Survival Outcomes Associated with First and Second-Line Palliative Systemic Therapies in Patients with Metastatic Bladder Cancer
Abstract
:1. Introduction
2. Materials and Methods
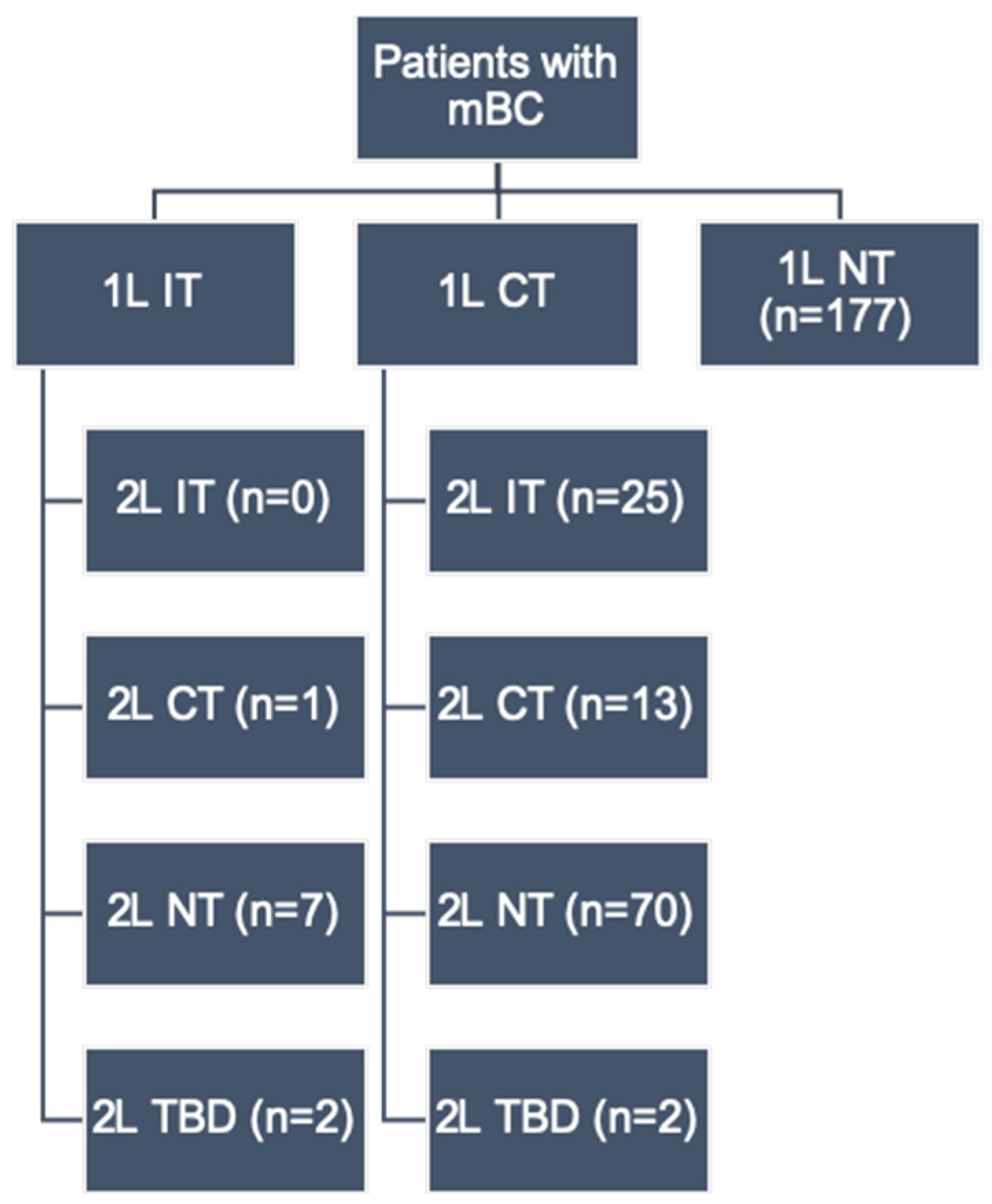
2.1. Study Design and Patient Population
2.2. Clinical Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Agents | 1L (N = 297), n (%) | 2L (N = 116), n (%) |
|---|---|---|
| Chemotherapy | 110 (37.0) | 14 (12.1) |
| Gemcitabine + Carboplatin | 53 (17.8) | 2 (5.2) |
| Gemcitabine + Cisplatin | 36 (12.1) | 0 (0.0) |
| Taxanes alone | 2 (0.7) | 7 (6.0) |
| Other a | 19 (6.4) | 5 (4.3) |
| Immunotherapy | 10 (3.4) | 25 (21.6) |
| Atezolizumab | 4 (1.3) | 16 (13.8) |
| Pembrolizumab | 4 (1.3) | 9 (7.8) |
