Quality of End-of-Life Cancer Care in Canada: A 12-Year Retrospective Analysis of Three Provinces’ Administrative Health Care Data Evaluating Changes over Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Study Design
2.2. Data Sources
2.3. Health Service Quality Indicators
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Quality Indicators
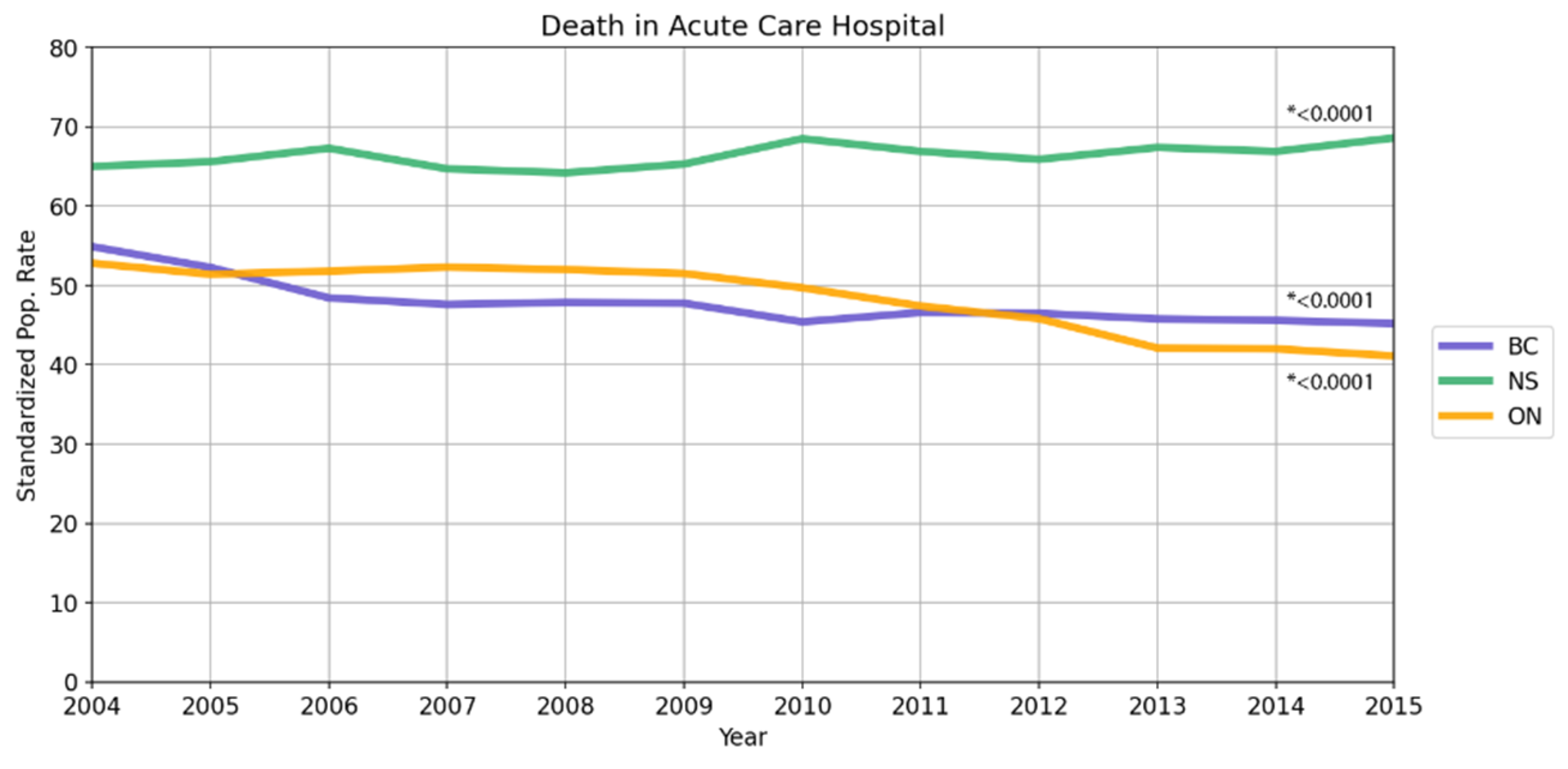
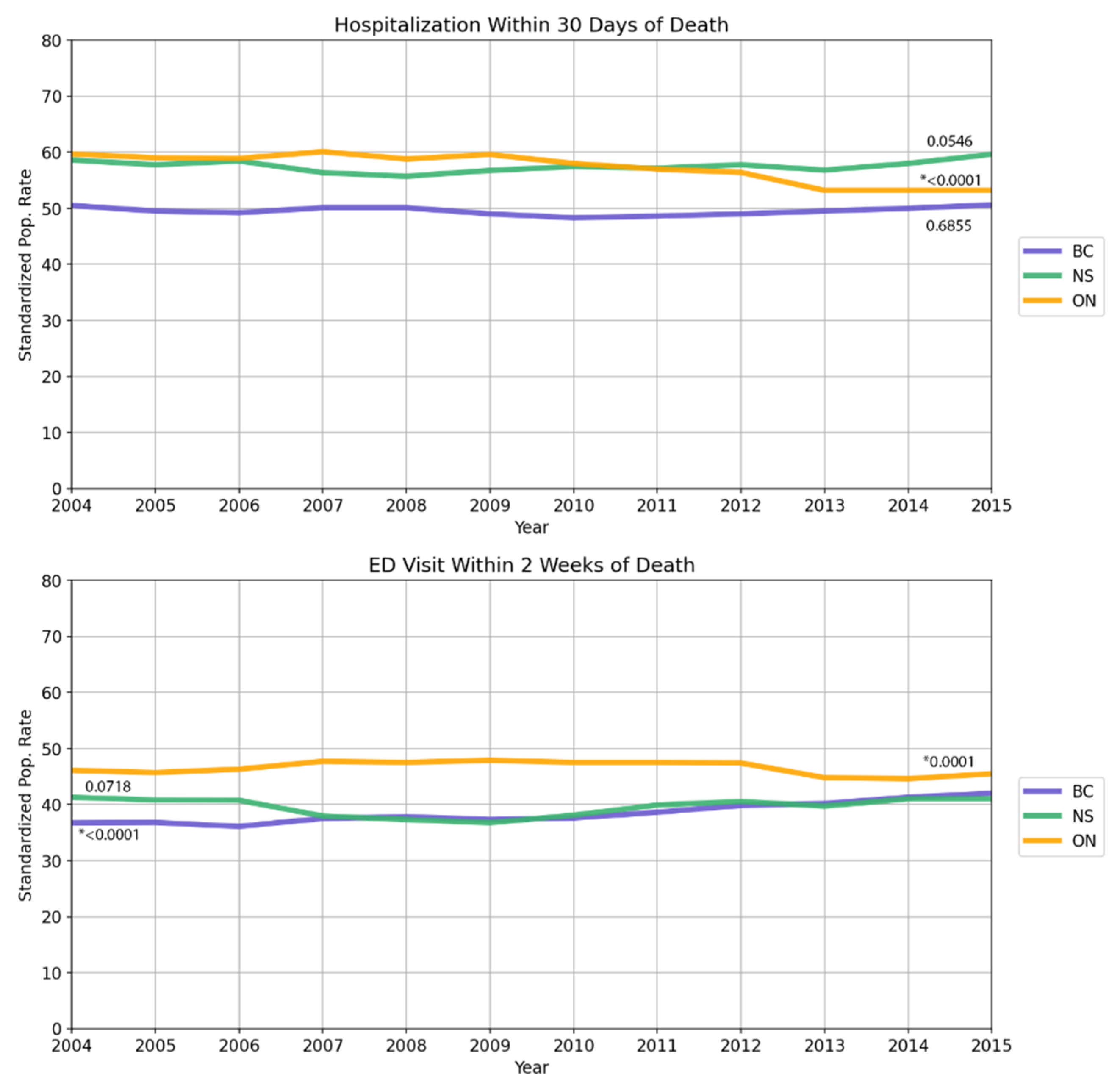
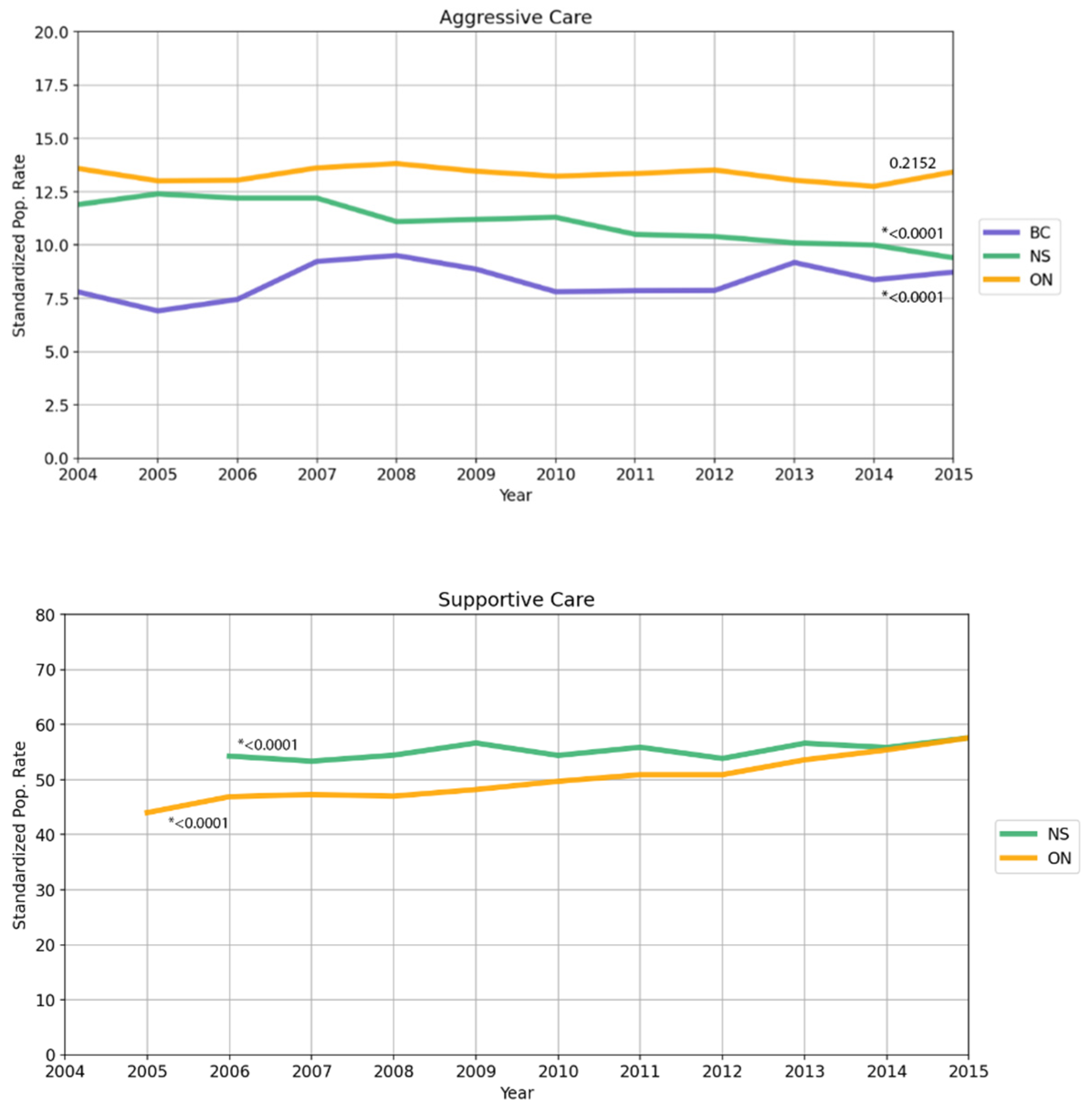
3.3. Data Trends over Time
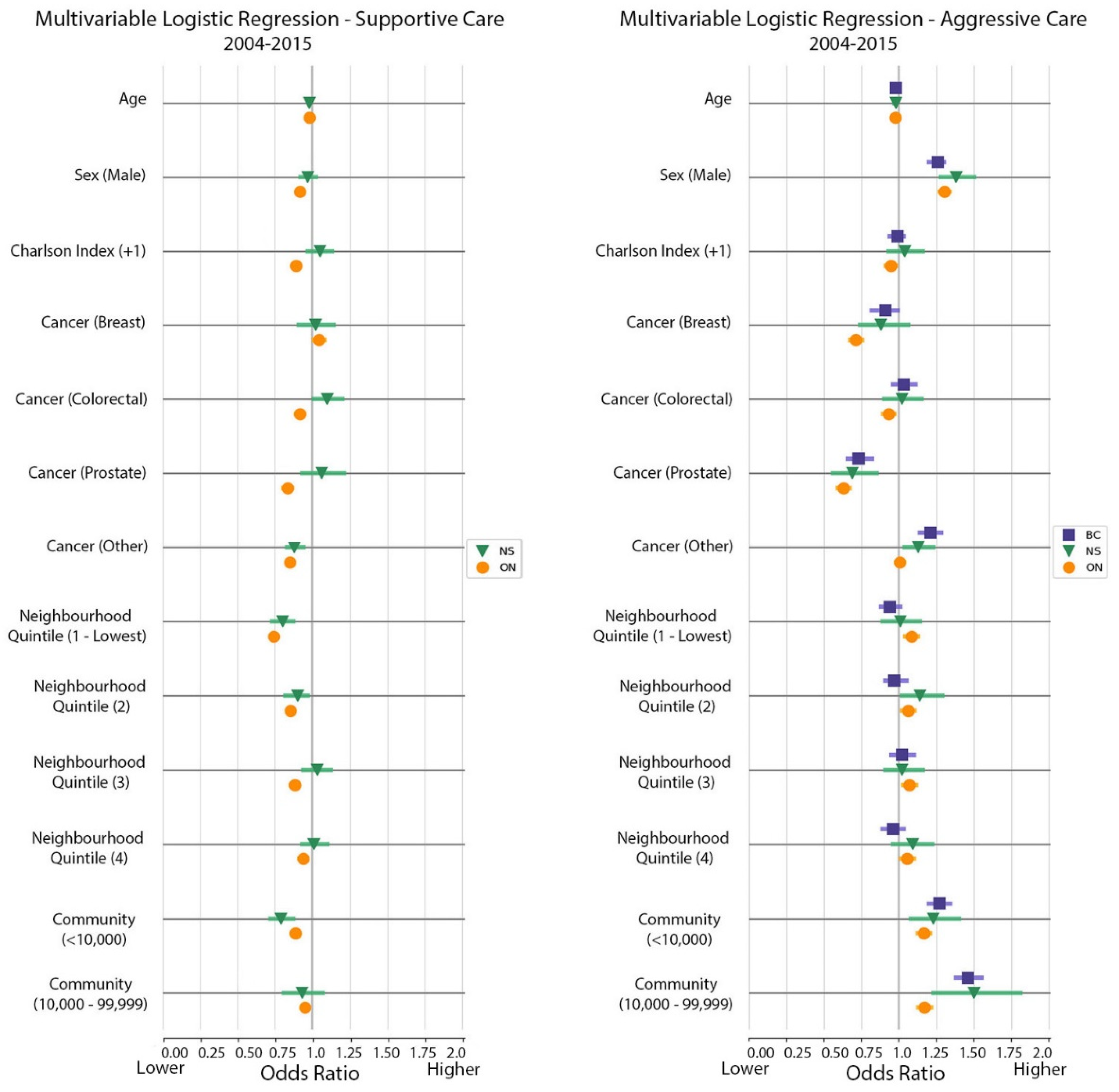
3.4. Multivariable Logistic Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristic | BC | ON | NS | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Study Population | 104,106 | 242,556 | 29,446 | 376,108 | ||||
| Age group | ||||||||
| 18–49 Years | 4984 | 4.8 | 12,782 | 5.3 | 1401 | 4.8 | 19,167 | 5.1 |
| 50–79 Years | 65,234 | 62.7 | 154,257 | 63.6 | 18,877 | 64.1 | 238,368 | 63.4 |
| 80+ Years | 33,878 | 32.5 | 75,517 | 31.1 | 9168 | 31.1 | 118,563 | 31.5 |
| Sex | ||||||||
| Male | 55,381 | 53.2 | 128,772 | 53.1 | 15,559 | 52.8 | 199,712 | 53.1 |
| Income quintile | ||||||||
| 1 (lowest) | 23,094 | 23.1 | 51,526 | 21.3 | 6300 | 21.7 | 80,920 | 21.9 |
| 2 | 20,459 | 20.5 | 51,569 | 21.4 | 6170 | 21.3 | 78,198 | 21.1 |
| 3 | 19,380 | 19.4 | 47,424 | 19.6 | 5864 | 20.2 | 72,668 | 19.6 |
| 4 | 18,418 | 18.4 | 46,496 | 19.3 | 5595 | 19.3 | 70,509 | 19.0 |
| 5 (highest) | 18,532 | 18.6 | 44,382 | 18.4 | 5070 | 17.5 | 67,984 | 18.4 |
| Community size | ||||||||
| >1,500,000 | 44,829 | 43.8 | 80,513 | 33.2 | 0 | 0 | 125,342 | 33.5 |
| 500,000–1,499,999 | 0 | 0 | 31,359 | 12.9 | 0 | 0 | 45,255 | 12.1 |
| 100,000–499,999 | 18,050 | 17.6 | 66,341 | 27.4 | 13,896 | 47.8 | 87,860 | 23.5 |
| 10,000–99,999 | 23,126 | 22.6 | 27,855 | 11.5 | 3469 | 11.9 | 62,681 | 16.8 |
| <10,000 | 16,363 | 16.0 | 36,306 | 15.0 | 11,700 | 40.3 | 53,050 | 14.2 |
| Cancer type | ||||||||
| Brain | 2856 | 2.8 | 6679 | 2.8 | 713 | 2.4 | 10,248 | 2.7 |
