Assessment of Risk of Bias in Osteosarcoma and Ewing’s Sarcoma Randomized Controlled Trials: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Title | Journal | Year | Reference | |
|---|---|---|---|---|
| 1 | Transfer factor versus combination chemotherapy: a preliminary report of a randomized postsurgical adjuvant treatment study in osteogenic sarcoma. | Annals of the New York Academy of Sciences | 1976 | [34] |
| 2 | Irradiation of the lungs as an adjuvant therapy in the treatment of osteosarcoma of the limbs. An E.O.R.T.C. randomized study. | European journal of cancer | 1978 | [35] |
| 3 | Delta-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. | Annals of internal medicine | 1979 | [36] |
| 4 | Ewing’s sarcoma of the vertebral column. | International journal of radiation oncology, biology, physics | 1981 | [37] |
| 5 | The role of radiation therapy in the management of non-metastatic Ewing’s sarcoma of bone. Report of the Intergroup Ewing’s Sarcoma Study. | International journal of radiation oncology, biology, physics | 1981 | [38] |
| 6 | Japanese experience with clinical trials of fast neutrons. | International journal of radiation oncology, biology, physics | 1982 | [39] |
| 7 | Toxicity associated with combination chemotherapy for osteosarcoma: a report of the cooperative osteosarcoma study (COSS 80). | Journal of cancer research and clinical oncology | 1983 | [40] |
| 8 | VM-26 and dimethyl triazeno imidazole carboxamide in Ewing’s sarcoma. | Australian paediatric journal | 1983 | [41] |
| 9 | Adjuvant chemotherapy in osteosarcoma-effects of cisplatinum, BCD, and fibroblast interferon in sequential combination with HD-MTX and adriamycin. Preliminary results of the COSS 80 study. | Journal of cancer research and clinical oncology | 1983 | [42] |
| 10 | The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. | The New England journal of medicine | 1986 | [43] |
| 11 | Adriamycin-methotrexate high dose versus adriamycin-methotrexate moderate dose as adjuvant chemotherapy for osteosarcoma of the extremities: a randomized study. | European journal of cancer & clinical oncology | 1986 | [44] |
| 12 | A trial of chemotherapy in patients with osteosarcoma (a report to the Medical Research Council by their Working Party on Bone Sarcoma. | British journal of cancer | 1986 | [45] |
| 13 | A randomized study comparing high-dose methotrexate with moderate-dose methotrexate as components of adjuvant chemotherapy in childhood nonmetastatic osteosarcoma: a report from the Childrens Cancer Study Group. | Medical and pediatric oncology | 1987 | [46] |
| 14 | The relationship of various aspects of surgical management to outcome in childhood nonmetastatic osteosarcoma: a report from the Childrens Cancer Study Group. | Journal of pediatric surgery | 1988 | [47] |
| 15 | Platinum disposition after intraarterial and intravenous infusion of cisplatin for osteosarcoma. Cooperative Osteosarcoma Study Group COSS. | Cancer chemotherapy and pharmacology | 1989 | [48] |
| 16 | Limb sparing versus amputation in osteosarcoma. Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. | Annals of oncology: official journal of the European Society for Medical Oncology | 1992 | [49] |
| 17 | Granulocyte-macrophage-colony stimulating factor for prevention of neutropenia and infections in children and adolescents with solid tumors. Results of a prospective randomized study. | Cancer | 1995 | [50] |
| 18 | Radiation therapy in Ewing’s sarcoma: an update of the CESS 86 trial. | International journal of radiation oncology, biology, physics | 1995 | [51] |
| 19 | Intra-arterial versus intravenous cisplatinum (in addition to systemic Adriamycin and high dose methotrexate) in the neoadjuvant treatment of osteosarcoma of the extremities. results of a randomized study. | Journal of chemotherapy (Florence, Italy) | 1996 | [52] |
| 20 | Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. | Annals of oncology: official journal of the European Society for Medical Oncology | 1997 | [53] |
| 21 | Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. | Lancet (London, England) | 1997 | [54] |
| 22 | A multidisciplinary study investigating radiotherapy in Ewing’s sarcoma: end results of POG #8346. Pediatric Oncology Group. | International journal of radiation oncology, biology, physics | 1998 | [55] |