| Nivolumab | 2 (0.7) | 0 (0.0) |
| No chemotherapy or immunotherapy | 177 (59.6) | 77 (66.4) |
| Overall Survival | 1L IT N = 10 | 1L CT N = 110 | NT (Reference) N = 177 |
|---|---|---|---|
| mOS (95% CI; months) | 11.10 (7.79–NA) | 12.76 (10.95–15.59) | 3.16 (2.69–3.66) |
| Log-rank test p-value | 0.0011 | <0.0001 | - |
| HR (95% CI) | 0.291 (0.136–0.624) | 0.310 (0.239–0.402) | - |
| Non-proportionality p-value | 0.2 | <0.0001 | - |
| RMST (95% CI; months) | 19.686 (8.619–30.753) | 16.909 (14.373–19.445) | 5.462 (4.333–6.591) |
| RMST difference (95% CI; months) | 14.224 (3.099–25.348) | 11.446 (8.670–14.222) | - |
| RMST difference p-value | 0.012 | <0.0001 | - |
| Overall Survival | 1L IT N = 10 | 1L CT (Reference) N = 110 |
|---|---|---|
| mOS (95% CI; months) | 5.03 (2.69–NA) | 9.13 (7.72–12.10) |
| HR (95% CI) | 0.911 (0.422–1.967) | - |
| Log-rank test p-value | 0.81 | - |
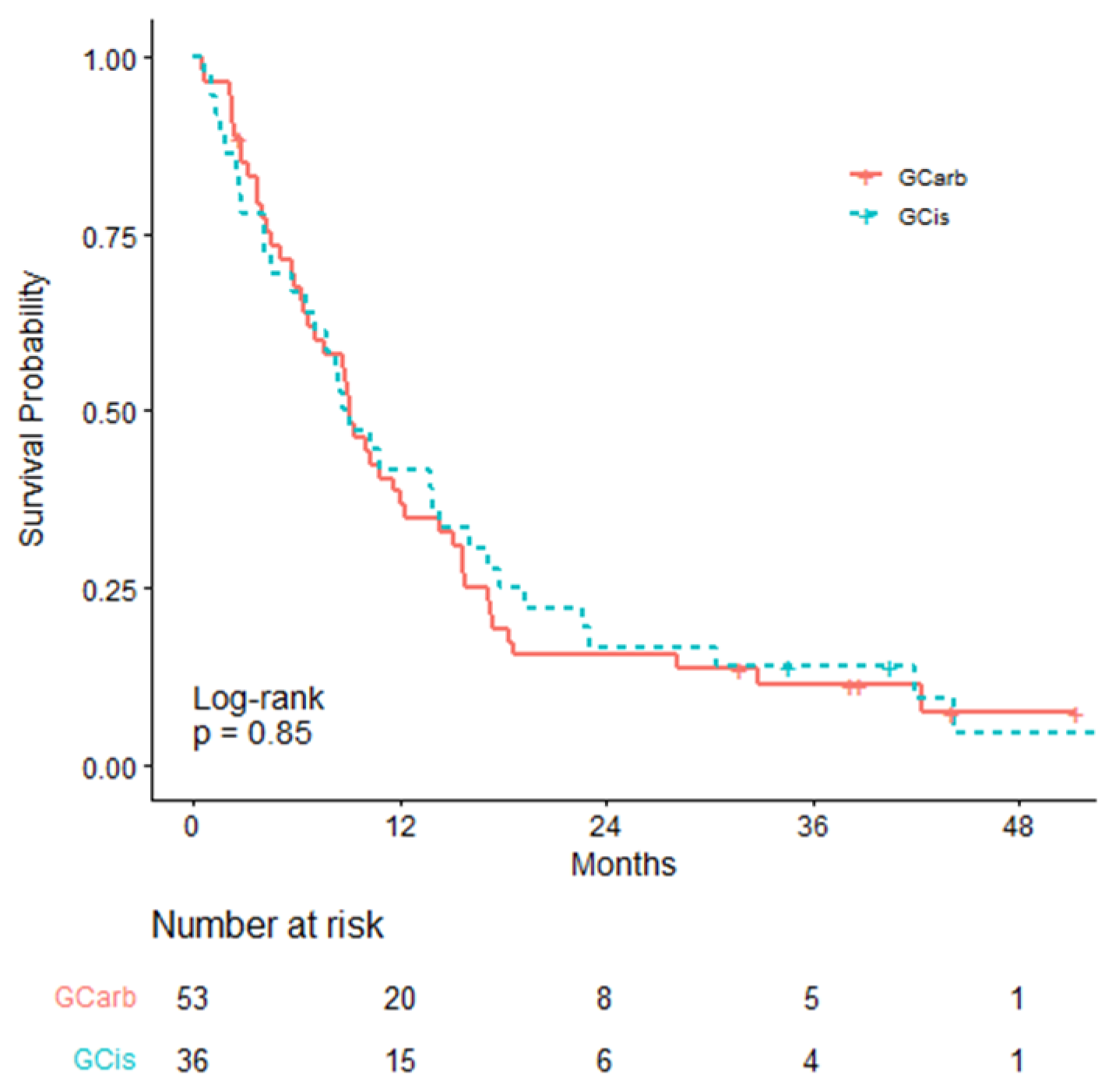
| Overall Survival | GCarb N = 53 | GCis (Reference) N = 36 |
|---|---|---|
| mOS (95% CI; months) | 9.13 (6.69–14.30) | 8.88 (6.53–16.10) |
| HR (95% CI) | 1.044 (0.668–1.633) | - |
| Log-rank test p-value | 0.85 | - |
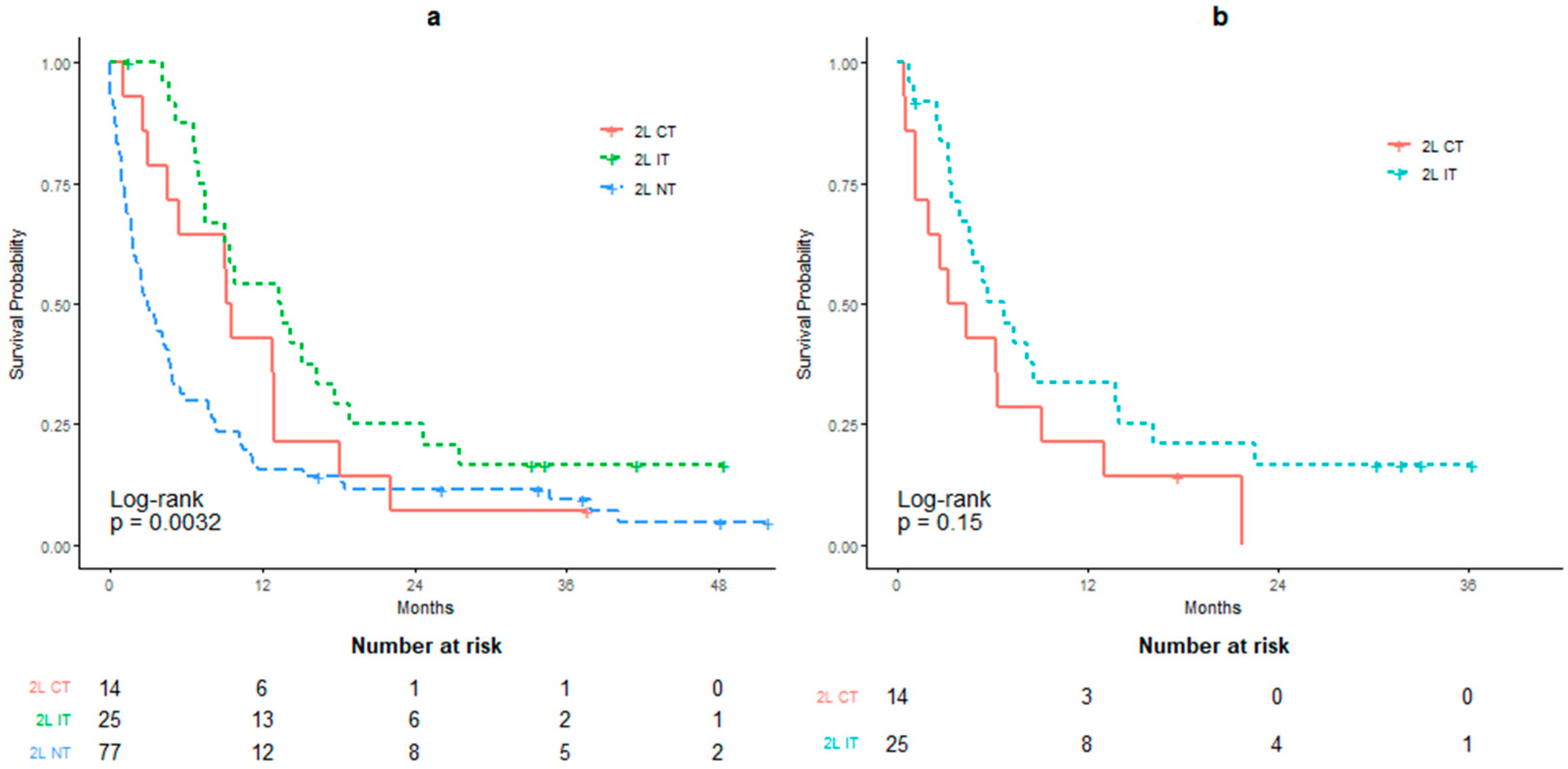
| Overall Survival | 2L IT N = 25 | 2L CT N = 14 | 2L NT (Reference) N = 77 |
|---|---|---|---|
| mOS (95% CI; months) | 13.43 (9.10–18.82) | 9.34 (5.46–22.10) | 2.92 (1.85–4.82) |
| Log-rank test p-value | 0.0047 | 0.1734 | - |
| HR (95% CI) | 0.454 (0.275–0.749) | 0.639 (0.352–1.160) | - |
| Non-proportionality p-value | 0.0002 | 0.0075 | - |
| RMST (95% CI; months) | 15.95 (11.67–20.24) | 11.36 (6.66–17.87) | 7.65 (5.20–10.09) |
| RMST difference (95% CI; months) | 8.31 (3.38–13.24) | 3.71 (−1.59–9.00) | - |
| RMST difference p-value | 0.001 | 0.170 | - |
| Overall Survival | 2L IT N = 25 | 2L CT (Reference) N = 14 |
|---|---|---|
| mOS (95% CI; months) | 6.72 (4.59–16.10) | 3.78 (1.99–NA) |
| HR (95% CI) | 0.595 (0.293–1.209) | - |
| Log-rank test p-value | 0.15 | - |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Key Statistics for Bladder Cancer. (8 January 2020). Available online: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html (accessed on 7 September 2020).
