Frontline Treatment of the Young, Fit Patient with CLL: A Canadian Perspective
Abstract
:1. Introduction
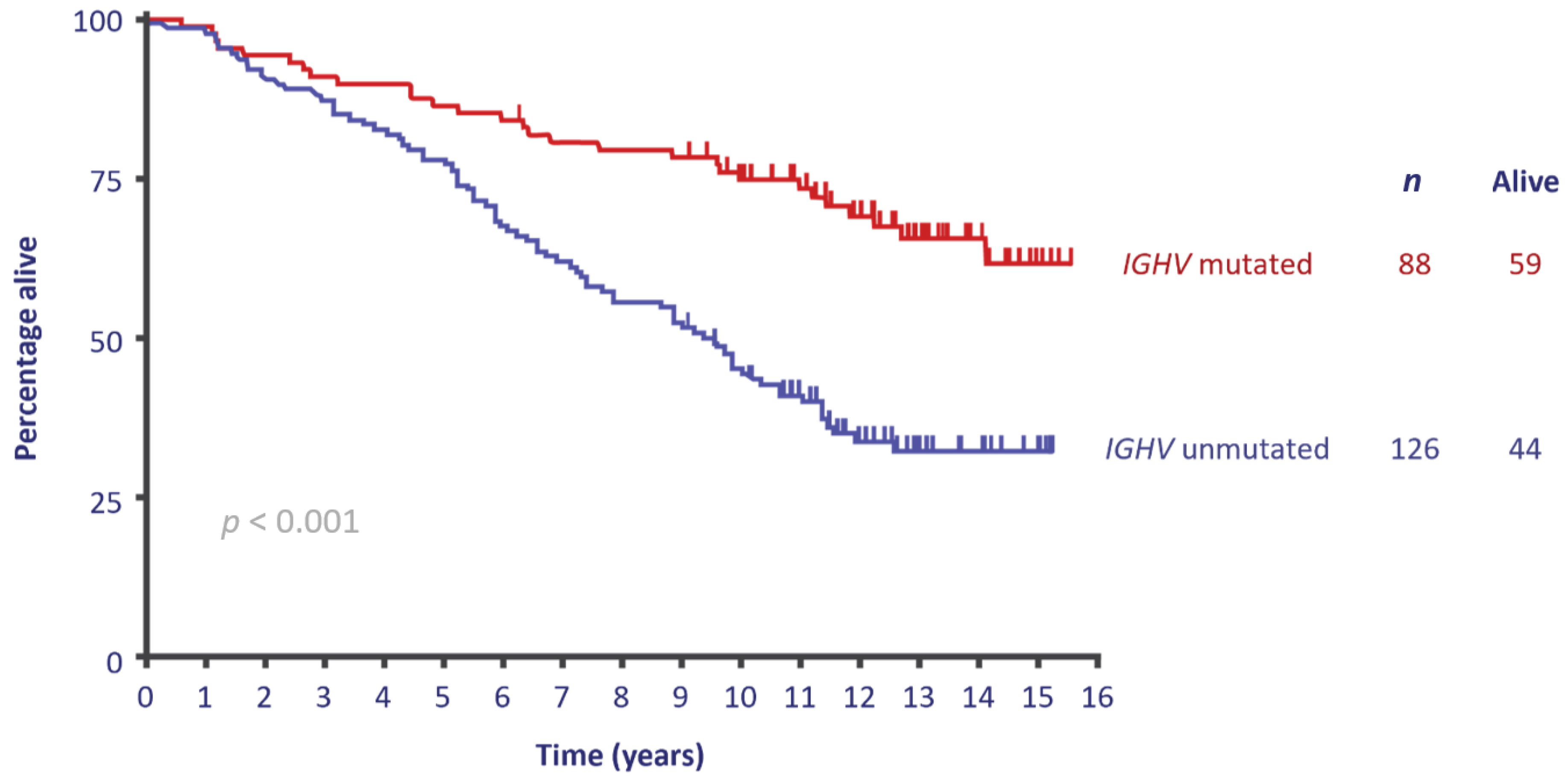
2. Defining the Young, Fit Patient with CLL
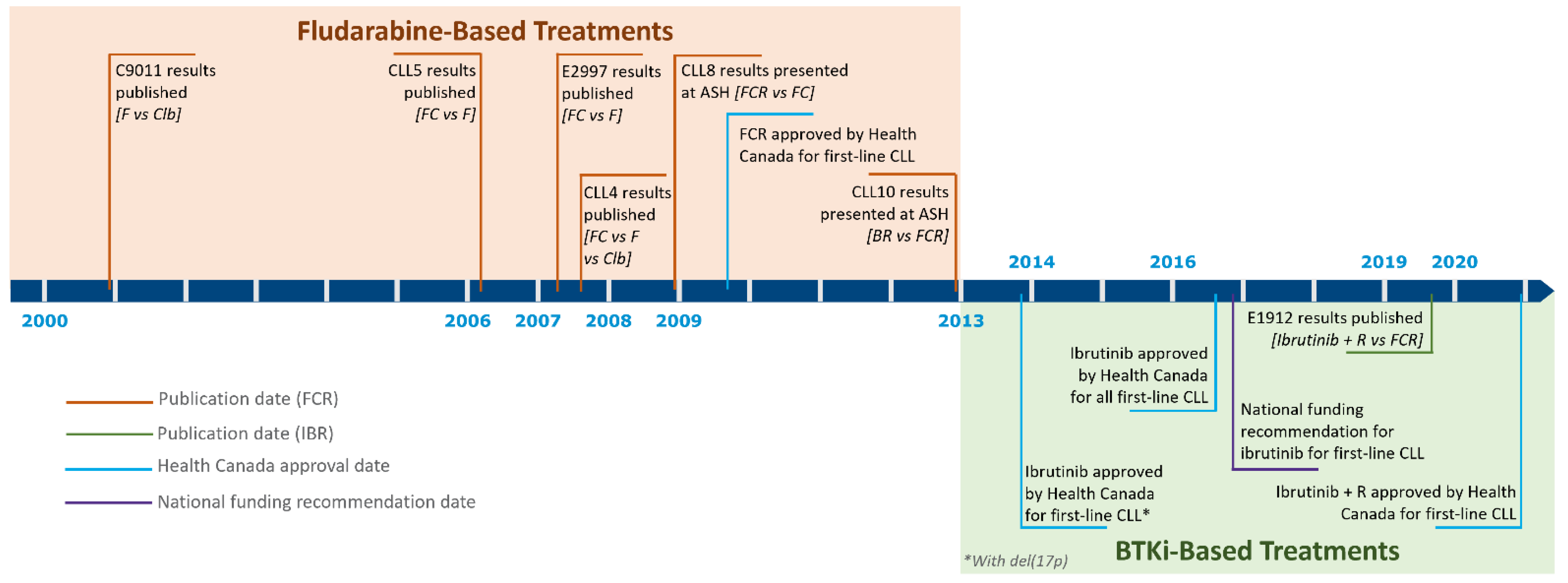
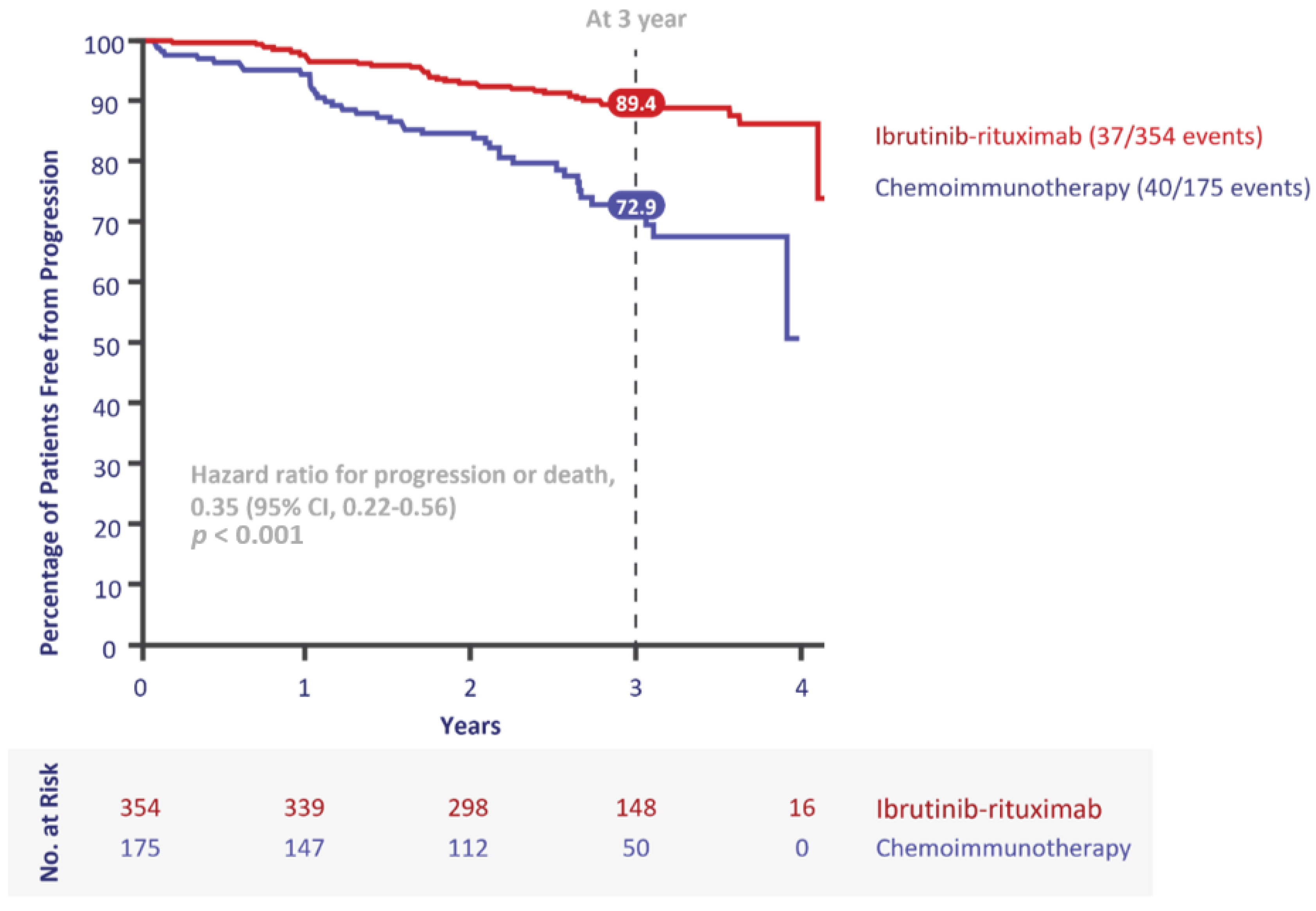
3. First-Line Treatment Evolution in the Young, Fit Population
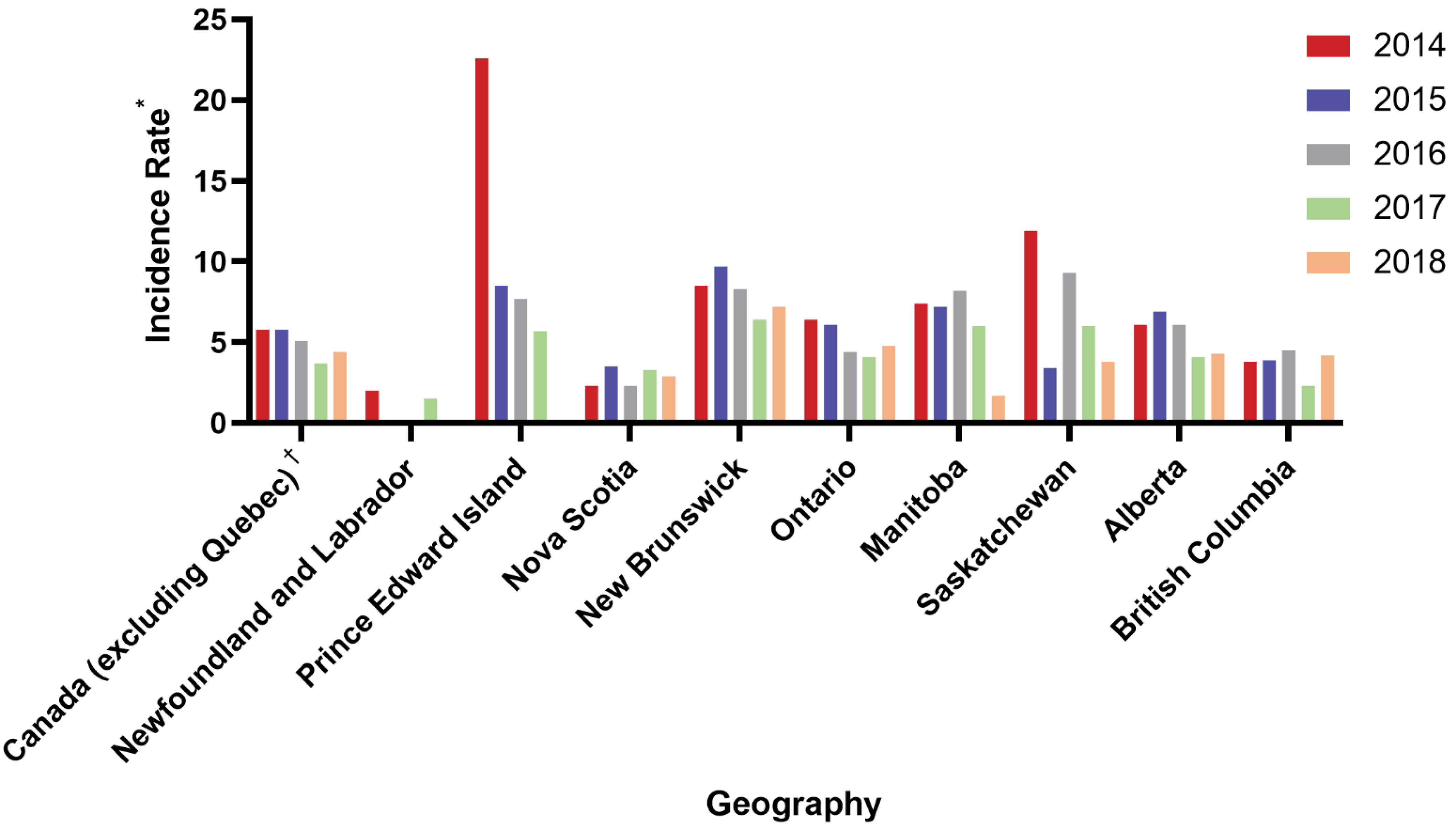
4. Provincial Differences in Coverage
5. The Canadian Landscape Today
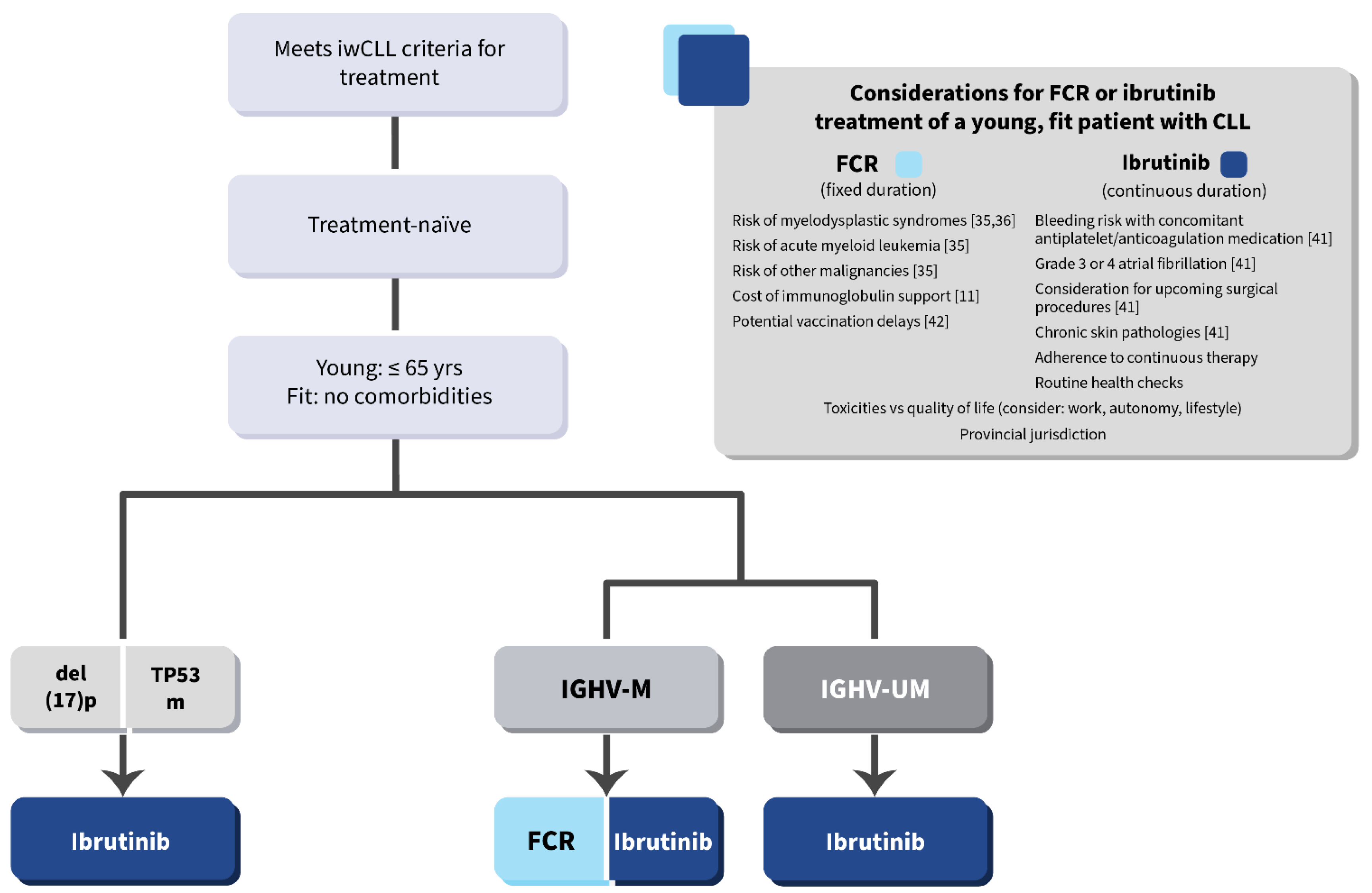