| 23 | The pharmacokinetics and metabolism of ifosfamide during bolus and infusional administration: a randomized cross-over study. | British journal of cancer | 1998 | [56] |
| 24 | Ewing sarcoma of the rib: results of an intergroup study with analysis of outcome by timing of resection. | The Journal of thoracic and cardiovascular surgery | 2000 | [57] |
| 25 | Osteosarcoma in preadolescent patients. | Clinical orthopaedics and related research | 2000 | [58] |
| 26 | Granisetron, tropisetron, and ondansetron in the prevention of acute emesis induced by a combination of cisplatin-Adriamycin and by high-dose ifosfamide delivered in multiple-day continuous infusions. | Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer | 2000 | [59] |
| 27 | The possible cost effectiveness of peripheral blood stem cell mobilization with cyclophosphamide and the late addition of G-CSF. | Journal of Korean medical science | 2000 | [60] |
| 28 | Second malignancies after ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. | Annals of oncology: official journal of the European Society for Medical Oncology | 2001 | [61] |
| 29 | A comparison of methods of loco-regional chemotherapy combined with systemic chemotherapy as neo-adjuvant treatment of osteosarcoma of the extremity. | European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology | 2001 | [62] |
| 30 | Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2003 | [63] |
| 31 | Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. | The New England journal of medicine | 2003 | [64] |
| 32 | Twenty-year follow-up of osteosarcoma of the extremity treated with adjuvant chemotherapy. | Journal of chemotherapy (Florence, Italy) | 2004 | [65] |
| 33 | Treatment of metastatic Ewing’s sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide—a Children’s Cancer Group and Pediatric Oncology Group study. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2004 | [66] |
| 34 | Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: early Chinese clinical experience. | Ultrasound in medicine & biology | 2004 | [67] |
| 35 | Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2005 | [68] |
| 36 | Ifosfamide, carboplatin, and etoposide (ICE) reinduction chemotherapy in a large cohort of children and adolescents with recurrent/refractory sarcoma: the Children’s Cancer Group (CCG) experience. | Pediatric blood & cancer | 2005 | [69] |
| 37 | Local control in pelvic Ewing sarcoma: analysis from INT-0091—a report from the Children’s Oncology Group. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2006 | [70] |
| 38 | Contribution to the treatment of nausea and emesis induced by chemotherapy in children and adolescents with osteosarcoma. | Sao Paulo medical journal = Revista paulista de medicina | 2006 | [71] |
| 39 | Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children’s Cancer Group Phase II Study 9457—a report from the Children’s Oncology Group. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2006 | [72] |
| 40 | Pharmacokinetics and pharmacodynamics of intravenous epoetin alfa in children with cancer. | Pediatric blood & cancer | 2006 | [73] |
| 41 | Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. | Pediatric hematology and oncology | 2007 | [74] |
| 42 | SFOP OS94: a randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. | European journal of cancer (Oxford, England: 1990) | 2007 | [75] |
| 43 | Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | [76] | |
| 44 | Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. | Journal of the National Cancer Institute | 2007 | [77] |
| 45 | Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children’s Oncology Group. | Blood | 2007 | [78] |
| 46 | Results of the EICESS-92 Study: two randomized trials of Ewing’s sarcoma treatment-cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2008 | [79] |
| 47 | Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children’s Oncology Group. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2008 | [80] |
| 48 | Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children’s Oncology Group. | Cancer | 2009 | [81] |
| 49 | Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children’s Oncology Group Study. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2009 | [82] |
| 50 | Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. | Clinical cancer research: an official journal of the American Association for Cancer Research | 2011 | [83] |