- Malkowicz, S.B.; van Poppel, H.; Mickisch, G.; Pansadoro, V.; Thüroff, J.; Soloway, M.S.; Chang, S.; Benson, M.; Fukui, I. Muscle-invasive urothelial carcinoma of the bladder. Urology 2007, 69 (Suppl. 1), 3–16. [Google Scholar] [CrossRef]
- National Institutes of Health National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER Cancer Stat Facts: Bladder Cancer. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 11 August 2017).
- Chalasani, V.; Chin, J.L.; Izawa, J.I. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can. Urol. Assoc. J. 2009, 3 (Suppl. 4), S193–S198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witjes, J.A.; Compérat, E.; Cowan, N.C.; De Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Van der Heijden, A.G.; Sherif, A. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Hahn, N.M.; Bellmunt, J.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.N.; et al. Treatment of patients with metastatic urothelial cancer “Unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powles, T.; Grivas, P.; Aragon-Ching, J.B.; Faroun, Y.; Kessler, E.R.; Tomita, Y.; Chakrabarti, D.; Laliberte, R.J.; Shnaidman, M.; Petrylak, D. A multicentre, international, randomised, open-label phase 3 trial of avelumab + best supportive care (BSC) vs. BSC alone as maintenance therapy after first-line platinum-based chemotherapy in patients with advanced urothelial cancer. J. Clin. Oncol. 2016, 27, vi292. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, L.F.; Edge, S.B.; Compton, C.C.; Gershenwald, E.J.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Feld, E.; Harton, J.; Meropol, N.J.; Adamson, B.J.; Cohen, A.; Parikh, R.B.; Galsky, M.D.; Narayan, V.; Christodouleas, J.; Vaghn, D.J.; et al. Effectiveness of first-line immune checkpoint blockade versus carboplatin-based chemotherapy for metastatic urothelial cancer. Eur. Urol. 2019, 76, 524–532. [Google Scholar] [CrossRef]
- Fisher, M.D.; Shenolikar, R.; Miller, P.J.; Fenton, M.; Walker, M.S. Treatment patterns and outcomes in stage IV bladder cancer in a community oncology setting: 2008–2015. Clin. Genitourin. Cancer 2018, 16, e1171–e1179. [Google Scholar] [CrossRef] [Green Version]
- Flannery, K.; Black-Shinn, J.; Boyd, M.; Robert, N.; Kamat, A. Second-line treatment patterns and outcomes of metastatic bladder cancer patients in clinical practice. Ann. Oncol. 2017, 28 (Suppl. 5), v295–v329. [Google Scholar] [CrossRef]