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rozman, C.; Montserrat, E. Chronic lymphocytic leukemia. N. Engl. J. Med. 1995, 333, 1052–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistics Canada. Table 13-10-0111-01. Number and Rates of New Cases of Primary Cancer, by Cancer Type, Age Group and Sex. Available online: https://doi.org/10.25318/1310011101-eng (accessed on 25 May 2021).
- Canadian Cancer Society. Chronic Lymphocytic Leukemia Statistics. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/leukemia-chronic-lymphocytic-cll/statistics/?region=on (accessed on 25 May 2021).
- Owen, C.; Gerrie, A.S.; Banerji, V.; Assouline, S.; Chen, C.; Robinson, K.S.; Lye, E.; Fraser, G. Canadian evidence-based guideline for the first-line treatment of chronic lymphocytic leukemia. Curr. Oncol. 2018, 25, e461–e474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shustik, C.; Bence-Bruckler, I.; Delage, R.; Owen, C.J.; Toze, C.L.; Coutre, S. Advances in the treatment of relapsed/refractory chronic lymphocytic leukemia. Ann. Hematol. 2017, 96, 1185–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.A.; Byrd, J.C. How will B-cell-receptor-targeted therapies change future CLL therapy? Blood 2014, 123, 1455–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib–rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef]
- Eichhorst, B.; Fink, A.M.; Bahlo, J.; Busch, R.; Kovacs, G.; Maurer, C.; Lange, E.; Köppler, H.; Kiehl, M.; Sökler, M.; et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016, 17, 928–942. [Google Scholar] [CrossRef]
- Fischer, K.; Bahlo, J.; Fink, A.M.; Goede, V.; Herling, C.D.; Cramer, P.; Langerbeins, P.; von Tresckow, J.; Engelke, A.; Maurer, C.; et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: Updated results of the CLL8 trial. Blood 2016, 127, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Leukemia & Lymphoma Society of Canada. CLL Staging. Available online: https://www.llscanada.org/leukemia/chronic-lymphocytic-leukemia/diagnosis/cll-staging (accessed on 25 May 2021).
