Current Attitudes toward Unfunded Cancer Therapies among Canadian Medical Oncologists
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Survey
2.3. Statistical Analysis
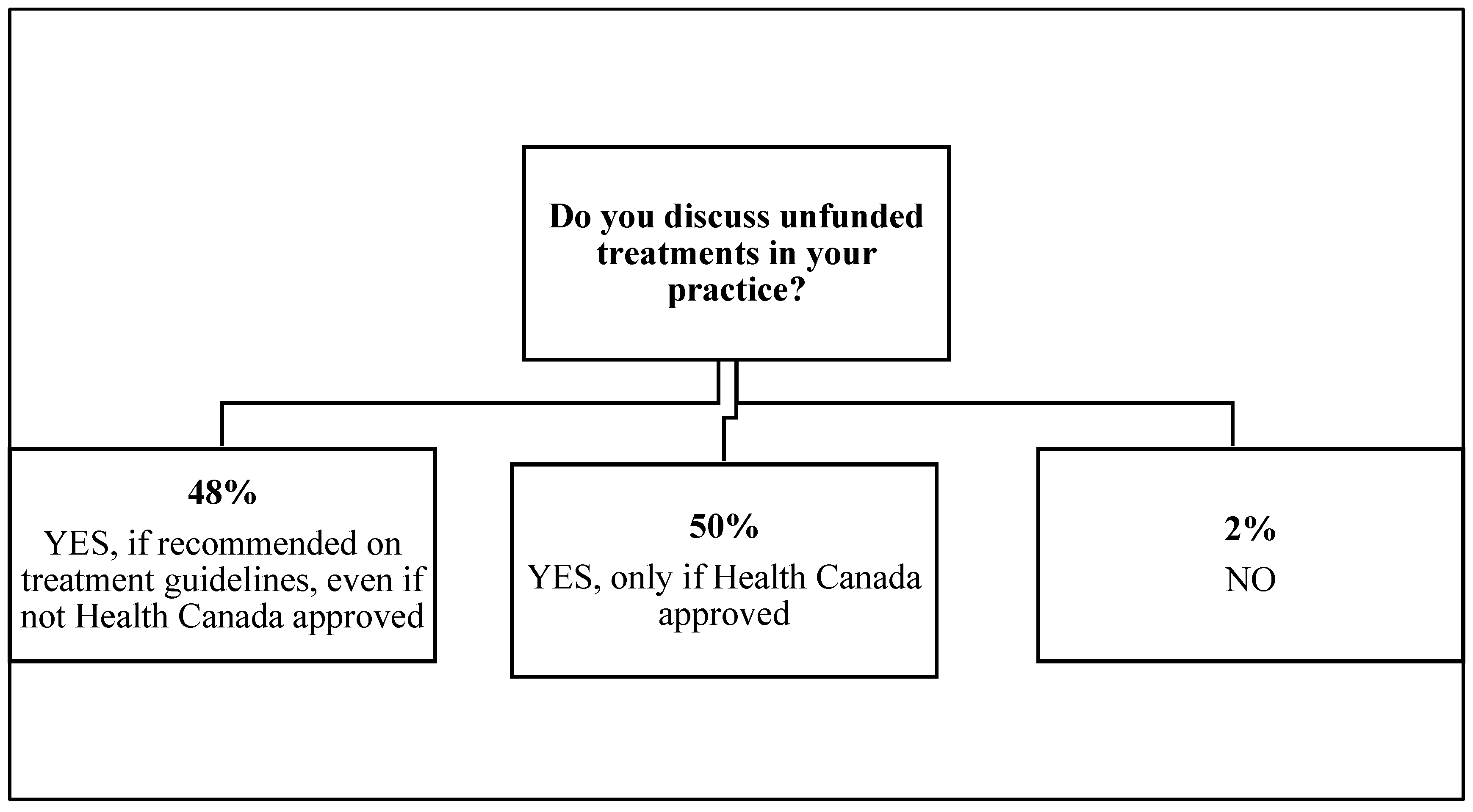
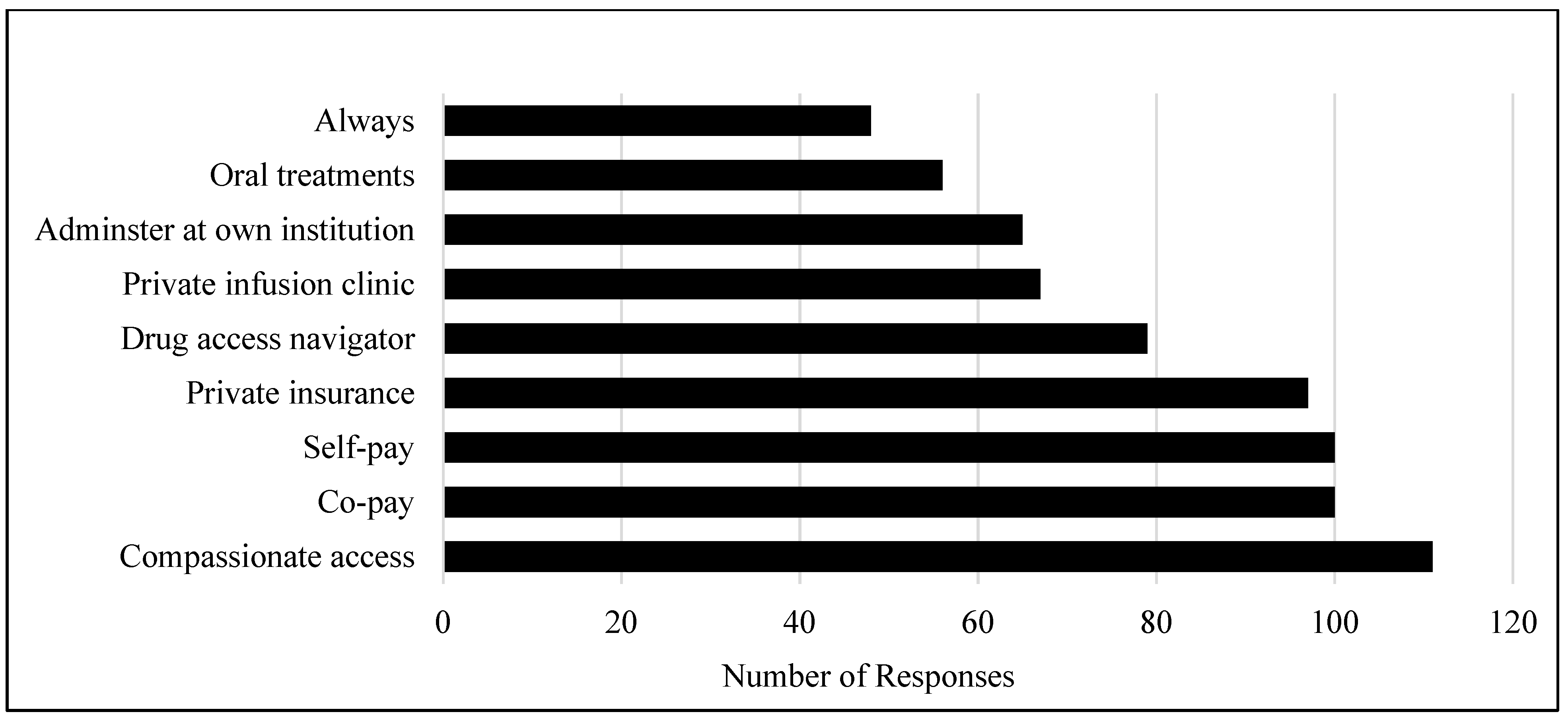
3. Results
4. Discussion
“I might bring it up even if I was not sure if they could pay, sometimes it’s hard to tell what financial resources people have…”
“Phase 1/2 trial data not sufficient…”
“I feel that it is important to give the patient all the appropriate available treatment options…but the degree to which I discuss a non-funded treatment can vary.”