| Breast | 7245 | 7.0 | 18,908 | 7.8 | 1949 | 6.6 | 28,102 | 7.5 |
| Colorectal | 12,781 | 12.4 | 30,397 | 12.5 | 3964 | 13.5 | 47,142 | 12.6 |
| Gynecological | 4483 | 4.3 | 11,138 | 4.6 | 1176 | 4.0 | 16,797 | 4.5 |
| Head and Neck | 2394 | 2.3 | 6650 | 2.7 | 625 | 2.1 | 9669 | 2.6 |
| Hematology | 9616 | 9.3 | 25,098 | 10.3 | 2190 | 7.4 | 36,904 | 9.8 |
| Lung | 26,447 | 25.7 | 66,784 | 27.5 | 8337 | 28.3 | 101,568 | 27.1 |
| Other | 13,164 | 12.8 | 21,879 | 9.0 | 4264 | 14.5 | 39,307 | 10.5 |
| Other Gastrointestinal | 11,864 | 11.5 | 26,497 | 10.9 | 2998 | 10.2 | 41,359 | 11.0 |
| Other Genitourinary | 5952 | 5.8 | 12,778 | 5.3 | 1705 | 5.8 | 20,435 | 5.4 |
| Prostate | 6304 | 6.1 | 15,748 | 6.5 | 1525 | 5.2 | 23,577 | 6.3 |
| Score on the Deyo-Charlson comorbidity index (from 24 months to 12 months before death) | ||||||||
| 0 | 55,008 | 52.8 | 116,225 | 47.9 | 14,492 | 49.2 | 185,725 | 49.4 |
| 1+ | 35,401 | 34.0 | 95,510 | 39.4 | 12,098 | 41.1 | 143,009 | 38.0 |
| Missing | 13,697 | 13.2 | 30,821 | 12.7 | 2859 | 9.7 | 47,377 | 12.6 |
| Indicator | BC | ON | NS | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Std | Std | Std | Crude | |||||
| c | 104,106 | 242,556 | 29,446 | 376,108 | ||||
| Death in acute care hospital or bed (overall) | ||||||||
| 49,687 | 47.7 | 119,037 | 48.9 | 19,627 | 66.5 | 188,351 | 50.1 | |
| New Hospitalization within 30 days of death | ||||||||
| ≥1 New admission | 51,701 | 49.5 | 129,091 | 57.6 | 16,857 | 57.6 | 197,649 | 55.4 |
| With new intensive care unit admission | 3136 | 3.0 | 16,685 | 8.1 | 1181 | 4.0 | 21,002 | 5.9 |
| All eligible patients (excluding those in hospital for the last 30 days of life) | 104,014 | 223,325 | 29,109 | 356,448 | ||||
| Emergency department visit within 30 days of death | ||||||||
| Source: CIHI Discharge Abstract Database | 40,200 | 38.5 | 104,428 | 46.7 | 11,571 | 39.7 | 156,199 | 43.8 |
| All eligible patients (excluding those in hospital for the last 30 days of life) | 104,014 | 223,325 | 29,109 | 356,448 | ||||