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [Green Version]
- International CLL-IPI Working Group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): A meta-analysis of individual patient data. Lancet Oncol. 2016, 17, 779–790. [Google Scholar] [CrossRef]
- Seftel, M.D.; Demers, A.A.; Banerji, V.; Gibson, S.B.; Morales, C.; Musto, G.; Pitz, M.W.; Johnston, J.B. High incidence of chronic lymphocytic leukemia (CLL) diagnosed by immunophenotyping: A population-based Canadian cohort. Leuk. Res. 2009, 33, 1463–1468. [Google Scholar] [CrossRef]
- Yosifov, D.Y.; Wolf, C.; Stilgenbauer, S.; Mertens, D. From biology to therapy: The CLL success story. Hemasphere 2019, 3, e175. [Google Scholar] [CrossRef] [PubMed]
- Andreani, G.; Carrà, G.; Lingua, M.F.; Maffeo, B.; Brancaccio, M.; Taulli, R.; Morotti, A. Tumor Suppressors in Chronic Lymphocytic Leukemia: From Lost Partners to Active Targets. Cancers 2020, 12, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, J.; Nadeu, F.; Colomer, D.; Campo, E. Chronic lymphocytic leukemia: From molecular pathogenesis to novel therapeutic strategies. Haematologica 2020, 105, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Catovsky, D.; Richards, S.; Matutes, E.; Oscier, D.; Dyer, M.; Bezares, R.; Pettitt, A.R.; Hamblin, T.; Milligan, D.W.; Child, J.A.; et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet 2007, 370, 230–239. [Google Scholar] [CrossRef]
- Eichhorst, B.F.; Busch, R.; Hopfinger, G.; Pasold, R.; Hensel, M.; Steinbrecher, C.; Siehl, S.; Jäger, U.; Bergmann, M.; Stilgenbauer, S.; et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006, 107, 885–891. [Google Scholar] [CrossRef]
- Flinn, I.W.; Neuberg, D.S.; Grever, M.R.; Dewald, G.W.; Bennett, J.M.; Paietta, E.M.; Hussein, M.A.; Appelbaum, F.R.; Larson, R.A.; Moore, D.F., Jr.; et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US intergroup trial E2997. J. Clin. Oncol. 2007, 25, 793–798. [Google Scholar] [CrossRef]
- Rai, K.R.; Peterson, B.L.; Appelbaum, F.R.; Tallman, M.S.; Belch, A.; Morrison, V.A.; Larson, R.A. Long-term survival analysis of the North American intergroup study C9011 comparing fludarabine (F) and chlorambucil (C) in previously untreated patients with chronic lymphocytic leukemia (CLL). Blood 2009, 114, 536. [Google Scholar] [CrossRef]
- Knauf, W.U.; Lissitchkov, T.; Aldaoud, A.; Liberati, A.M.; Loscertales, J.; Herbrecht, R.; Juliusson, G.; Postner, G.; Gercheva, L.; Goranov, S.; et al. Bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia: Updated results of a randomized phase III trial. Br. J. Haematol. 2012, 159, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.R.; Peterson, B.L.; Appelbaum, F.R.; Kolitz, J.; Elias, L.; Shepherd, L.; Hines, J.; Threatte, G.A.; Larson, R.A.; Cheson, B.D.; et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2000, 343, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Dmoszynska, A.; Solal-Céligny, P.; Warzocha, K.; Loscertales, J.; Catalano, J.; Afanasiev, B.V.; Larratt, L.; Geisler, C.H.; Montillo, M.; et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J. Clin. Oncol. 2010, 28, 1756–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, P.A.; Tam, C.S.; O’Brien, S.M.; Wierda, W.G.; Stingo, F.; Plunkett, W.; Smith, S.C.; Kantarjian, H.M.; Freireich, E.J.; Keating, M.J. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 2016, 127, 303–309. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.M.; Barrientos, J.C.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib and rituximab provides superior clinical outcome compared to FCR in younger patients with chronic lymphocytic leukemia (CLL): Extended follow-up from the E1912 trial. Blood 2019, 134, 33. [Google Scholar] [CrossRef]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Devereux, S.; Grosicki, S.; et al. Up to seven years of follow-up in the RESONATE-2 study of first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. In Proceedings of the 2021 American Society of Clinical Oncology Annual Meeting, Virtual/Online, 4–8 June 2021. Poster 7523. [Google Scholar]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.; Sharman, J.P.; Wierda, W.; Zhao, W.; Heerema, N.A.; et al. Ibrutinib Treatment for First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia: Final Analysis of the Pivotal Phase Ib/II PCYC-1102 Study. Clin. Cancer Res. 2020, 26, 3918–3927. [Google Scholar] [CrossRef] [Green Version]
- Canadian Agency for Drugs and Technologies in Health. Pan-Canadian Oncology Drug Review. Provincial Funding Summary: Ibrutinib (Imbruvica) for Chronic Lymphocytic Leukemia/Small Lymphocytic Leukemia (Previously Untreated) (pCODR 10085). 2020, pp. 1–2. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr-provfund_ibrutinib_imbruvica-cll-sll-preun.pdf (accessed on 12 May 2021).