“I would try to present information in a manner that wouldn’t be a torment to the patient, like dangling something unobtainable that might be life-saving”
“I am very concerned with burdening patients with a possible treatment that they cannot afford/access. Has potential to cause harm.”
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Oliveira, C.; Weir, S.; Rangrej, J.; Krahn, M.D.; Mittmann, N.; Hoch, J.S.; Chan, K.K.W.; Peacock, S. The economic burden of cancer care in Canada: A population-based cost study. CMAJ Open 2018, 6, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- CADTH. The pCODR Expert Review Committee (pERC). Available online: https://www.cadth.ca/collaboration-and-outreach/advisory-bodies/pcodr-expert-review-committee-perc (accessed on 7 May 2021).
- Trudeau, M.E.; Chambers, A.; Christiansen, K.; Mai, H. Pan-Canadian Oncology Drug Review (pCODR): A unique model to support harmonization of cancer drug funding decisions in Canada. J. Clin. Oncol. 2018, 36, 41. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 12 August 2021).
- Chan, K.K.; Wong, B.; Siu, L.L.; Straus, S.E.; Chang, J.; Berry, S.R. Less than ideal: How oncologists practice with limited drug access. J. Oncol. Pract. 2012, 8, 190–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mellor, J.D.; Van Koeverden, P.; Yip, S.W.; Thakerar, A.; Kirsa, S.W.; Michael, M. Access to anticancer drugs: Many evidence-based treatments are off-label and unfunded by the Pharmaceutical Benefits Scheme. Intern. Med. J. 2012, 42, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Trinkaus, M.; Hogeveen, S.; Mamdani, M.; Berry, S.R.; Jang, R.W.; Hoch, J.S.; Simmons, C. Overcoming obstacles in accessing unfunded oral chemotherapy: Physician experience and challenges. J. Oncol. Pract. 2013, 9, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.R.; Hubay, S.; Soibelman, H.; Martin, D.K. The effect of priority setting decisions for new cancer drugs on medical oncologists’ practice in Ontario: A qualitative study. BMC Health Serv. Res. 2007, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Karikios, D.J.; Mileshkin, L.; Martin, A.; Ferraro, D.; Stockler, M.R. Discussing and prescribing expensive unfunded anticancer drugs in Australia. ESMO Open 2017, 2, e000170. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.; Schofield, P.; Mileshkin, L.; Agalianos, E.; Savulescu, J.; Zalcberg, J.; Jefford, M. Do oncologists discuss expensive anti-cancer drugs with their patients? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006, 17, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.R.; Evans, W.K.; Strevel, E.L.; Bell, C.M. Variation and consternation: Access to unfunded cancer drugs in Canada. J. Oncol. Pract. 2012, 8, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Chafe, R.; Culyer, A.; Dobrow, M.; Coyte, P.C.; Sawka, C.; O’Reilly, S.; Laing, K.; Trudeau, M.; Smith, S.; Hoch, J.S.; et al. Access to cancer drugs in Canada: Looking beyond coverage decisions. Healthc. Policy 2011, 6, 27–36. [Google Scholar] [CrossRef][Green Version]
- Jenkins, V.A.; Trapala, I.S.; Parlour, L.; Langridge, C.I.; Fallowfield, L.J. The views of patients and the general public about expensive anti-cancer drugs in the NHS: A questionnaire-based study. JRSM Short Rep. 2011, 2, 69. [Google Scholar] [CrossRef]