| Home visit within 6 months of death | ||||||||
| By a registered nurse | 156,118 | 64.9 | 12,804 | 51.6 | ||||
| By a personal support worker | 98,992 | 41.2 | 5047 | 20.5 | ||||
| Having a palliative nursing or personal support worker home care visit within 6 months of death | 98,534 | 46.0 | 13,514 | 54.9 | ||||
| All eligible patients (excluding those in hospital for the last 30 days of life) | 240,500 | - | 24,630 | - | ||||
| Physician house call within 2 weeks of death | ||||||||
| Patients who received a house call | 53,521 | 27.3 | 3586 | 12.4 | ||||
| All eligible patients (excluding those in hospital for the last 14 days of life) | 196,061 | - | 28,964 | - | ||||
| Aggressive care | ||||||||
| At least one care factor listed below: | 8776 | 8.3 | 29,802 | 13.2 | 3283 | 11.1 | 41,861 | 11.7 |
| Having at least 2 ED visits within 30 days of death | 3872 | 10,201 | 1000 | 15,073 | 4.2 | |||
| Having at least 2 new hospitalizations within 30 days of death | 6913 | 15,023 | 2235 | 24,171 | 6.8 | |||
| Being in the ICU within 30 days of death | 7122 | 18,293 | 1181 | 26,596 | 7.5 | |||
| All eligible patients (excluding those in hospital for the last 30 days of life) | 104,014 | 223,325 | 29,109 | 356,448 | - | |||
| Supportive care | ||||||||
| Utilizing at least one service below: | 107,353 | 50.1 | 13,701 | 55.3 | ||||
| Having a physician house call within 2 weeks of death | 48,238 | 22.5 | 3586 | |||||
| Having palliative nursing or personal support worker home care visit within 6 months of death | 98,534 | 46.0 | 13,514 | 54.7 | ||||
| All eligible patients (excluding those in hospital for the last 6 months of life) | 214,389 | 24,630 | ||||||
| Factor | BC | ON | NS (New Health Zone) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CL | OR | 95% CL | OR | 95% CL | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||
| Age (years) | 0.98 | 0.97 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.97 | 0.98 |
| Male (reference: Female) | 1.26 | 1.20 | 1.30 | 1.31 | 1.27 | 1.34 | 1.38 | 1.28 | 1.5 |