- Lukenbill, J.; Kalaycio, M. Fludarabine: A review of the clear benefits and potential harms. Leuk. Res. 2013, 37, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; O’Brien, S.; Wierda, W.; Kantarjian, H.; Wen, S.; Do, K.A.; Thomas, D.A.; Cortes, J.; Lerner, S.; Keating, M.J. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008, 112, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Borner, M.; Scheithauer, W.; Twelves, C.; Maroun, J.; Wilke, H. Answering patients’ needs: Oral alternatives to intravenous therapy. Oncologist 2001, 6, 12–16. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Jatoi, A. Oral cancer chemotherapy: The critical interplay between patient education and patient safety. Curr. Oncol. Rep. 2010, 12, 247–252. [Google Scholar] [CrossRef]
- Tucker, S.J.; Pelusi, J. Capecitabine versus 5-FU in metastatic colorectal cancer: Considerations for treatment decision-making. Community Oncol. 2006, 3, 19–27. [Google Scholar] [CrossRef]
- Wood, L. A review on adherence management in patients on oral cancer therapies. Eur. J. Oncol. Nursing 2012, 16, 432–438. [Google Scholar] [CrossRef]
- Janssen Inc. Imbruvica® (ibrutinib) Product Monograph; Janssen Inc.: Beerse, Belgium, 28 May 2021. [Google Scholar]
- Clinical Guidance on COVID-19 Vaccines for People with Solid Cancers. Available online: http://www.bccdc.ca/Health-Info-Site/Documents/COVID-19_vaccine/Solid_Cancer_Clinical_Guidance.pdf (accessed on 18 April 2021).
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.M.; Yenerel, M.N.; Illes, A.; Kay, N.E.; et al. First results of a head-to-head trial of acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia. J. Clin. Oncol. 2021, 39, 7500. [Google Scholar] [CrossRef]
- Gu, D.; Tang, H.; Wu, J.; Li, J.; Miao, Y. Targeting Bruton tyrosine kinase using non-covalent inhibitors in B cell malignancies. J. Hematol. Oncol. 2021, 14, 40. [Google Scholar] [CrossRef]
- Hillmen, P.; Hillmen, B.; Brown, J.R.; Lamanna, N.; O’Brien, S.; Tam, C.S.; Qiu, L.; Kazmierczak, M.; Zhou, K.; Simkovic, M.; et al. First interim analysis of alpine study: Results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/ small lymphocytic lymphoma. In Proceedings of the 2021 European Hematology Association Virtual Congress, 9–17 June 2021. Oral Abstract LB1900. [Google Scholar]
- Mato, A.R.; Pagel, J.M.; Coombs, C.C.; Shah, N.N.; Lamanna, N.; Leck-Marańda, E.; Eyre, T.A.; Woyach, J.A.; Wierda, W.G.; Cheah, C.Y.; et al. LOXO-305, a next generation, highly selective, non-covalent BTK inhibitor in previously treated CLL/SLL: Results from the phase 1/2 BRUIN study. In Proceedings of the 2020 American Society of Hematology Annual Meeting & Exposition, Virtual/Online, 7 December 2020. Abstract 542. [Google Scholar]
- Al-Sawaf, O.; Zhang, C.; Robrecht, S.; Tandon, M.; Panchal, A.; Fink, A.M.; Tausch, E.; Ritgen, M.; Kreuzer, K.A.; Kim, S.Y.; et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 4-year follow-up analysis of the randomized CLL14 study. In Proceedings of the 2021 European Hematology Association Virtual Congress, 9–17 June 2021. Abstract S146. [Google Scholar]