| 51 | Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2012 | [84] |
| 52 | Long-term results (>25 years) of a randomized, prospective clinical trial evaluating chemotherapy in patients with high-grade, operable osteosarcoma. | Cancer | 2012 | [85] |
| 53 | Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian sarcoma group trial ISG/OS-1. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2012 | [86] |
| 54 | Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: results of the randomized noninferiority Euro-EWING99-R1 trial. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2014 | [87] |
| 55 | Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. | Clinical orthopaedics and related research | 2014 | [88] |
| 56 | Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients with Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2015 | [89] |
| 57 | Impact of gender on efficacy and acute toxicity of alkylating agent -based chemotherapy in Ewing sarcoma: secondary analysis of the Euro-Ewing99-R1 trial. | European journal of cancer (Oxford, England: 1990) | 2015 | [90] |
| 58 | Local control in Ewing sarcoma of the chest wall: results of the EURO-EWING 99 trial. | Annals of surgical oncology | 2015 | [91] |
| 59 | Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. | The Lancet. Oncology | 2016 | [92] |
| 60 | Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. | The Lancet. Oncology | 2016 | [93] |
| 61 | Glucagon Decreases IGF-1 Bioactivity in Humans, Independently of Insulin, by Modulating Its Binding Proteins. | The Journal of clinical endocrinology and metabolism | 2017 | [94] |
| 62 | Metronomic Chemotherapy vs. Best Supportive Care in Progressive Pediatric Solid Malignant Tumors: A Randomized Clinical Trial. | JAMA oncology | 2017 | [95] |
| 63 | Results of a randomized, prospective clinical trial evaluating metronomic chemotherapy in nonmetastatic patients with high-grade, operable osteosarcomas of the extremities: A report from the Latin American Group of Osteosarcoma Treatment. | Cancer | 2017 | [96] |
| 64 | The role of FDG PET/CT in patients treated with neoadjuvant chemotherapy for localized bone sarcomas. | European journal of nuclear medicine and molecular imaging | 2017 | [97] |
| 65 | Significance of neoadjuvant chemotherapy (NACT) in limb salvage treatment of osteosarcoma and its effect on GLS1 expression. | European review for medical and pharmacological sciences | 2018 | [98] |
| 66 | Ewing’s Sarcoma of the Head and Neck: Margins are not just for surgeons. | Cancer medicine | 2018 | [99] |
| 67 | Comprehensive Treatment and Rehabilitation of Patients with Osteosarcoma of the Mandible. | Implant dentistry | 2018 | [100] |
| 68 | Pantoprazole, an Inhibitor of the Organic Cation Transporter 2, Does Not Ameliorate Cisplatin-Related Ototoxicity or Nephrotoxicity in Children and Adolescents with Newly Diagnosed Osteosarcoma Treated with Methotrexate, Doxorubicin, and Cisplatin. | The Oncologist | 2018 | [101] |
| 69 | Gabapentin as an Adjuvant Therapy for Prevention of Acute Phantom-Limb Pain in Pediatric Patients Undergoing Amputation for Malignant Bone Tumors: A Prospective Double-Blind Randomized Controlled Trial. | Journal of pain and symptom management | 2018 | [102] |
| 70 | Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. | European journal of cancer (Oxford, England: 1990) | 2018 | [103] |
| 71 | Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. | The Lancet. Oncology | 2019 | [104] |
| 72 | Effects of mindfulness-based stress reduction combined with music therapy on pain, anxiety, and sleep quality in patients with osteosarcoma. | Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999) | 2019 | [105] |
| 73 | Addition of Zoledronate to Chemotherapy in Patients with Osteosarcoma Treated with Limb-Sparing Surgery: A Phase III Clinical Trial. | Medical science monitor: international medical journal of experimental and clinical research | 2019 | [106] |
| Study Stratification | (N) | Overall Percentages of Risk of Bias in All Domains | Domain with the Most “High-Risk” Ratings | Domain with the Most “Unclear-Risk” Ratings | |||
|---|---|---|---|---|---|---|---|
| Low Risk | Unclear Risk | High-Risk | |||||
| Type of Sarcoma | Osteosarcoma (OA) | 46 | 49.6% (137/276) | 43.1% (119/276) | 7.2% (20/276) | Blinding of participants and personnel | Blinding of participants and personnel |