| Charlson comorbidity index 1+ (reference: 0 or missing) | 0.99 | 0.94 | 1.03 | 0.95 | 0.91 | 0.98 | 1.04 | 0.93 | 1.16 |
| Cancer type (reference: Lung) | |||||||||
| Breast | 0.91 | 0.82 | 0.99 | 0.71 | 0.68 | 0.75 | 0.88 | 0.74 | 1.06 |
| Colorectal | 1.03 | 0.96 | 1.11 | 0.93 | 0.89 | 0.97 | 1.02 | 0.9 | 1.15 |
| Prostate | 0.73 | 0.66 | 0.82 | 0.63 | 0.59 | 0.67 | 0.69 | 0.56 | 0.85 |
| Other | 1.21 | 1.14 | 1.28 | 1.01 | 0.98 | 1.04 | 1.13 | 1.04 | 1.23 |
| Neighborhood income quintile (reference: 5, highest) | |||||||||
| 1 (lowest) | 0.94 | 0.88 | 1.01 | 1.09 | 1.04 | 1.13 | 1.01 | 0.89 | 1.14 |
| 2 | 0.98 | 0.91 | 1.05 | 1.06 | 1.02 | 1.10 | 1.14 | 1.02 | 1.29 |
| 3 | 1.02 | 0.95 | 1.1 | 1.07 | 1.03 | 1.11 | 1.02 | 0.91 | 1.16 |
| 4 | 0.96 | 0.89 | 1.03 | 1.05 | 1.013 | 1.10 | 1.09 | 0.96 | 1.22 |
| Community size (reference: ≥100,000) | |||||||||
| <10,000 | 1.27 | 1.21 | 1.34 | 1.17 | 1.13 | 1.21 | 1.23 | 1.08 | 1.4 |
| 10,000–99,999 | 1.47 | 1.38 | 1.55 | 1.17 | 1.13 | 1.22 | 1.5 | 1.23 | 1.81 |
| Factor | ON | NS (New Health Zone) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CL | OR | 95% CL | |||
| Lower | Upper | Lower | Upper | |||
| Age (years) | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 |
| Male (reference: Female) | 0.92 | 0.90 | 0.93 | 0.97 | 0.92 | 1.02 |
| Charlson comorbidity index 1+ (reference: 0 or missing) | 0.89 | 0.87 | 0.91 | 1.05 | 0.97 | 1.13 |
| Cancer type | ||||||
| Breast | 1.04 | 1.01 | 1.08 | 1.02 | 0.91 | 1.14 |
| Colorectal | 0.92 | 0.89 | 0.95 | 1.10 | 1.01 | 1.20 |
| Prostate | 0.84 | 0.80 | 0.87 | 1.06 | 0.93 | 1.21 |
| Other | 0.85 | 0.83 | 0.87 | 0.88 | 0.83 | 0.94 |
| Lung | 1 | - | - | 1 | - | - |
| Neighborhood income quintile (reference: 5, highest) | ||||||
| 1 (lowest) | 0.74 | 0.72 | 0.76 | 0.80 | 0.73 | 0.87 |
| 2 | 0.85 | 0.83 | 0.88 | 0.90 | 0.82 | 0.97 |
| 3 | 0.88 | 0.86 | 0.91 | 1.03 | 0.94 | 1.12 |
| 4 | 0.94 | 0.91 | 0.96 | 1.01 | 0.93 | 1.10 |
| Community size (reference: ≥100,000) | ||||||
