Abstract
Robot-assisted radical cystectomy (RARC) is replacing open radical cystectomy (ORC) and requires clamping of the ureters, resulting in a predisposition to postrenal acute kidney injury (AKI). We investigated the association between ureteral clamping or its duration and acute/chronic postoperative kidney function. Patients who underwent radical cystectomy (robotic or open) at two tertiary institutions during 2002–2021 were retrospectively enrolled. In those who underwent RARC, the maximum postoperative percentage serum creatinine level (%sCre) change was plotted against ureteral clamping duration. They were divided into two groups using the median clamping time (210 min), and the maximum %sCre change and percentage estimated glomerular filtration rate (%eGFR) change at 3–6 months (chronic) were compared between the ORC (no clamp), RARC < 210, and RARC ≥ 210 groups. In 44 RARC patients, a weak correlation was observed between the duration of ureteral clamping and %Cre change (R2 = 0.22, p = 0.001). Baseline serum creatinine levels were comparable between the groups. However, %sCre change was significantly larger in the RARC ≥ 210 group (N = 17, +32.1%) than those in the RARC < 210 (N = 27, +6.1%) and ORC (N = 76, +9.5%) groups (both, p < 0.001). Chronic %eGFR change was comparable between the groups. Longer clamping of the ureter during RARC may precipitate AKI; therefore, the clamping duration should be minimized.
1. Introduction
Radical cystectomy (RC) remains the gold standard treatment for patients with muscle-invasive or high-risk non-muscle-invasive urinary carcinoma of the bladder and is recommended in various guidelines globally [1,2]. While open RC (ORC) is still relevant, minimally invasive surgery, such as robot-assisted RC (RARC), results in less blood loss and better postoperative recovery [3].
One of the distinct features of minimally invasive surgery in comparison with the open approach in RC is the clamping of ureters during the intracorporeal procedures. Laboratory studies have reported that ureteral obstruction increases the intratubular pressure and may worsen renal function [4]. In the phase 3 RAZOR trial, the rate of acute renal failure (as defined in that study) was 11%, and no difference in its incidence was observed in comparison with ORC [3]. Additionally, mid-term renal function is reported to be comparable between ORC and RARC or across different urinary diversions [5]. However, the level of evidence is not very strong, and therefore, maximum effort is warranted to avoid acute kidney injury (AKI) [5]. Both AKI and long-term renal function outcomes are attributed to various factors, including preoperative renal function, hypertension, and diabetes; therefore, it is quite challenging to evaluate the effects of ureteral clamping on short-term renal function.
In this study, we aimed to assess the effects of the duration of ureteral clamping time (UCL) during RARC on the postoperative renal function with a focus on the acute phase (AKI) and the chronic phase.
2. Materials and Methods
2.1. Patient Selection
We retrospectively recruited 199 patients who underwent RC at two tertiary institutions, which included those who underwent RARC between April 2018 and July 2021 (N = 83) and those who underwent ORC between May 2004 and July 2021 (N = 116). After excluding patients who underwent simultaneous nephroureterectomy (N = 32), those with end-stage renal failure (N = 8), those without complete operative video recording during RARC (N = 32, as UCL could not be measured), and those with insufficient clinical or follow-up data (N = 7), 120 (44 RARC, 76 ORC) patients were finally analyzed. All clinical and laboratory data were obtained from the institutions’ electronic databases and patient medical records.
2.2. Study Design
First, in patients who underwent RARC, the duration of UCL was plotted against the maximum postoperative percentage change in serum creatinine (%sCre change) to assess acute kidney failure. UCL was also evaluated in relation to the maximum postoperative percentage change in estimated glomerular filtration rate (%eGFR change) at 3–6 months to assess chronic renal function. Next, these RARC patients were classified into groups as is stated in Section 2.5. Statistical Analysis. The following outcomes were assessed between the RARC groups of patients, as well as between the two RARC and ORC groups: postoperative maximum sCre; %sCre change; AKI according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (serum creatinine compared with preoperative reference: stage 1: 1.5–2.0 times; stage 2: 2.0–2.9 times; and stage 3: ≥3.0 times [6]); eGFR at 3–6 months postoperatively; %eGFR change at 3–6 months; operative time (OT); estimated blood loss (EBL, mL). We utilized sCre to evaluate AKI and eGFR for chronic renal function change as is suggested in the international guidelines [6,7].