| Ewing Sarcoma (ES) | 23 | 46.0% (74/161) | 51.6% (83/161) | 2.5% (4/161) | Blinding of participants and personnel | Blinding of outcome assessment | |
| RCTs containing both OA and ES | 4 | 53.6% (15/28) | 46.2% (13/28) | 0% (0/28) | - | Blinding of participant, personnel, and outcome assessment | |
| Type of Intervention | Medical RCTs | 69 | 47.6% (230/483) | 47.4% (229/483) | 5.0% (24/483) | Blinding of participants and personnel | Blinding of outcome assessment |
| Surgical RCTs | 4 | 42.9% (12/28) | 53.6% (15/28) | 3.6% (1/28) | Random Sequence Generation | Allocation sequence concealment, and blinding of participant, personnel, and outcome assessment | |
| Presence/Absence of Metastasis | RCTs solely on metastatic disease | 5 | 51.4% (18/35) | 48.6% (17/35) | 0% (0/35) | - | Blinding of participant, personnel, and outcome assessment |
| RCTs solely on non-metastatic disease | 5 | 45.7% (16/35) | 54.3% (19/35) | 0% (0/35) | - | Allocation sequence concealment, and blinding of participant, personnel, and outcome assessment | |
| RCT not stratified based on presence or absence of metastasis | 63 | 47.0% (203/432) | 47.2% (204/432) | 5.8% (25/432) | Blinding of participant, personnel, and outcome assessment | Blinding of participant, personnel, and outcome assessment | |
| Study | Type of Sarcoma | Intervention | Journal | Year |
|---|---|---|---|---|
| Local control in pelvic Ewing sarcoma: analysis from INT-0091—a report from the Children’s Oncology Group. | Ewing Sarcoma | Medical | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2006 |
| Second malignancies after ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. | Ewing Sarcoma | Medical | Annals of oncology: official journal of the European Society for Medical Oncology | 2001 |
| Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. | Osteosarcoma | Medical | Lancet (London, England) | 1997 |
| Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. | Osteosarcoma | Medical | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2007 |
| Japanese experience with clinical trials of fast neutrons. | Osteosarcoma | Medical | International journal of radiation oncology, biology, physics | 1982 |
| Pharmacokinetics and pharmacodynamics of intravenous epoetin alfa in children with cancer. | Osteosarcoma | Medical | Pediatric blood & cancer | 2006 |
| Limb sparing versus amputation in osteosarcoma. Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. | Osteosarcoma | Surgical | Annals of oncology: official journal of the European Society for Medical Oncology | 1992 |
| Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: results of the randomized noninferiority Euro-EWING99-R1 trial. | Ewing Sarcoma | Medical | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2014 |
| Contribution to the treatment of nausea and emesis induced by chemotherapy in children and adolescents with osteosarcoma. | Osteosarcoma | Medical | Sao Paulo medical journal = Revista paulista de medicina | 2006 |
| Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. | Osteosarcoma | Medical | Journal of the National Cancer Institute | 2007 |
| Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients with Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. | Osteosarcoma | Medical | Journal of clinical oncology: official journal of the American Society of Clinical Oncology | 2015 |
| Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. | Osteosarcoma | Medical | The Lancet. Oncology | 2016 |
| Effects of mindfulness-based stress reduction combined with music therapy on pain, anxiety, and sleep quality in patients with osteosarcoma. | Osteosarcoma | Medical | Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999) | 2019 |
| Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. | Osteosarcoma | Medical | The Lancet. Oncology | 2016 |
| Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. | Osteosarcoma | Medical | Clinical orthopaedics and related research | 2014 |
References