- Mileshkin, L.; Schofield, P.E.; Jefford, M.; Agalianos, E.; Levine, M.; Herschtal, A.; Savulescu, J.; Thomson, J.A.; Zalcberg, J.R. To tell or not to tell: The community wants to know about expensive anticancer drugs as a potential treatment option. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5830–5837. [Google Scholar] [CrossRef]
- Schrag, D.; Hanger, M. Medical oncologists’ views on communicating with patients about chemotherapy costs: A pilot survey. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 233–237. [Google Scholar] [CrossRef]
- Altomare, I.; Irwin, B.; Zafar, S.Y.; Houck, K.; Maloney, B.; Greenup, R.; Peppercorn, J. Physician Experience and Attitudes Toward Addressing the Cost of Cancer Care. J. Oncol. Pract. 2016, 12, 247–288. [Google Scholar] [CrossRef]
| Demographic | N (%) |
|---|---|
| Gender | |
| Female | 62 (53) |
| Male | 53 (46) |
| Not disclosed | 1 (1) |
| Province of practice | |
| British Columbia | 41 (35) |
| Ontario | 31 (27) |
| Alberta | 13 (11) |
| Quebec | 12 (10) |
| Manitoba | 6 (5) |
| New Brunswick | 6 (5) |
| Nova Scotia | 3 (3) |
| Saskatchewan | 2 (2) |
| Newfoundland | 1 (1) |
| Prince Edward Island | 1 (1) |
| Practice setting | |
| Comprehensive Cancer Center | 102 (88) |
| Community | 14 (12) |
| Private Practice | 0 (0) |
| Disease site | |
| Gastrointestinal | 55 (47) |
| Breast | 55 (47) |
| Genitourinary | 34 (29) |
| Lung | 34 (29) |
| Gynecologic | 22 (19) |
| Melanoma | 22 (19) |
| Sarcoma | 18 (16) |
| Head and Neck | 19 (16) |
| Hematology/Lymphoma | 18 (16) |
| Other | 20 (17) |
| Years in practice | |
| <5 years | 22 (19) |
| 5–10 years | 27 (23) |
| 10–15 years | 13 (11) |
| >15 years | 54 (47) |
| Previous training outside Canada | |
| Yes | 45 (39) |
| No | 71 (61) |
| Variable | Odds Ratio (95% Confidence Interval) | p-Value |
|---|---|---|
| Ontario vs. British Columbia | 1.53 (0.47–4.97) | 0.47 |
| Quebec vs. British Columbia | 0.70 (0.12–3.91) | 0.68 |
| Atlantic vs. British Columbia | 0.99 (0.16–6.19) | 0.99 |
| Prairies vs. British Columbia | 0.20 (0.04–0.87) | 0.03 * |
| Community vs. Comprehensive Cancer Center | 0.17 (0.03–0.91) | 0.04 * |
| Male vs. Female | 2.35 (0.92–5.97) | 0.07 |
| Years in practice 5–10 y vs. <5 y | 0.40 (0.10–1.58) | 0.19 |
| Years in practice 10–15 y vs. <5 y | 0.24 (0.04–1.27) | 0.09 |
| Years in practice >15 y vs. <5 y | 0.14 (0.04–0.50) | 0.002 * |
| Institution permits administration (Only if manufacturer access program vs. Yes | 0.43 (0.12–1.59) | 0.20 |
| Institution permits unfunded treatment, No vs. Yes | 0.49 (0.14–1.74) | 0.27 |
| Workload (No/Minimal vs Moderate/Significant #) | 0.42 (0.15–1.16) | 0.09 |
| Drug access navigator available, No vs. Yes | 2.29 (0.52–10.15) | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.K.; Gondara, L.; Gill, S. Current Attitudes toward Unfunded Cancer Therapies among Canadian Medical Oncologists. Curr. Oncol. 2021, 28, 4748-4755. https://doi.org/10.3390/curroncol28060400
Wong SK, Gondara L, Gill S. Current Attitudes toward Unfunded Cancer Therapies among Canadian Medical Oncologists. Current Oncology. 2021; 28(6):4748-4755. https://doi.org/10.3390/curroncol28060400
Chicago/Turabian StyleWong, Selina K., Lovedeep Gondara, and Sharlene Gill. 2021. "Current Attitudes toward Unfunded Cancer Therapies among Canadian Medical Oncologists" Current Oncology 28, no. 6: 4748-4755. https://doi.org/10.3390/curroncol28060400
APA StyleWong, S. K., Gondara, L., & Gill, S. (2021). Current Attitudes toward Unfunded Cancer Therapies among Canadian Medical Oncologists. Current Oncology, 28(6), 4748-4755. https://doi.org/10.3390/curroncol28060400