Finally, the relationship between possible factors and %sCre change or %eGFR change at 3–6 months were each analyzed.
2.3. Surgery and Perioperative Care
Both RARC and ORC were performed as described previously [8,9,10,11,12]. Since 2018 (Japanese national insurance system coverage), RARC has been the preferred surgery except for patients with contraindications, such as those with a history of multiple abdominal operations. The type of urinary diversion, including intracorporeal urinary diversion (ICUD) or extracorporeal urinary diversion (ECUD) during RARC, was determined by the operating surgical team based on the patient’s preoperative status, including comorbidities, surgical history, and preference. In both RARC and ORC, cystectomy was performed first, followed by lymph node dissection and urinary diversion. In RARC, the ureter was clamped halfway through cystectomy and unclamped during urinary diversion after pelvic lymphadenectomy, which was performed with an extended template in a majority of cases [13]. UCL was measured retrospectively using the recorded operation video and defined as the duration between the last clamping of either of the ureters and the unclamping of the first ureter. In ORC, the ureter was catheterized using a 6-Fr single-J catheter immediately after ligation. Therefore, there was no UCL. The number of surgeons involved included 10 in ORC and six in RARC.
Postoperative management was generally performed according to the Enhanced Recovery After Surgery (ERAS) protocol [14]. Briefly, fluid intake was started on the day of the surgery, and oral nutrition was initiated as soon as it could be tolerated by patients in both RARC and ORC groups. Parental nutrition was initiated at the physicians’ discretion if oral nutrition could not be initiated within 5–7 days.
2.4. Neoadjuvant and Adjuvant Chemotherapy
Neoadjuvant chemotherapy was considered when the patients were fit and when lymph node or surrounding tissue involvement was suspected in imaging studies. Adjuvant chemotherapy was offered when lymph node metastasis or local invasion was confirmed in the surgical specimen. All final decisions were made by the surgical team after considering the patients’ comorbidities and preferences. While several chemotherapy regimens were used, all were based on either cisplatin or carboplatin.
2.5. Statistical Analysis
Continuous variables were analyzed using the Mann–Whitney U-test and expressed as medians and interquartile range (IQR). β-coefficient was calculated using linear regression models. Categorical variables were analyzed using the χ2 or Fisher’s exact tests with odds ratio (OR) and 95% confidence interval. The median UCL value was used as the cutoff to classify the RARC patients into two groups. The patients were also classified into three groups according to UCL quartiles (shortest quartile, longest quartile, and between). Assessment of the relationship between possible factors and %sCre change or %eGFR change at 3–6 months were first performed using univariate analysis using the following variables: age, baseline eGFR, presence of hydronephrosis at the time of surgery, hypertension (on medications), diabetes, OT, EBL, perioperative urinary tract infections (for %eGFR change only), and UCL. Multivariate analyses were also performed in cases in which ≥ two significant factors were identified. Multivariate linear regression models were used to adjust the β-coefficient in cases when two or more significant factors were identified univariate analysis. All analyses were performed using JMP v14.0 (SAS Institute, Cary, NC, USA). Differences were considered statistically significant at p-values < 0.05.
3. Results
3.1. Patient Characteristics
The patients’ characteristics are summarized in Table 1. The median age at surgery in the RARC group was 74.3 years (IQR, 69.0–80.0), and 77.3% were male. All preoperative parameters were equally distributed between both sexes, except for the presence of hydronephrosis (Male: 26.1%, Female: 46.9%, p = 0.034). The median preoperative eGFR was 52.0 (45.1–70.1) mL/min/1.73 m2. The prevalence of hypertension and diabetes were 27.3% and 29.5%, respectively. Hydronephrosis was observed in 36.4% of patients. The type of urinary diversion used included ileal conduit (79.5%), orthotopic neobladder (4.5%), and ureterocutaneostomy (15.9%); additionally, 28 (63.6%) procedures used ICUD, and 18 (40.9%) procedures used ECUD.