| <10,000 | 0.89 | 0.87 | 0.91 | 0.79 | 0.72 | 0.87 |
| 10,000–99,999 | 0.95 | 0.93 | 0.98 | 0.93 | 0.81 | 1.07 |
References
- Currow, D.C.; Agar, M.R.; Phillips, J.L. Role of hospice care at the end of life for people with cancer. J. Clin. Oncol. 2020, 38, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Barbera, L.; Seow, H.; Sutradhar, R.; Chu, A.; Burge, F.; Fassbender, K.; McGrail, K.; Lawson, B.; Liu, Y.; Pataky, R.; et al. Quality of end-of-life cancer care in Canada: A retrospective four-province study using administrative health care data. Curr. Oncol. 2015, 22, 341–355. [Google Scholar] [CrossRef]
- Singer, P.A.; Bowman, K.W. Quality end-of-life care: A global perspective. BMC Palliat. Care 2002, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- De Schreye, R.; Smets, T.; Annemans, L.; Deliens, L.; Gielen, B.; De Gendt, C.; Cohen, J. Applying quality indicators for administrative databases to evaluate end-of-life care for cancer patients in Belgium. Health Aff. 2017, 36, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.N.; Shekelle, P.G.; Davies, H.T.O.; Smith, P.C. Public reporting on quality in the United States and the United Kingdom. Health Aff. 2003, 22, 134–148. [Google Scholar] [CrossRef]
- Lindskog, M.; Tavelin, B.; Lundström, S. Old age as risk indicator for poor end-of-life care quality—A population-based study of cancer deaths from the Swedish Register of Palliative Care. Eur. J. Cancer 2015, 51, 1331–1339. [Google Scholar] [CrossRef]
- Miyashita, M.; Nakamura, A.; Morita, T.; Bito, S. Identification of quality indicators of end-of-life cancer care from medical chart review using a modified Delphi method in Japan. Am. J. Hosp. Palliat. Med. 2008, 25, 33–38. [Google Scholar] [CrossRef]
- Henson, L.A.; Edmonds, P.; Johnston, A.; Johnson, H.E.; Ling, C.N.Y.; Sklavounos, A.; Ellis-Smith, C.; Gao, W. Population-Based Quality Indicators for End-of-Life Cancer Care: A Systematic Review. JAMA Oncol. 2020, 6, 142–150. [Google Scholar] [CrossRef]