- Bothwell, L.E.; Podolsky, S.H. The Emergence of the Randomized, Controlled Trial. N. Engl. J. Med. 2016, 375, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Farrah, K.; Young, K.; Tunis, M.C.; Zhao, L. Risk of bias tools in systematic reviews of health interventions: An analysis of PROSPERO-registered protocols. Syst. Rev. 2019, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naci, H.; Davis, C.; Savović, J.; Higgins, J.P.T.; Sterne, J.A.C.; Gyawali, B.; Romo-Sandoval, X.; Handley, N.; Booth, C.M. Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: Cross sectional analysis. BMJ 2019, 366, l5221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurusamy, K.S.; Gluud, C.; Nikolova, D.; Davidson, B.R. Assessment of risk of bias in randomized clinical trials in surgery. Br. J. Surg. 2009, 96, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane. 2021. Available online: https://www.training.cochrane.org/handbook (accessed on 22 September 2021).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savović, J.; Akl, E.A.; Hróbjartsson, A. Financial conflicts of interest in clinical research. Intensive Care Med. 2018, 44, 1767–1769. [Google Scholar] [CrossRef] [Green Version]
- Lundh, A.; Lexchin, J.; Mintzes, B.; Schroll, J.B.; Bero, L. Industry sponsorship and research outcome. Cochrane Database Syst. Rev. 2017, 2, MR000033. [Google Scholar] [CrossRef] [Green Version]
- Logviss, K.; Krievins, D.; Purvina, S. Characteristics of clinical trials in rare vs. common diseases: A register-based Latvian study. PLoS ONE 2018, 13, e0194494. [Google Scholar] [CrossRef]
- Rees, C.A.; Pica, N.; Monuteaux, M.C.; Bourgeois, F.T. Noncompletion and nonpublication of trials studying rare diseases: A cross-sectional analysis. PLoS Med. 2019. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Karanicolas, P.J.; Thoma, A.; Simunovic, M.; Bhandari, M.; Devereaux, P.J.; Anvari, M.; Adili, A.; Guyatt, G. Randomized controlled trials of surgical interventions. Ann. Surg. 2010, 251, 409–416. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; The CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010, 7, e1000251. [Google Scholar] [CrossRef]
- Chess, L.E.; Gagnier, J. Risk of bias of randomized controlled trials published in orthopaedic journals. BMC Med. Res. Methodol. 2013, 13, 76. [Google Scholar] [CrossRef] [Green Version]
- Joksimovic, L.; Koucheki, R.; Popovic, M.; Ahmed, Y.; Schlenker, M.B.; Ahmed, I.I.K. Risk of bias assessment of randomised controlled trials in high-impact ophthalmology journals and general medical journals: A systematic review. Br. J. Ophthalmol. 2017, 101, 1309–1314. [Google Scholar] [CrossRef]
- Voineskos, S.H.; Coroneos, C.J.; Ziolkowski, N.I.; Kaur, M.N.; Banfield, L.; Meade, M.O.; Thoma, A.; Chung, K.C.; Bhandari, M. A Systematic Review of Surgical Randomized Controlled Trials: Part I. Risk of Bias and Outcomes: Common Pitfalls Plastic Surgeons Can Overcome. Plast. Reconstr. Surg. 2016, 137, 696–706. [Google Scholar] [CrossRef]
- Hariton, E.; Locascio, J.J. Randomised controlled trials—The gold standard for effectiveness research: Study design: Randomised controlled trials. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1716. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.F.; Grimes, D.A. Generation of allocation sequences in randomised trials: Chance, not choice. Lancet 2002, 359, 515–519. [Google Scholar] [CrossRef]
- Moher, D.; Pham, B.; Jones, A.; Cook, D.J.; Jadad, A.R.; Moher, M.; Tugwell, P.; Klassen, T.P. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998, 352, 609–613. [Google Scholar] [CrossRef]
- Dettori, J. The random allocation process: Two things you need to know. Evid. Based. Spine Care. J. 2010, 1, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Altman, D.G.; Schulz, K.F. Statistics notes: Concealing treatment allocation in randomised trials. BMJ 2001, 323, 446–447. [Google Scholar] [CrossRef] [Green Version]
- Montori, V.M.; Bhandari, M.; Devereaux, P.J.; Manns, B.J.; Ghali, W.A.; Guyatt, G.H. In the dark: The reporting of blinding status in randomized controlled trials. J. Clin. Epidemiol. 2002, 55, 787–790. [Google Scholar] [CrossRef]
- Probst, P.; Zaschke, S.; Heger, P.; Harnoss, J.C.; Hüttner, F.J.; Mihaljevic, A.L.; Knebel, P.; Diener, M.K. Evidence-based recommendations for blinding in surgical trials. Langenbeck’s Arch. Surg. 2019, 404, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Prophylactic antibiotic regimens in tumour surgery (PARITY): A pilot multicentre randomised controlled trial. Bone Joint Res. 2015, 4, 154–162. [CrossRef] [PubMed]
- Gazendam, A.; Bozzo, A.; Schneider, P.; Giglio, V.; Wilson, D.; Ghert, M. Recruitment patterns in a large international randomized controlled trial of perioperative care in cancer patients. Trials 2021, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Busse, J.W.; Jackowski, D.; Montori, V.M.; Schünemann, H.; Sprague, S.; Mears, D.; Schemitsch, E.H.; Heels-Ansdell, D.; Devereaux, P.J. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ 2004, 170, 477–480. [Google Scholar] [PubMed]