Table 1.
Patient characteristics.
Patients who underwent RARC were significantly older (74.3 (69.0–80.0) years) than those who underwent ORC (69.2 (58.5–75.6) years, p = 0.001) and had marginally lower eGFR (52.0 (45.1–70.1) vs. 62.5 (47.6–81.2) mL/min/1.73 m2, respectively, p = 0.065). There were also significant differences in the American Society of Anesthesiologists (ASA) score (p = 0.043) and the type of urinary diversion (p < 0.001); more patients were treated with a neobladder in the ORC group than those in the RARC group. All other baseline characteristics were comparable between the RARC and ORC groups of patients.
3.2. Association between UCL and Acute/Chronic Phase Renal Function Change in RARC
Figure 1 illustrates the relationship between UCL (209.1 (155.2–254.4) min) and %sCre change. A weak but significant correlation was observed between the two parameters (R2 = 0.22, p = 0.001). Figure 2 illustrates the relationship between UCL and %eGFR change at 3–6 months, which demonstrated no significant correlation (R2 = 0.03, p = 0.282). In those who underwent ileal conduit, there was no difference in the UCL between the intracorporeal (n = 19, 229.9 (195.8–256.7) min) and extracorporeal (203.1 (166.5–255.9) min) procedures (p = 0.227). The median and average UCL was 209.1 (155.2–254.4) and 206.6 (±66.7) min, respectively. We, therefore, set the median cutoff value of UCL at 210 min, and patients who underwent RARC were divided into the RARC < 210 and RARC ≥ 210 groups. Additionally, 155 and 245 min were used as a cutoff value of shortest and longest quartiles.
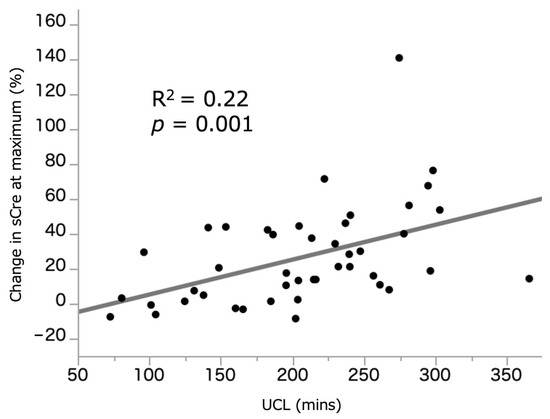
Figure 1.
Correlation between UCL and %sCre change at maximum. sCre: serum creatinine. UCL: ureteral clamping time.
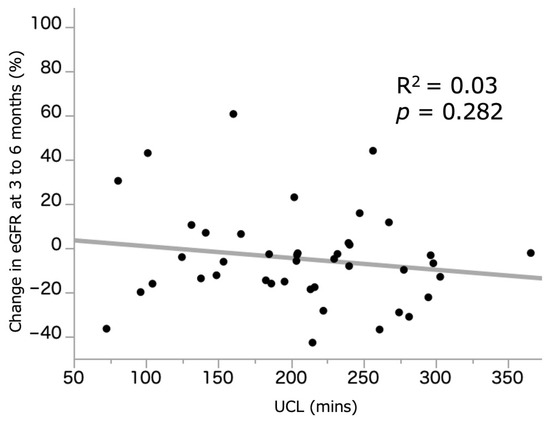
Figure 2.
Correlation between UCL and %eGFR change at 3 to 6 months. eGFR: estimated glomerular filtration rate. UCL: ureteral clamping time.