- Fowler, R.; Hammer, M. View of End-of-Life Care in Canada. Available online: https://cimonline.ca/index.php/cim/article/view/19723/16336 (accessed on 17 May 2021).
- Framework on Palliative Care in Canada. Available online: https://www.canada.ca/en/health-canada/services/health-care-system/reports-publications/palliative-care/framework-palliative-care-canada.html (accessed on 6 September 2021).
- Discharge Abstract Database (Hospital Separations). Available online: https://www.popdata.bc.ca/data/health/dad (accessed on 6 September 2021).
- Home & Community Care (Continuing Care). Available online: http://www.popdata.bc.ca/data (accessed on 6 September 2021).
- BC Cancer Registry Data V2. Available online: https://www.popdata.bc.ca/data/health/bccancer (accessed on 6 September 2021).
- Ladha, K.S.; Zhao, K.; Quraishi, S.A.; Kurth, T.; Eikermann, M.; Kaafarani, H.M.; Klein, E.N.; Seethala, R.; Lee, J. The Deyo-Charlson and Elixhauser-van Walraven Comorbidity Indices as predictors of mortality in critically ill patients. BMJ Open 2015, 5, 8990. [Google Scholar] [CrossRef]
- Moens, K.; Higginson, I.J.; Harding, R. Are There Differences in the Prevalence of Palliative Care-Related Problems in People Living with Advanced Cancer and Eight Non-Cancer Conditions? A Systematic Review. J. Pain Symptom Manag. 2014, 48, 660–677. [Google Scholar] [CrossRef]
- Rocque, G.B.; Barnett, A.E.; Illig, L.C.; Eickhoff, J.C.; Bailey, H.H.; Campbell, T.C.; Stewart, J.A.; Cleary, J.F. Inpatient hospitalization of oncology patients: Are we missing an opportunity for end-of-life care? J. Oncol. Pract. 2013, 9, 51–54. [Google Scholar] [CrossRef]
- De Roo, M.L.; Miccinesi, G.; Onwuteaka-Philipsen, B.D.; Van Den Noortgate, N.; Van den Block, L.; Bonacchi, A.; Donker, G.A.; Lozano Alonso, J.E.; Moreels, S.; Deliens, L.; et al. Actual and Preferred Place of Death of Home-Dwelling Patients in Four European Countries: Making Sense of Quality Indicators. PLoS ONE 2014, 9, e93762. [Google Scholar] [CrossRef]
