapira-Frommer, R.; et al. Efficacy and safety of niraparib as maintenance treatment in older patients (>/=70years) with recurrent ovarian cancer: Results from the ENGOT-OV16/NOVA trial. Gynecol. Oncol. 2019, 152, 560–567. [Google Scholar] [CrossRef] [Green Version]
- Dockery, L.E.; Tew, W.P.; Ding, K.; Moore, K.N. Tolerance and toxicity of the PARP inhibitor olaparib in older women with epithelial ovarian cancer. Gynecol. Oncol. 2017, 147, 509–513. [Google Scholar] [CrossRef]
- Oza, A.M.; Matulonis, U.A.; Malander, S.; Hudgens, S.; Sehouli, J.; Del Campo, J.M.; Berton-Rigaud, D.; Banerjee, S.; Scambia, G.; Berek, J.S.; et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): Results from a double-blind, phase 3, randomised controlled trial. Lancet. Oncol. 2018, 19, 1117–1125. [Google Scholar] [CrossRef]
- Ethier, J.-L.; Lheureux, S.; Oza, A.M. The role of niraparib for the treatment of ovarian cancer. Futur. Oncol. 2018, 14, 2565–2577. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Wu, H.L.; Shi, H.Y.; Su, L.; Zhang, X. The efficacy and safety of olaparib in the treatment of cancers: A meta-analysis of randomized controlled trials. Cancer. Manag. Res. 2018, 10, 2553–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Schutz, V.C.; Gomes, L.M.; Mariano, R.C.; de Almeida, D.V.; Pimenta, J.M.; Molin, G.Z.D.; Kater, F.R.; Yamamura, R.; Neto, N.F.C.; Maluf, F.C.; et al. Risk of fatigue and anemia in patients with advanced cancer treated with olaparib: A meta-analysis of randomized controlled trials. Crit. Rev. Oncol. 2019, 141, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Feng, L.J.; Zhang, X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2017, 11, 3009–3017. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Meng, J.; Wang, G. Risk of selected gastrointestinal toxicities associated with poly (ADP-ribose) polymerase (PARP) inhibitors in the treatment of ovarian cancer: A meta-analysis of published trials. Drug Des. Devel. Ther. 2018, 12, 3013–3019. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.R.; Heap, K.J.; Carroll, S.; Travers, K.; Harrow, B.; Westin, S.N. Real-world adverse events with niraparib 200 mg/day maintenance therapy in ovarian cancer: A retrospective study. Future Oncol. 2019, 15, 4197–4206. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Hendrickson, A.E.W.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Mirza, M.R.; Bergmann, T.K.; Mau-Sorensen, M.; Christensen, R.D.; Avall-Lundqvist, E.; Birrer, M.J.; Jørgensen, M.; Roed, H.; Malander, S.; Nielsen, F.; et al. A phase I study of the PARP inhibitor niraparib in combination with bevacizumab in platinum-sensitive epithelial ovarian cancer: NSGO AVANOVA1/ENGOT-OV24. Cancer Chemother Pharmacol. 2019, 84, 791–798. [Google Scholar] [CrossRef]
- Mirza, M.R.; Lundqvist, E.A.; Birrer, M.J.; de Pont Christensen, R.; Nyvang, G.B.; Malander, S.; Anttila, M.; Werner, T.L.; Lund, B.; Lindahl, G.; et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): A randomised, phase 2, superiority trial. Lancet Oncol. 2019, 20, 1409–1419. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Waggoner, S.; Vidal, G.A.; Mita, M.; Moroney, J.W.; Holloway, R.; Van Le, L.; Sachdev, J.C.; Chapman-Davis, E.; Colon-Otero, G.; et al. Single-Arm Phases 1 and 2 Trial of Niraparib in Combination With Pembrolizumab in Patients With Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol. 2019, 5, 1141–1149. [Google Scholar] [CrossRef] [Green Version]
| NOVA Study Mirza et al. 2016 [12] | PRIMA Study González-Martín et al. 2019 [13] | NORA Study Wu et al. 2020 [14] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| gBRCAm (+) 203 Total n. of patients | gBRCAm (−) 350 Total n. of patients | HRD (+) 373 Total n. of patients | Non-HRD(+) 249 Total n. of patients | 177 Total n. of patients | 88 Total n. of patients | |||||
| Niraparib | Placebo | Niraparib | Placebo | Niraparib | Placebo | Niraparib | Placebo | Niraparib | Placebo | |
| Number of patients | 138 | 65 | 234 | 116 | 247 | 126 | 240 | 120 | 177 | 88 |
| Number of patients who discontinued treatment | 91 | 61 | 188 | 104 | 126 | 83 | 184 | 93 | 101 | 77 |
| Number of patients receiving ongoing treatment at data cut off | 47 | 4 | 46 | 12 | 121 | 42 | 56 | 27 | 76 | 11 |
| Median age, years | 57 | 58 | 63 | 61 | 58 | 58 | 66 | 66 | 53 | 55 |
| Primary location tumor | ||||||||||
| Ovary | 122 | 53 | 192 | 96 | 201 | 105 | 187 | 96 | 174 | 86 |
| Other | 16 | 12 | 42 | 19 | 46 | 21 | 53 | 24 | 3 | 2 |
| Response to platinum-based chemotherapy | ||||||||||
| Complete | 71 | 33 | 117 | 60 | 185 | 93 | 152 | 79 | 121 | 60 |
| Partial | 67 | 32 | 117 | 56 | 62 | 33 | 88 | 41 | 56 | 28 |
| Number to previous platinum-based regiments | ||||||||||
| 1 | 1 | 0 | 0 | 0 | NR | NR | NR | NR | NR | NR |
| 2 | 70 | 30 | 155 | 77 | NR | NR | NR | NR | NR | NR |
| ≥3 | 67 | 35 | 79 | 38 | NR | NR | NR | NR | NR | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagkali, A.; Mamais, I.; Michalinos, A.; Agouridis, A.P. Safety Profile of Niraparib as Maintenance Therapy for Ovarian Cancer: A Systematic Review and Meta-Analysis. Curr. Oncol. 2022, 29, 321-336. https://doi.org/10.3390/curroncol29010029
Pagkali A, Mamais I, Michalinos A, Agouridis AP. Safety Profile of Niraparib as Maintenance Therapy for Ovarian Cancer: A Systematic Review and Meta-Analysis. Current Oncology. 2022; 29(1):321-336. https://doi.org/10.3390/curroncol29010029
Chicago/Turabian StylePagkali, Antonia, Ioannis Mamais, Adamantios Michalinos, and Aris P. Agouridis. 2022. "Safety Profile of Niraparib as Maintenance Therapy for Ovarian Cancer: A Systematic Review and Meta-Analysis" Current Oncology 29, no. 1: 321-336. https://doi.org/10.3390/curroncol29010029
APA StylePagkali, A., Mamais, I., Michalinos, A., & Agouridis, A. P. (2022). Safety Profile of Niraparib as Maintenance Therapy for Ovarian Cancer: A Systematic Review and Meta-Analysis. Current Oncology, 29(1), 321-336. https://doi.org/10.3390/curroncol29010029