The %sCre change was significantly larger in the RARC ≥ 210 group (32.1 (15.5–54.3)%) when compared with those in the RARC < 210 group (6.1 (−1.3 to 32.0)%, p < 0.001) and ORC group (9.5 (0.2–23.9)%, p < 0.001). There was no significant difference between the RARC < 210 and ORC groups (p = 0.983) (Figure 3, Table 2). Incidence of AKI (any grade) was also significantly higher in the RARC ≥ 210 group (31.8%), compared with those in the RARC < 210 (0.0%, p < 0.001) and ORC (2.6%, p < 0.001) groups. In contrast, %eGFR change at 3–6 months was −8.2 (−25.4 to −0.4)%, −5.0 (−15.2 to 7.7)%, and −7.9 (−18.3 to −0.2)% in the RARC ≥ 210, RARC < 210, and ORC groups, respectively, with no significant differences between them (Figure 4, Table 2).
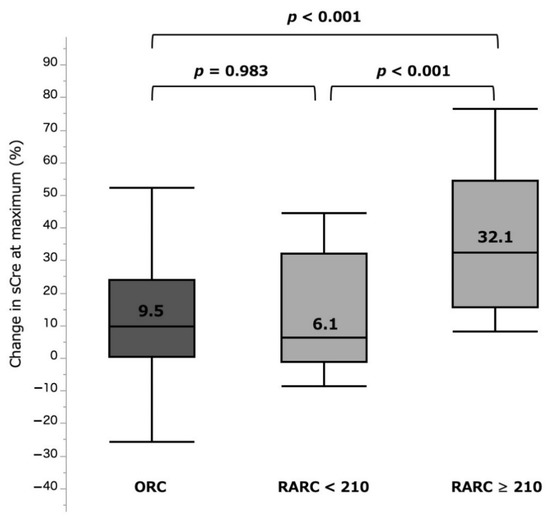
Figure 3.
%sCre change at maximum by three surgical types. ORC: open radical cystectomy. RARC: robot-assisted radical cystectomy. sCre: serum creatinine.

Table 2.
Surgical outcomes.
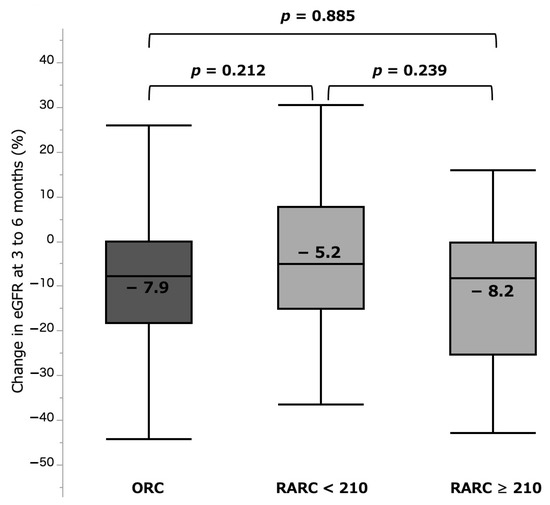
Figure 4.
%eGFR change at 3 to 6 months by three surgical types. eGFR: estimated glomerular filtration rate. ORC: open radical cystectomy. RARC: robot-assisted radical cystectomy.
In comparative analyses of the groups of quartiles, there were further distinct differences of 4.8% vs. 40.0% for shortest and longest quartile groups (p = 0.013) at the acute phase (Figure 5). For the chronic phase, the shortest group (−6.2%) showed less eGFR loss than the longest (−9.9%) quartile group, but the difference was again not significant (p = 0.555, Figure 6).
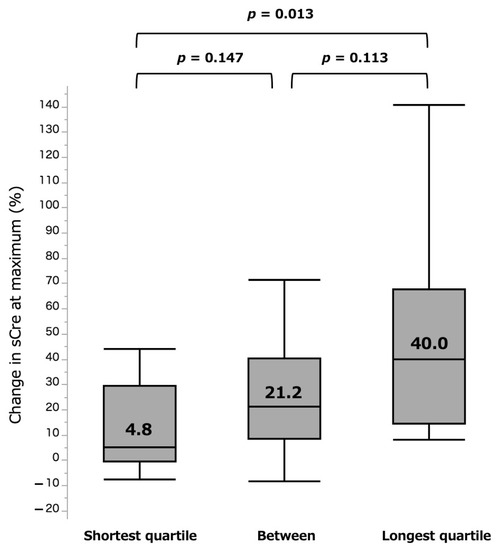
Figure 5.