- Burge, F.; Lawson, B.; Johnston, G.; Asada, Y.; McIntyre, P.F.; Flowerdew, G. Preferred and Actual Location of Death: What Factors Enable a Preferred Home Death? J. Palliat. Med. 2015, 18, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- DeCaria, K.; Dudgeon, D.; Green, E.; Moxam, R.S.; Rahal, R.; Niu, J.; Bryant, H. Acute care hospitalization near the end of life for cancer patients who die in hospital in Canada. Curr. Oncol. 2017, 24, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Seow, H.; Barbera, L.; Howell, D.; Dy, S.M. Did Ontario’s end-of-life care strategy reduce acute care service use? Healthc. Q. 2010, 13, 93–100. [Google Scholar] [CrossRef]
- Barbera, L.; Seow, H.; Sutradhar, R.; Chu, A.; Burge, F.; Fassbender, K.; McGrail, K.; Lawson, B.; Liu, Y.; Pataky, R. Quality Indicators of End-of-Life Care in Patients with Cancer: What Rate Is Right? J. Oncol. Pract. 2015, 11, e279–e287. [Google Scholar] [CrossRef]
- Tanuseputro, P.; Beach, S.; Chalifoux, M.; Wodchis, W.P.; Hsu, A.T.; Seow, H.; Manuel, D.G. Associations between physician home visits for the dying and place of death: A population-based retrospective cohort study. PLoS ONE 2018, 13, e0191322. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Lau, Z.Y.; Ong, W.S.; Yang, G.M.; Tan, K.B.; Ong, M.E.H.; Wong, T.H. Sociodemographic and clinical factors for non-hospital deaths among cancer patients: A nationwide population-based cohort study. PLoS ONE 2020, 15, e0232219. [Google Scholar] [CrossRef]
- Kalseth, J.; Halvorsen, T. Relationship of place of death with care capacity and accessibility: A multilevel population study of system effects on place of death in Norway. BMC Health Serv. Res. 2020, 20, 454. [Google Scholar] [CrossRef]
- Cancers System Performance: 2017 Report. Available online: https://s22457.pcdn.co/wp-content/uploads/2019/12/2017_cancer_system_performance_report_EN.pdf (accessed on 6 September 2021).