- Flannery, K.; Boyd, M.; Black-Shinn, J.; Robert, N.; Kamat, A.M. Outcomes in patients with metastatic bladder cancer in the USA: A retrospective electronic medical record study. Future Oncol. 2019, 15, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Dogliotti, L.; Cartenì, G.; Siena, S.; Bertetto, O.; Martoni, A.; Bono, A.; Amadori, D.; Onat, H.; Marini, L. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: Results of a randomized phase 2 trial. Eur. Urol. 2007, 52, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; von der Maase, H.; Mead, G.M.; Skoneczna, I.; De Santis, M.; Daugaard, G.; Boehle, A.; Chevreau, C.; Paz-Ares, L.; Laufman, L.R.; et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012, 30, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 2009, 27, 4454–4461. [Google Scholar] [CrossRef]
- Tsang, E.S.; Forbes, C.; Chi, K.N.; Eigl, B.J.; Parimi, S. Second-line systemic therapies for metastatic urothelial carcinoma: A population-based cohort analysis. Curr. Oncol. 2019, 26, e260–e265. [Google Scholar] [CrossRef] [Green Version]
- Furubayashi, N.; Negishi, T.; Yamashita, T.; Kusano, S.; Taguchi, K.; Shimokawa, M.; Nakamura, M. The combination of paclitaxel and carboplatin as second-line chemotherapy can be a preferred regimen for patients with urothelial carcinoma after the failure of gemcitabine and cisplatin chemotherapy. Mol. Clin. Oncol. 2017, 7, 1112–1118. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Ross, R.W.; Jacobus, S.; Vaishampayan, U.; Yu, E.Y.; Quinn, D.I.; Hahn, N.M.; Hutson, T.E.; Sponpavde, G.; Morrissey, S.C.; et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J. Clin. Oncol. 2012, 30, 507–512. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Galsky, M.D. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of > 2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; Van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]

| Variable | Overall (N = 297) | |
|---|---|---|
| Gender (Male), number of patients | 228 (77%) | |
| Age at metastasis in years, median (range) | 73 (36–98) | |
| Grade, number of pts (%) | High | 272 (92%) |
| Intermediate | 7 (3%) | |
| Low | 5 (2%) | |
| Unknown | 13 (4%) | |
| Histology, number of pts (%) | Urothelial | 231 (78%) |
| Squamous | 5 (2%) | |
| Neuroendocrine | 14 (4%) | |