%sCre change at maximum by three surgical types. sCre: serum creatinine.
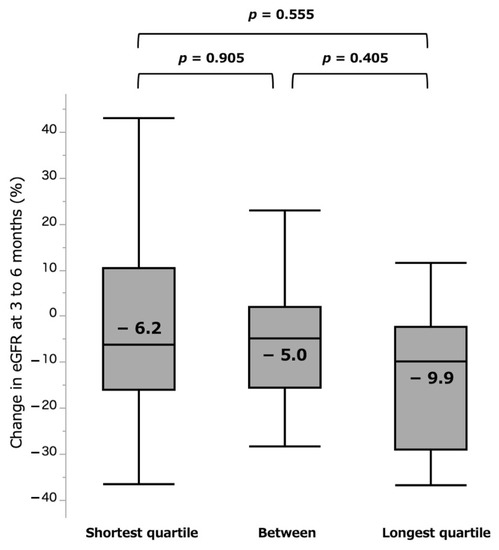
Figure 6.
%eGFR change at 3 to 6 months by quartile groups. eGFR: estimated glomerular filtration rate.
3.3. Relationship between UCL and Other Outcomes
Surgical time was significantly shorter in the RARC < 210 group (392.0 (278.8–452.8) min), compared with that in the RARC > 210 (466.0 (430.8–536.5) min, p < 0.001) and ORC (463.0 (356.0–548.0) min, p = 0.003) groups. There was no significant difference between the RARC ≥ 210 and ORC groups (p = 0.395). EBL was the lowest in the RARC < 210 (103.5 (52.3–262.5) mL) group in comparison with the RARC ≥ 210 (275.0 (160.3–470.0) mL, p < 0.001) and ORC (1080.0 (527.5–2417.5) mL, p < 0.001) groups. The difference between the RARC ≥ 210 and ORC groups was also statistically significant (p < 0.001) (Table 2).
3.4. Factors Affecting the Acute/Chronic Phase Renal Function Change in RARC
We analyzed the variables that may be related to acute %sCre change and chronic %eGFR change. In univariate analysis of acute %sCre change, UCL (continuous value, β-coefficient 0.20 (standard error (SE) 0.06), p = 0.001) age (continuous value, β-coefficient 0.59 (SE 0.34), p = 0.061), and surgical time (continuous value, β-coefficient 0.09 (SE 0.04), p = 0.025) were identified as significant factors. In multivariate analysis, UCL was demonstrated to be independently associated (β-coefficient 0.25, (SE 0.11), p = 0.023) (Table 3). In univariate analysis of chronic %eGFR change, only preoperative baseline eGFR was identified as an associated factor (β-coefficient −0.43 (SE 0.18), p = 0.019) (Table 4).

Table 3.
Univariate and multivariate linear regression for change in sCre at maximum (%).

Table 4.
Linear regression for change in eGFR at 6 months (%).
4. Discussion
In this study, a significant association between UCL and %sCre change was observed in the AKI phase. When stratified into two groups based on the median UCL time (210 min), patients with UCL ≥ 210 min had a greater increase in %sCre and were more likely to develop AKI, compared with those with UCL < 210 min or who underwent ORC. No association between UCL and chronic (3–6 months) renal function was evident.
Acute kidney damage caused by urinary tract obstruction is recognized as postrenal AKI. There are several proposed mechanisms for this phenomenon, including increased intratubular pressure resulting in a decline in GFR [4], and that obstruction may lead to impairment of renal circulation and inflammation [15], both of which contribute directly to GFR loss [16]. These reactions later result in fibrotic changes in the renal parenchyma [17], thus precipitating the chronic loss of renal function. Animal laboratory model data suggest a relationship between the duration of ureteral obstruction and the severity of the damage. In rats, renal function evaluated using 99mTc-dimercaptosuccinic acid (DMSA) uptake decreased from 35% at baseline (before ureter ligation) to 13% after 24 h and further to 1.5% after 31 days. If the ureter was freed after 10 days, DMSA uptake recovered from 7% to 15%, whereas the recovery was only from 1.5% to 2% when ligation was prolonged to 30 days [18].