- Salas, A.S.; Watanabe, S.M.; Tarumi, Y.; Wildeman, T.; García, A.M.H.; Adewale, B.; Duggleby, W. Social disparities and symptom burden in populations with advanced cancer: Specialist palliative care providers’ perspectives. Support. Care Cancer 2019, 27, 4733–4744. [Google Scholar] [CrossRef]
- Barbera, L.; Sussman, J.; Viola, R.; Husain, A.; Howell, D.; Librach, S.L.; Walker, H.; Sutradhar, R.; Chartier, C.; Paszat, L. Factors associated with end-of-life health service use in patients dying of cancer. Healthc. Policy 2010, 5, e125. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.F.; Seow, H.; Sutradhar, R.; Peacock, S.; Chan, K.K.-W.; Burge, F.; McGrail, K.; Raymakers, A.; Lawson, B.; Barbera, L. Quality of End-of-Life Cancer Care in Canada: A 12-Year Retrospective Analysis of Three Provinces’ Administrative Health Care Data Evaluating Changes over Time. Curr. Oncol. 2021, 28, 4673-4685. https://doi.org/10.3390/curroncol28060394
Khan AF, Seow H, Sutradhar R, Peacock S, Chan KK-W, Burge F, McGrail K, Raymakers A, Lawson B, Barbera L. Quality of End-of-Life Cancer Care in Canada: A 12-Year Retrospective Analysis of Three Provinces’ Administrative Health Care Data Evaluating Changes over Time. Current Oncology. 2021; 28(6):4673-4685. https://doi.org/10.3390/curroncol28060394
Chicago/Turabian StyleKhan, Amanda Farah, Hsien Seow, Rinku Sutradhar, Stuart Peacock, Kelvin Kar-Wing Chan, Fred Burge, Kim McGrail, Adam Raymakers, Beverley Lawson, and Lisa Barbera. 2021. "Quality of End-of-Life Cancer Care in Canada: A 12-Year Retrospective Analysis of Three Provinces’ Administrative Health Care Data Evaluating Changes over Time" Current Oncology 28, no. 6: 4673-4685. https://doi.org/10.3390/curroncol28060394
APA StyleKhan, A. F., Seow, H., Sutradhar, R., Peacock, S., Chan, K. K.-W., Burge, F., McGrail, K., Raymakers, A., Lawson, B., & Barbera, L. (2021). Quality of End-of-Life Cancer Care in Canada: A 12-Year Retrospective Analysis of Three Provinces’ Administrative Health Care Data Evaluating Changes over Time. Current Oncology, 28(6), 4673-4685. https://doi.org/10.3390/curroncol28060394