- Kater, A.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-duration ibrutinib and venetoclax (I+V) versus chlorambucil plus obinutuzumab (Clb+O) for first-line (1L) chronic lymphocytic leukemia (CLL): Primary analysis of the phase 3 glow study. In Proceedings of the 2021 European Hematology Association Virtual Congress, 9–17 June 2021. Abstract LB1902. [Google Scholar]
- Ghia, P.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.; Opat, S.; Barr, P.M.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-duration (FD) first-line treatment (Tx) with ibrutinib (I) plus venetoclax (V) for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): Primary analysis of the FD cohort of the phase 2 captivate study. In Proceedings of the 2021 American Society of Clinical Oncology Annual Meeting, Virtual, 4–8 June 2021. Oral Abstract 7501. [Google Scholar]

| Trial | Agents | ORR | Median PFS | Median OS | Del(17p) Inclusion | Median Age, Years | n | Median Follow-Up, Years |
|---|---|---|---|---|---|---|---|---|
| CLL4 [23] | FC vs. F vs. Clb | 94% vs. 80% vs. 72% | 36% vs. 10% vs. 10% at 5 yrs | 34.2% vs. 36.6% vs. 30.0% events | 7% vs. 8% vs. 6% | 65 vs. 64 vs. 65 | 777 | 3.4 |
| CLL5 [24] | FC vs. F | 94% vs. 83% | 48 vs. 20 months | 80.3% vs. 80.7% at 3 yrs | NA | 58 vs. 59 | 375 | 1.8 |
| E2997 [25] | FC vs. F | 74.3% vs. 59.5% | 31.6 vs. 19.2 months | 79% vs. 80% est at 2 yrs | 10% vs. 9% | 61 vs. 61 | 278 | 2.17 |
| C9011 [26] | F vs. Clb | 63% vs. 37% at 5.2 yrs | 20 vs. 13 months | 63 vs. 59 months | NA | 64 vs. 62 | 544 | ~15 |
| Knauf et al. [27] | B vs. Clb | 34.6% vs. 29.9% | 21.2 vs. 8.8 months | NR vs. 78.8 months | NA | 63 vs. 64 | 319 | 4.5 |
| CLL8 [9] | FCR vs. FC | 90% vs. 80% | 56.8 vs. 32.9 months | NR vs. 86 months | 7% vs. 10% | 61 | 817 | 5.9 |
| CLL10 ≤ 65 years [8] | BR vs. FCR | 98% vs. 95% | 38.5 vs. 53.6 months | NA | 0% | ≤ 65 | 367 | 3.1 |
| ECOG-ACRIN 1912 [7] | Ibr + R vs. FCR | 95.8% vs. 81.1% | 89.4% vs. 72.9% at 3 yrs | 98.8% vs. 91.5% at 3 yrs | 0% | 56.7 | 529 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costello, J.; Kang, M.; Banerji, V. Frontline Treatment of the Young, Fit Patient with CLL: A Canadian Perspective. Curr. Oncol. 2021, 28, 3825-3835. https://doi.org/10.3390/curroncol28050326
Costello J, Kang M, Banerji V. Frontline Treatment of the Young, Fit Patient with CLL: A Canadian Perspective. Current Oncology. 2021; 28(5):3825-3835. https://doi.org/10.3390/curroncol28050326
Chicago/Turabian StyleCostello, Jacqueline, Matthew Kang, and Versha Banerji. 2021. "Frontline Treatment of the Young, Fit Patient with CLL: A Canadian Perspective" Current Oncology 28, no. 5: 3825-3835. https://doi.org/10.3390/curroncol28050326
APA StyleCostello, J., Kang, M., & Banerji, V. (2021). Frontline Treatment of the Young, Fit Patient with CLL: A Canadian Perspective. Current Oncology, 28(5), 3825-3835. https://doi.org/10.3390/curroncol28050326