However, in humans, the exact relationship between kidney damage and ureteral obstruction, bilateral obstruction particularly, has been poorly investigated largely due to the difficulty in collecting patient data under such conditions. Furthermore, whether very limited UCL, as short as 100–300 min (as described in our study), affects acute renal function was unknown. The current finding that UCL is correlated with acute kidney damage is a novel addition to the existing knowledge. We set the UCL cutoff value at 210 min to identify significant differences in renal function and AKI incidence. There is no rationale yet to support this cutoff value; therefore, further studies are warranted to identify the optimal UCL threshold. Furthermore, the finding that eGFR recovery at 3–6 months was comparable between the groups irrespective of UCL is partly in line with previous laboratory data on return of renal function following ureteral obstruction of 4–14 days [19,20].
There was one patient in the RARC group who had a nephrostomy placed prior to RC and left open during the operation. UCL duration for this patient was 278 min (i.e., >210), and he experienced a rather large increase in sCre (40.0%). Theoretically, the risk of AKI would be considerably low if one kidney was freed from postrenal occlusion [21], but this specific finding does not necessarily support this supposition. We believe extensive research is required to answer if the preoperative placement of nephrostomy can protect patients from AKI.
There is a growing body of evidence to support AKI prevention for multiple purposes. Strong interconnection has been reported between AKI and chronic kidney disease (CKD) [22]. Additionally, AKI has been reported to have independent effects on perioperative complications and mortality [23]. In terms of CKD progression, we could not detect any differences in renal function at 3–6 months postoperatively between the groups. This finding is distinct from those of studies that have investigated the association between AKI and CKD after partial nephrectomy, in which AKI is possibly caused by loss of nephrons and ischemia [24]. The differences in the pathophysiology may explain this discrepancy, while the small sample size and short follow-up period could have also contributed to it.
Apart from ureteral obstruction, RC is a procedure with an inherent considerable risk of damage to renal function. Furrer et al. reported that 11% of patients in their ORC cohort experienced AKI [25]. This number is comparable to the one observed in the current study. Lone et al. reported that 64% of patients who underwent either ORC, RARC with ICUD, or RARC with ECUD experienced a decline in eGFR ≥ 10 mL/min/1.73 m2 with no differences between the surgery type [5]. It should be borne in mind that UCL in RC has not been extensively studied since it was only after the utilization of laparoscopic and robotic technologies that surgeons began ureteral clamping to continue intracorporeal procedures after ligating the ureters [9].
Data have revealed possible gender discrepancies in bladder cancer management and outcomes. In a recent review, females were shown for significantly longer postoperative stay, operative time, more blood loss, and a higher rate of mortality or complications [26]. In the present study, the preoperative parameters were comparable between males and females. Furthermore, %sCre change at acute phase was larger for males (14.0%) compared with females (9.7%) with no significance (p = 0.141), while occurrence of AKI were more frequently observed among males (10.2% vs. 0.0%, p = 0.016). %eGFR change at chronic phase was comparable between sexes (non-significant). As a result, we could not detect any signs that females are more prone to AKI after RC. We believe a further focus on these disparities is needed.
After confirming that AKI is likely to occur in those with prolonged UCL, we should emphasize that preventing AKI after RC is of paramount importance due to several reasons. First, AKI in itself is a significant risk factor for both short-term and long-term cardiovascular events [27]. Second, patients with bladder cancer are generally older (over 70 years) at the time of diagnosis [28] and may be prone to CKD following AKI [29,30]. In fact, the median age of our patients was ≥70 years. Third, renal function impairment may limit the therapeutic options during the perioperative period, thus possibly resulting in serious adverse events. Therefore, UCL time should be minimized to reduce the risk of postoperative AKI in RARC. The time required to deliver safe and effective RARC (as well as ICUD) requires a certain learning curve [31,32]. In cases of planned lymphadenectomy, performing lymphadenectomy before cystectomy may serve as a possible strategy to shorten UCL time. As all patients in this study underwent lymphadenectomy after cystectomy, further investigations are required to justify the optimal sequence. At the least, ureteral clamping should be withheld until the bladder is mobilized as much as possible. For procedures of urinary diversion (ICUD or ECUD) used in the ileal conduit, we did not observe any difference in the UCL time between the techniques. This finding may show that trained surgeons with adequate training can perform ICUD with UCL as efficiently as the traditional ECUD.