- Beyari, M.M.; Hak, A.; Li, C.S.; Lamfon, H.A. Conflict of interest reporting in dentistry randomized controlled trials: A systematic review. J. Evid. Based. Dent. Pract. 2014, 14, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Jones, A.; Lepage, L. Use of the CONSORT statement and quality of reports of randomized trials. J. Am. Med. Assoc. 2001. [Google Scholar] [CrossRef] [PubMed]
- Malek, F.; Somerson, J.S.; Mitchel, S.; Williams, R.P. Does limb-salvage surgery offer patients better quality of life and functional capacity than amputation? Clin. Orthop. Relat. Res. 2012, 470, 2000–2006. [Google Scholar] [CrossRef] [Green Version]
- Ayerza, M.A.; Farfalli, G.L.; Aponte-Tinao, L.; Muscolo, D.L. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin. Orthop. Relat. Res. 2010, 468, 2854–2859. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Anderson, P.; Lazar, A.J.; Burdett, E.; Raymond, K.; Ludwig, J.A. Ewing’s sarcoma: Standard and experimental treatment options. Curr. Treat. Options Oncol. 2009, 10, 126–140. [Google Scholar] [CrossRef]
- Cripe, T.P. Ewing Sarcoma: An Eponym Window to History. Sarcoma 2011, 2011, 457532. [Google Scholar] [CrossRef]
- Ekhtiari, S.; Gazendam, A.M.; Nucci, N.W.; Kruse, C.C.; Bhandari, M. The Fragility of Statistically Significant Findings From Randomized Controlled Trials in Hip and Knee Arthroplasty. J. Arthroplasty 2021, 36, 2211–2218. [Google Scholar] [CrossRef]
- Ivins, J.C.; Ritts, R.E.; Pritchard, D.J.; Gilchrist, G.S.; Miller, G.C.; Taylor, W.F. Transfer factor versus combination chemotherapy: A preliminary report of a randomized postsurgical adjuvant treatment study in osteogenic sarcoma. Ann. N. Y. Acad. Sci. 1976, 277, 558–574. [Google Scholar] [CrossRef]
- Breur, K.; Cohen, P.; Schweisguth, O.; Hart, A.M. Irradiation of the lungs as an adjuvant therapy in the treatment of osteosarcoma of the limbs. An E.O.R.T.C. randomized study. Eur. J. Cancer 1978, 14, 461–471. [Google Scholar] [CrossRef]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Simon, R.M.; Rosenberg, S.A. Delata-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann. Intern. Med. 1979, 91, 819–824. [Google Scholar] [CrossRef]
- Pilepich, M.V.; Vietti, T.J.; Nesbit, M.E.; Tefft, M.; Kissane, J.; Burgert, O.; Prichard, D.; Gehan, E.A. Ewing’s sarcoma of the vertebral column. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 27–31. [Google Scholar] [CrossRef]
- Perez, C.A.; Tefft, M.; Nesbit, M.; Burgert, E.O.J.; Vietti, T.; Kissane, J.; Pritchard, D.J.; Gehan, E.A. The role of radiation therapy in the management of non-metastatic Ewing’s sarcoma of bone. Report of the Intergroup Ewing’s Sarcoma Study. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 141–149. [Google Scholar] [CrossRef]
- Tsunemoto, H.; Arai, T.; Morita, S.; Ishikawa, T.; Aoki, Y.; Takada, N.; Kamata, S. Japanese experience with clinical trials of fast neutrons. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 2169–2172. [Google Scholar] [CrossRef]
- Jürgens, H.; Beron, G.; Winkler, K. Toxicity associated with combination chemotherapy for osteosarcoma: A report of the cooperative osteosarcoma study (COSS 80). J. Cancer Res. Clin. Oncol. 1983, 106, 14–18. [Google Scholar] [CrossRef]
- Campbell, A.M.; Ekert, H.; Waters, K.D. VM-26 and dimethyl triazeno imidazole carboxamide in Ewing’s sarcoma. Aust. Paediatr. J. 1983, 19, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Beron, G.; Kotz, R.; Salzer-Kuntschik, M.; Beck, J.; Beck, W.; Brandeis, W.; Ebell, W.; Erttmann, R.; Göbel, U.; et al. Adjuvant chemotherapy in osteosarcoma - effects of cisplatinum, BCD, and fibroblast interferon in sequential combination with HD-MTX and adriamycin. Preliminary results of the COSS 80 study. J. Cancer Res. Clin. Oncol. 1983, 106, 1–7. [Google Scholar] [CrossRef]
- Link, M.P.; Goorin, A.M.; Miser, A.W.; Green, A.A.; Pratt, C.B.; Belasco, J.B.; Pritchard, J.; Malpas, J.S.; Baker, A.R.; Kirkpatrick, J.A. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N. Engl. J. Med. 1986, 314, 1600–1606. [Google Scholar] [CrossRef]
