Is Elevation of Alkaline Phosphatase a Predictive Factor of Response to Alectinib in NSCLC?
Abstract
:1. Introduction
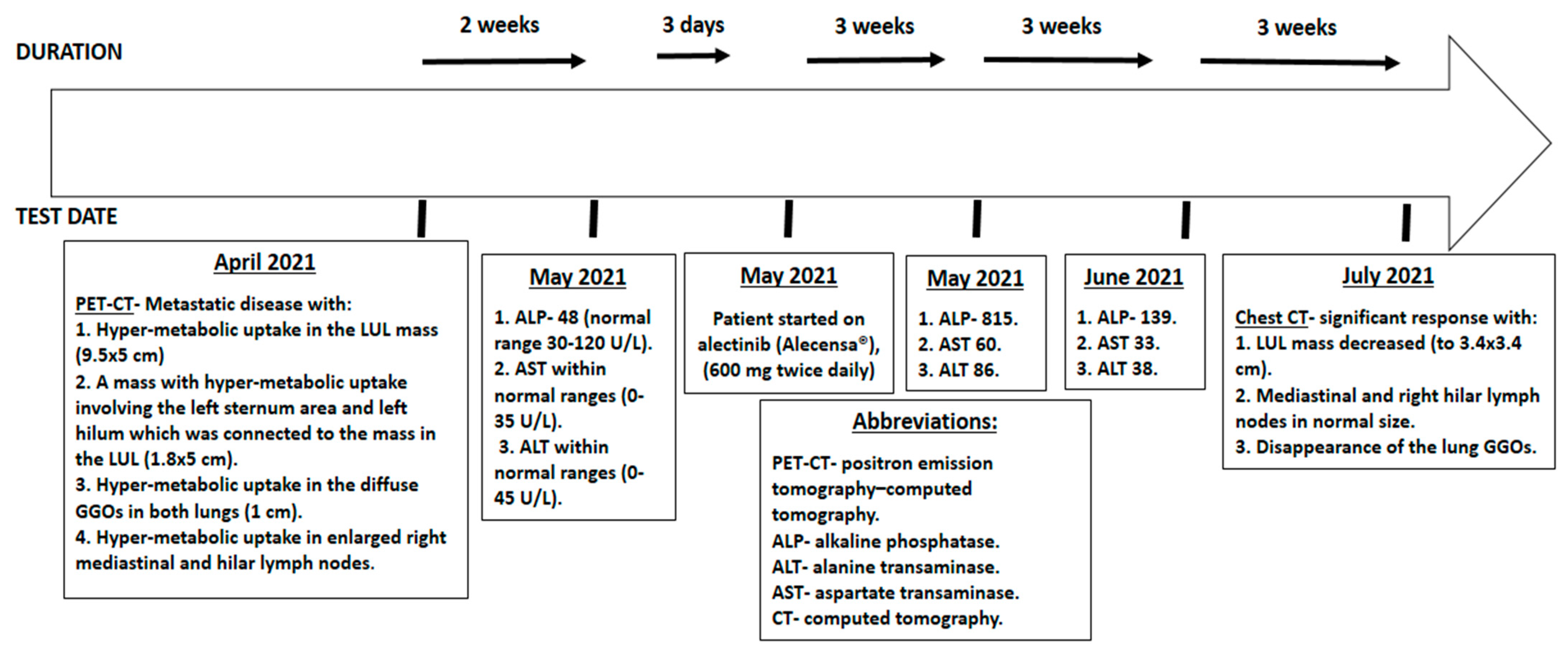
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, B.M.; Mortensen, J.; Hansen, H.; Vilmann, P.; Larsen, S.S.; Loft, A.; Bertelsen, A.K.; Ravn, J.; Clementsen, P.F.; Høegholm, A.; et al. Multimodality approach to mediastinal staging in non-small cell lung cancer. Faults and benefits of PET-CT: A randomised trial. Thorax 2011, 66, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addeo, A.; Tabbò, F.; Robinson, T.; Buffoni, L.; Novello, S. Precision medicine in ALK rearranged NSCLC: A rapidly evolving scenario. Crit. Rev. Oncol. Hematol. 2018, 122, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Rodig, S.J.; Mino-Kenudson, M.; Dacic, S.; Yeap, B.Y.; Shaw, A.; Barletta, J.A.; Stubbs, H.; Law, K.; Lindeman, N.; Mark, E.; et al. Unique Clinicopathologic Features Characterize ALK-Rearranged Lung Adenocarcinoma in the Western Population. Clin. Cancer Res. 2009, 15, 5216–5223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.; Gerber, D.E. ALK alterations and inhibition in lung cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2017; Volume 42, pp. 81–88. [Google Scholar]
- Awad, M.M.; Shaw, A.T. ALK inhibitors in non-small cell lung cancer: Crizotinib and beyond. Clin. Adv. Hematol. Oncol. H&O 2014, 12, 429–439. [Google Scholar]
- Kodama, T.; Tsukaguchi, T.; Yoshida, M.; Kondoh, O.; Sakamoto, H. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett. 2014, 351, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Alecensa European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/alecensa (accessed on 20 September 2020).