Our study has some limitations. This was a retrospective cohort study involving a relatively small number of patients, which may have introduced selection bias. Second, the effects of urinary diversion on UCL time or renal function could not be evaluated due to the small number of patients in the RARC cohort. Third, interpretation of our analysis between the RARC and ORC cohort ought to be carried out with caution, as their clinical background differed in several aspects. Further studies, ideally prospective and with longer follow-up periods, are warranted to corroborate our findings.
5. Conclusions
This retrospective analysis showed that UCL time during RARC was significantly associated with acute renal function loss and AKI. Although its effects on chronic renal function were limited, effort should be incorporated to minimize the UCL time and protect patients from potential AKI and its sequelae.
Author Contributions
Conceptualization, Y.I., T.K. and H.I.; methodology, Y.I and T.K; formal analysis, Y.I.; investigation, Y.I.; resources, Y.I.; data curation, Y.I.; writing—original draft preparation, Y.I.; writing—review and editing, Y.I. and T.K.; supervision, K.Y., J.I., K.T. and T.T.; project administration, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Internal Ethics Review Board of Tokyo Women’s Medical University (Approval ID: 2020-0108).
Informed Consent Statement
Patient consent was waived due to the retrospective and observational study design.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors thank Nobuko Hata (Department of Urology, Tokyo Women’s Medical University) for her secretarial work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of non-metastatic muscle-invasive bladder cancer: Aua/asco/astro/suo guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Parekh, D.J.; Reis, I.M.; Castle, E.P.; Gonzalgo, M.L.; Woods, M.E.; Svatek, R.S.; Weizer, A.Z.; Konety, B.R.; Tollefson, M.; Krupski, T.L.; et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (razor): An open-label, randomised, phase 3, non-inferiority trial. Lancet 2018, 391, 2525–2536. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [PubMed] [Green Version]
- Lone, Z.; Murthy, P.B.; Zhang, J.H.; Ericson, K.J.; Thomas, L.; Khanna, A.; Haber, G.P.; Lee, B.H. Comparison of renal function after open radical cystectomy, extracorporeal robot assisted radical cystectomy, and intracorporeal robot assisted radical cystectomy. Urol. Oncol. 2021, 39, 301.e301–301.e309. [Google Scholar] [CrossRef]
- KDIGO. Kdigo clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–38. [Google Scholar]
- KDIGO. Kdigo 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1. [Google Scholar]
- Wein, A.J.; Kavoussi, L.R.; Partin, A.W.; Peters, C. Transurethral and open surgery for bladder cancer. In Campbell-Walsh Urology, 11th ed.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Wein, A.J.; Kavoussi, L.R.; Partin, A.W.; Peters, C. Robotic and laparoscopic bladder surgery. In Campbell-Walsh Urology, 11th ed.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Wein, A.J.; Kavoussi, L.R.; Partin, A.W.; Peters, C. Use of intestinal segments in urinary diversion. In Campbell-Walsh Urology; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Wein, A.J.; Kavoussi, L.R.; Partin, A.W.; Peters, C. Orthotopic urinary diversion. In Campbell-Walsh Urology; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Wein, A.J.; Kavoussi, L.R.; Partin, A.W.; Peters, C. Minimally invasive urinary diversion. In Campbell-Walsh Urology; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Dhar, N.B.; Klein, E.A.; Reuther, A.M.; Thalmann, G.N.; Madersbacher, S.; Studer, U.E. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J. Urol. 2008, 179, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hubner, M.; Kassouf, W.; Muller, S.; Baldini, G.; Carli, F.; et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced recovery after surgery (eras((r))) society recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, N.J.; Young, L.S.; Kirwan, C.N.; O’Neill, A.J.; Bouchier-Hayes, D.M.; Sweeney, P.; Watson, R.W.; Fitzpatrick, J.M. Nitric oxide in unilateral ureteral obstruction: Effect on regional renal blood flow. Kidney Int. 2001, 59, 1059–1065. [Google Scholar] [CrossRef] [Green Version]