- Bacci, G.; Gherlinzoni, F.; Picci, P.; Van Horn, J.R.; Jaffe, N.; Guerra, A.; Ruggieri, P.; Biagini, R.; Capanna, R.; Toni, A. Adriamycin-methotrexate high dose versus adriamycin-methotrexate moderate dose as adjuvant chemotherapy for osteosarcoma of the extremities: A randomized study. Eur. J. Cancer Clin. Oncol. 1986, 22, 1337–1345. [Google Scholar] [CrossRef]
- A trial of chemotherapy in patients with osteosarcoma (a report to the Medical Research Council by their Working Party on Bone Sarcoma. Br. J. Cancer 1986, 53, 513–518. [CrossRef]
- Krailo, M.; Ertel, I.; Makley, J.; Fryer, C.J.; Baum, E.; Weetman, R.; Yunis, E.; Barnes, L.; Bleyer, W.A.; Hammond, G.D. A randomized study comparing high-dose methotrexate with moderate-dose methotrexate as components of adjuvant chemotherapy in childhood nonmetastatic osteosarcoma: A report from the Childrens Cancer Study Group. Med. Pediatr. Oncol. 1987, 15, 69–77. [Google Scholar] [CrossRef]
- Makley, J.T.; Krailo, M.; Ertel, I.J.; Fryer, C.J.; Baum, E.S.; Weetman, R.M.; Yunis, E.J.; Bleyer, W.A.; Hammond, G.D. The relationship of various aspects of surgical management to outcome in childhood nonmetastatic osteosarcoma: A report from the Childrens Cancer Study Group. J. Pediatr. Surg. 1988, 23, 146–151. [Google Scholar] [CrossRef]
- Bielack, S.S.; Erttmann, R.; Looft, G.; Purfürst, C.; Delling, G.; Winkler, K.; Landbeck, G. Platinum disposition after intraarterial and intravenous infusion of cisplatin for osteosarcoma. Cooperative Osteosarcoma Study Group COSS. Cancer Chemother. Pharmacol. 1989, 24, 376–380. [Google Scholar] [CrossRef]
- Gherlinzoni, F.; Picci, P.; Bacci, G.; Campanacci, D. Limb sparing versus amputation in osteosarcoma. Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. Ann. Oncol. 1992, 3, S23–S27. [Google Scholar] [CrossRef]
- Burdach, S.E.; Müschenich, M.; Josephs, W.; Frisch, J.; Schulz, G.; Jürgens, H.; Göbel, U. Granulocyte-macrophage-colony stimulating factor for prevention of neutropenia and infections in children and adolescents with solid tumors. Results of a prospective randomized study. Cancer 1995, 76, 510–516. [Google Scholar] [CrossRef]
- Dunst, J.; Jürgens, H.; Sauer, R.; Pape, H.; Paulussen, M.; Winkelmann, W.; Rübe, C. Radiation therapy in Ewing’s sarcoma: An update of the CESS 86 trial. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 919–930. [Google Scholar] [CrossRef]
- Bacci, G.; Ruggieri, P.; Picci, P.; Mercuri, M.; Ferraro, A.; Tella, G.; Ferrari, S.; Bertoni, F.; Comandone, A. Intra-arterial versus intravenous cisplatinum (in addition to systemic Adriamycin and high dose methotrexate) in the neoadjuvant treatment of osteosarcoma of the extremities. results of a randomized study. J. Chemother. 1996, 8, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Bacci, G.; Picci, P.; Mercuri, M.; Briccoli, A.; Pinto, D.; Gasbarrini, A.; Tienghi, A.; Brach del Prever, A. Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Ann. Oncol. 1997, 8, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Souhami, R.L.; Craft, A.W.; Van der Eijken, J.W.; Nooij, M.; Spooner, D.; Bramwell, V.H.; Wierzbicki, R.; Malcolm, A.J.; Kirkpatrick, A.; Uscinska, B.M.; et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the European Osteosarcoma Intergroup. Lancet 1997, 350, 911–917. [Google Scholar] [CrossRef]
- Donaldson, S.S.; Torrey, M.; Link, M.P.; Glicksman, A.; Gilula, L.; Laurie, F.; Manning, J.; Neff, J.; Reinus, W.; Thompson, E.; et al. A multidisciplinary study investigating radiotherapy in Ewing’s sarcoma: End results of POG #8346. Pediatric Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 125–135. [Google Scholar] [CrossRef]
- Singer, J.M.; Hartley, J.M.; Brennan, C.; Nicholson, P.W.; Souhami, R.L. The pharmacokinetics and metabolism of ifosfamide during bolus and infusional administration: A randomized cross-over study. Br. J. Cancer 1998, 77, 978–984. [Google Scholar] [CrossRef] [Green Version]
- Shamberger, R.C.; Laquaglia, M.P.; Krailo, M.D.; Miser, J.S.; Pritchard, D.J.; Gebhardt, M.C.; Healey, J.H.; Tarbell, N.J.; Fryer, C.J.; Meyers, P.A.; et al. Ewing sarcoma of the rib: Results of an intergroup study with analysis of outcome by timing of resection. J. Thorac. Cardiovasc. Surg. 2000, 119, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Rytting, M.; Pearson, P.; Raymond, A.K.; Ayala, A.; Murray, J.; Yasko, A.W.; Johnson, M.; Jaffe, N. Osteosarcoma in Preadolescent Patients. Clin. Orthop. Relat. Res. 2000, 373. [Google Scholar] [CrossRef]