- Alecensa (Alectinib) FDA Approval History Drugs.com. Available online: https://www.drugs.com/history/alecensa.html (accessed on 20 September 2020).
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 49806720, Alectinib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alectinib (accessed on 9 September 2021).
- Gadgeel, S.M.; Gandhi, L.; Riely, G.J.; Chiappori, A.A.; West, H.L.; Azada, M.C.; Morcos, P.N.; Lee, R.-M.; Garcia, L.; Yu, L.; et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014, 15, 1119–1128. [Google Scholar] [CrossRef]
- Lowe, D.; Sanvictores, T.; John, S. Alkaline Phosphatase. (Updated 11 August 2021 Au). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459201/ (accessed on 11 August 2021).
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alecensa.com. 2020. ALECENSA® (Alectinib) First-Line Safety Profile. Available online: https://www.alecensa.com/hcp/alk-mnsclc-clinical-overview/1l-safety-profile.html (accessed on 20 September 2020).
- Accessdata.fda.gov. 2018; Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208434s004lbl.pdf (accessed on 20 September 2020).
- Shalata, W.; Massalha, I.; Agbarya, A. Is alectinib-induced elevation of creatine phosphokinase a predictive factor for response? Report of two cases and review of the literature. Anti-Cancer Drugs 2021, 32, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Peled, N.; Gabizon, I.; Abu Saleh, O.; Kian, W.; Yakobson, A. Associated Myocarditis: A Predictive Factor for Response? Case Rep. Oncol. 2020, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Barbaryan, A.; Zdunek, T.; Khan, M.; Voore, P.; Mirrakhimov, A.E. Spontaneous tumor lysis syndrome in a patient with cholangiocarcinoma. J. Gastrointest. Oncol. 2014, 5, E46–E49. [Google Scholar] [CrossRef] [PubMed]
- Durani, U.; Hogan, W.J. Emergencies in haematology: Tumour lysis syndrome. Br. J. Haematol. 2020, 188, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.S. Laboratory Values and Interpretation of Results. ScienceDirect. 25 July 2013. Available online: https://www.sciencedirect.com/science/article/pii/B9780323498302000032 (accessed on 1 January 2018).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalata, W.; Yakobson, A.; Steckbeck, R.; Jama, A.A.; Abu Saleh, O.; Agbarya, A. Is Elevation of Alkaline Phosphatase a Predictive Factor of Response to Alectinib in NSCLC? Curr. Oncol. 2022, 29, 173-177. https://doi.org/10.3390/curroncol29010016
Shalata W, Yakobson A, Steckbeck R, Jama AA, Abu Saleh O, Agbarya A. Is Elevation of Alkaline Phosphatase a Predictive Factor of Response to Alectinib in NSCLC? Current Oncology. 2022; 29(1):173-177. https://doi.org/10.3390/curroncol29010016
Chicago/Turabian StyleShalata, Walid, Alexander Yakobson, Rachel Steckbeck, Ashraf Abu Jama, Omar Abu Saleh, and Abed Agbarya. 2022. "Is Elevation of Alkaline Phosphatase a Predictive Factor of Response to Alectinib in NSCLC?" Current Oncology 29, no. 1: 173-177. https://doi.org/10.3390/curroncol29010016
APA StyleShalata, W., Yakobson, A., Steckbeck, R., Jama, A. A., Abu Saleh, O., & Agbarya, A. (2022). Is Elevation of Alkaline Phosphatase a Predictive Factor of Response to Alectinib in NSCLC? Current Oncology, 29(1), 173-177. https://doi.org/10.3390/curroncol29010016