| Glandular | 2 (<1%) | |
| Mixed | 44 (15%) | |
| Undifferentiated/other | 1 (<1%) | |
| Lymphovascular invasion, number of pts (%) | 108 (36%) | |
| Stage at initial diagnosis, number of pts (%) | II | 75 (25%) |
| III | 91 (31%) | |
| IV | 130 (44%) | |
| Radical cystectomy, number of pts (%) | 137 (46%) | |
| Radical radiotherapy, number of pts (%) | 27 (9%) | |
| Prior curative chemotherapy use, number of pts (%) | 73 (25%) | |
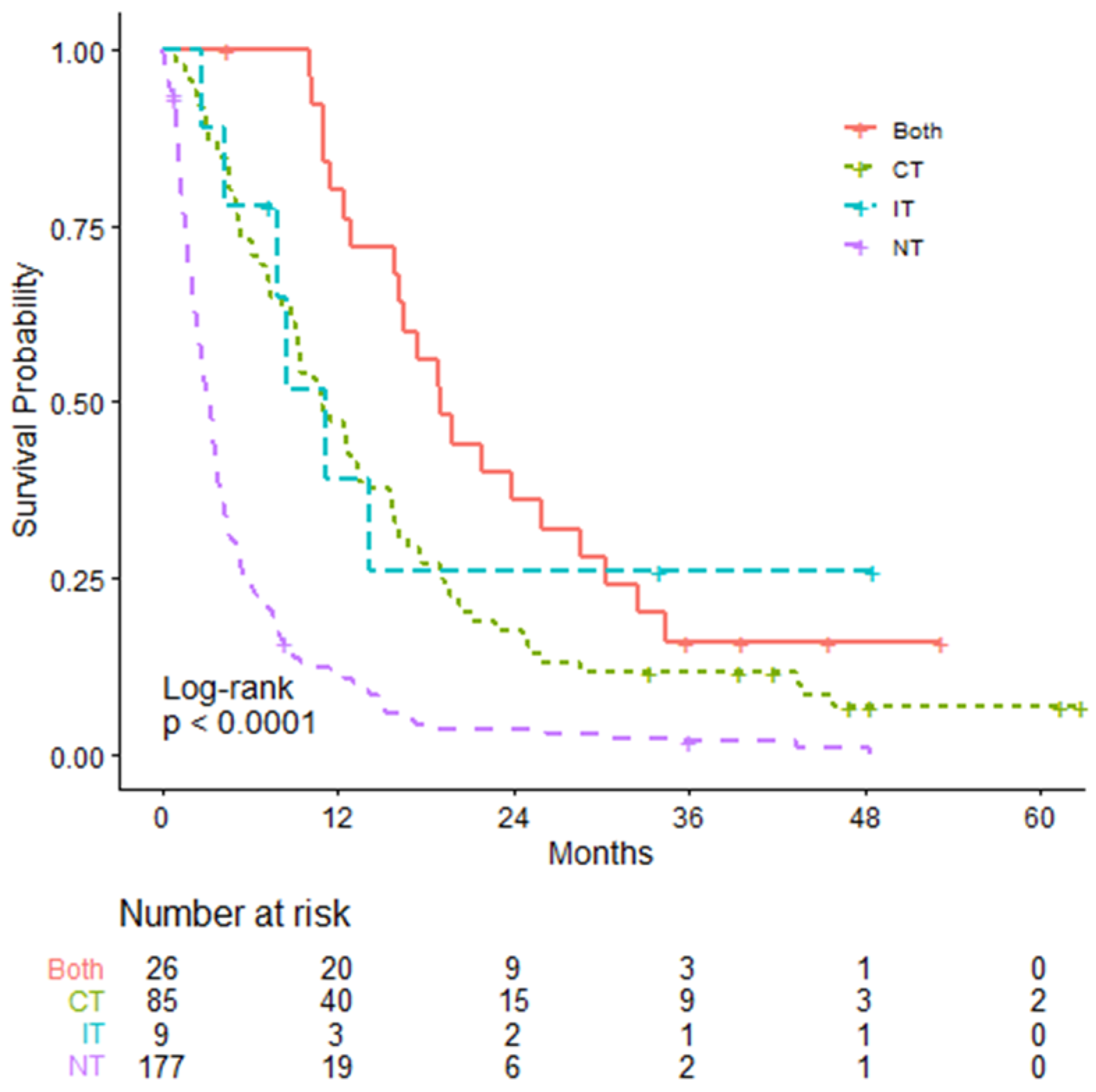
| Overall Survival | IT Only N = 9 | CT Only N = 85 | Both CT & IT N = 26 | NT (Reference) N = 177 |
|---|---|---|---|---|
| mOS (95% CI; months) | 11.10 (7.79–NA) | 10.82 (9.26–13.59) | 18.99 (16.23–30.26) | 3.16 (2.69–3.66) |
| Log-rank test p-value | 0.004 | <0.0001 | <0.0001 | - |
| HR (95% CI) | 0.296 (0.13–0.67) | 0.362 (0.27–0.48) | 0.231 (0.15–0.37) | - |
| Non-proportionality p-value | 0.29 | 0.0002 | 0.0001 | - |
| RMST (95% CI; months) | 18.61 (6.31–30.91) | 15.07 (12.27–17.87) | 23.68 (18.73–28.63) | 5.46 (4.33–6.59) |
| RMST difference (95% CI; months) | 13.15 (0.80–25.50) | 9.60 (6.58–12.62) | 18.22 (13.14–23.29) | - |
| RMST difference p-value | 0.037 | <0.0001 | <0.0001 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beigi, A.; Vafaei-Nodeh, S.; Huang, L.; Sun, S.Z.; Ko, J.J. Survival Outcomes Associated with First and Second-Line Palliative Systemic Therapies in Patients with Metastatic Bladder Cancer. Curr. Oncol. 2021, 28, 3812-3824. https://doi.org/10.3390/curroncol28050325
Beigi A, Vafaei-Nodeh S, Huang L, Sun SZ, Ko JJ. Survival Outcomes Associated with First and Second-Line Palliative Systemic Therapies in Patients with Metastatic Bladder Cancer. Current Oncology. 2021; 28(5):3812-3824. https://doi.org/10.3390/curroncol28050325
Chicago/Turabian StyleBeigi, Arshia, Saba Vafaei-Nodeh, Longlong Huang, Shaun Z. Sun, and Jenny J. Ko. 2021. "Survival Outcomes Associated with First and Second-Line Palliative Systemic Therapies in Patients with Metastatic Bladder Cancer" Current Oncology 28, no. 5: 3812-3824. https://doi.org/10.3390/curroncol28050325
APA StyleBeigi, A., Vafaei-Nodeh, S., Huang, L., Sun, S. Z., & Ko, J. J. (2021). Survival Outcomes Associated with First and Second-Line Palliative Systemic Therapies in Patients with Metastatic Bladder Cancer. Current Oncology, 28(5), 3812-3824. https://doi.org/10.3390/curroncol28050325