- ahlberg, J.; Karlberg, L.; Persson, A.E. Total and regional renal blood flow during complete unilateral ureteral obstruction. Acta Physiol. Scand. 1984, 121, 111–118. [Google Scholar] [CrossRef]
- Washino, S.; Hosohata, K.; Miyagawa, T. Roles played by biomarkers of kidney injury in patients with upper urinary tract obstruction. Int. J. Mol. Sci. 2020, 21, 5490. [Google Scholar] [CrossRef]
- Schelfhout, W.; Simons, M.; Oosterlinck, W.; De Sy, W.A. Evaluation of 99mtc-dimercaptosuccinic acid renal uptake as an index of individual kidney function after acute ureteral obstruction and desobstruction. An experimental study in rats. Eur. Urol. 1983, 9, 221–226. [Google Scholar] [CrossRef]
- Vaughan, E.D., Jr.; Gillenwater, J.Y. Recovery following complete chronic unilateral ureteral occlusion: Functional, radiographic and pathologic alterations. J. Urol. 1971, 106, 27–35. [Google Scholar] [CrossRef]
- Fink, R.L.; Caridis, D.T.; Chmiel, R.; Ryan, G. Renal impairment and its reversibility following variable periods of complete ureteric obstruction. Aust. N. Z. J. Surg. 1980, 50, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Spanou, L. Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin. Biochemist. Rev. 2016, 37, 85–98. [Google Scholar]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gameiro, J.; Fonseca, J.A.; Neves, M.; Jorge, S.; Lopes, J.A. Acute kidney injury in major abdominal surgery: Incidence, risk factors, pathogenesis and outcomes. Ann. Intensive Care 2018, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Bravi, C.A.; Vertosick, E.; Benfante, N.; Tin, A.; Sjoberg, D.; Hakimi, A.A.; Touijer, K.; Montorsi, F.; Eastham, J.; Russo, P.; et al. Impact of acute kidney injury and its duration on long-term renal function after partial nephrectomy. Eur. Urol. 2019, 76, 398–403. [Google Scholar] [CrossRef]
- Furrer, M.A.; Schneider, M.P.; Burkhard, F.C.; Wuethrich, P.Y. Incidence and perioperative risk factors for early acute kidney injury after radical cystectomy and urinary diversion. Urol. Oncol. 2018, 36, 306.e317–306.e323. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Righetto, M.; Baggio, G. Spotlight on gender-specific disparities in bladder cancer. Urologia 2020, 87, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Rossignol, P. Cardiovascular consequences of acute kidney injury. N. Engl. J. Med. 2020, 382, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D. Seer Cancer Statistics Review, 1975–2017. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 23 March 2021).
- Schmitt, R.; Coca, S.; Kanbay, M.; Tinetti, M.E.; Cantley, L.G.; Parikh, C.R. Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am. J. Kidney Dis. 2008, 52, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of esrd among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, J.W.; Tyritzis, S.; Nyberg, T.; Schumacher, M.C.; Laurin, O.; Adding, C.; Jonsson, M.; Khazaeli, D.; Steineck, G.; Wiklund, P.; et al. Robot-assisted radical cystectomy (rarc) with intracorporeal neobladder—What is the effect of the learning curve on outcomes? BJU Int. 2014, 113, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Tuderti, G.; Mastroianni, R.; Brassetti, A.; Bove, A.M.; Misuraca, L.; Anceschi, U.; Ferriero, M.; Gallucci, M.; Simone, G. Robot-assisted radical cystectomy with intracorporeal neobladder: Impact of learning curve and long-term assessment of functional outcomes. Minerva Urol. Nefrol. 2020. Online ahead of print. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).