- Forni, C.; Ferrari, S.; Loro, L.; Mazzei, T.; Beghelli, C.; Biolchini, A.; Simoni, P.; Tremosini, M.; Strazzari, S.; Puggioli, C.; et al. Granisetron, tropisetron, and ondansetron in the prevention of acute emesis induced by a combination of cisplatin-Adriamycin and by high-dose ifosfamide delivered in multiple-day continuous infusions. Support. care cancer 2000, 8, 131–133. [Google Scholar] [CrossRef]
- Min, Y.J.; Kim, S.W.; Suh, C.; Park, J.; Kim, H.J.; Kim, J.G.; Kim, T.W.; Lee, J.H.; Kim, S.B.; Lee, K.H.; et al. The possible cost effectiveness of peripheral blood stem cell mobilization with cyclophosphamide and the late addition of G-CSF. J. Korean Med. Sci. 2000, 15, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Paulussen, M.; Ahrens, S.; Lehnert, M.; Taeger, D.; Hense, H.W.; Wagner, A.; Dunst, J.; Harms, D.; Reiter, A.; Henze, G.; et al. Second malignancies after ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann. Oncol. 2001, 12, 1619–1630. [Google Scholar] [CrossRef]
- Bacci, G.; Ferrari, S.; Tienghi, A.; Bertoni, F.; Mercuri, M.; Longhi, A.; Fiorentini, G.; Forni, C.; Bacchini, P.; Rimondini, S.; et al. A comparison of methods of loco-regional chemotherapy combined with systemic chemotherapy as neo-adjuvant treatment of osteosarcoma of the extremity. Eur. J. Surg. Oncol. 2001, 27, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Goorin, A.M.; Schwartzentruber, D.J.; Devidas, M.; Gebhardt, M.C.; Ayala, A.G.; Harris, M.B.; Helman, L.J.; Grier, H.E.; Link, M.P. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J. Clin. Oncol. 2003, 21, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Grier, H.E.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.H.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; Perlman, E.J.; Meyers, P.A.; et al. Addition of Ifosfamide and Etoposide to Standard Chemotherapy for Ewing’s Sarcoma and Primitive Neuroectodermal Tumor of Bone. N. Engl. J. Med. 2003, 348, 694–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhi, A.; Pasini, E.; Bertoni, F.; Pignotti, E.; Ferrari, C.; Bacci, G. Twenty-year follow-up of osteosarcoma of the extremity treated with adjuvant chemotherapy. J. Chemother. 2004, 16, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Miser, J.S.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.H.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; Perlman, E.J.; Meyers, P.A.; et al. Treatment of metastatic Ewing’s sarcoma or primitive neuroectodermal tumor of bone: Evaluation of combination ifosfamide and etoposide--a Children’s Cancer Group and Pediatric Oncology Group study. J. Clin. Oncol. 2004, 22, 2873–2876. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.-B.; Chen, W.-Z.; Zou, J.-Z.; Bai, J.; Zhu, H.; Li, K.-Q.; Xie, F.-L.; Jin, C.-B.; Su, H.-B.; et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med. Biol. 2004, 30, 245–260. [Google Scholar] [CrossRef]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.; Kleinerman, E.S.; Betcher, D.; Bernstein, M.L.; Conrad, E.; Ferguson, W.; Gebhardt, M.; Goorin, A.M.; et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J. Clin. Oncol. 2005, 23, 2004–2011. [Google Scholar] [CrossRef]
- Van Winkle, P.; Angiolillo, A.; Krailo, M.; Cheung, Y.-K.; Anderson, B.; Davenport, V.; Reaman, G.; Cairo, M.S. Ifosfamide, carboplatin, and etoposide (ICE) reinduction chemotherapy in a large cohort of children and adolescents with recurrent/refractory sarcoma: The Children’s Cancer Group (CCG) experience. Pediatr. Blood Cancer 2005, 44, 338–347. [Google Scholar] [CrossRef]
- Yock, T.I.; Krailo, M.; Fryer, C.J.; Donaldson, S.S.; Miser, J.S.; Chen, Z.; Bernstein, M.; Laurie, F.; Gebhardt, M.C.; Grier, H.E.; et al. Local control in pelvic Ewing sarcoma: Analysis from INT-0091—A report from the Children’s Oncology Group. J. Clin. Oncol. 2006, 24, 3838–3843. [Google Scholar] [CrossRef]
- Luisi, F.A.V.; Petrilli, A.S.; Tanaka, C.; Caran, E.M.M. Contribution to the treatment of nausea and emesis induced by chemotherapy in children and adolescents with osteosarcoma. Sao Paulo Med. J. 2006, 124, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, M.L.; Devidas, M.; Lafreniere, D.; Souid, A.-K.; Meyers, P.A.; Gebhardt, M.; Stine, K.; Nicholas, R.; Perlman, E.J.; Dubowy, R.; et al. Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children’s Cancer Group Phase II Study 9457--a report from the Children’s Oncology Group. J. Clin. Oncol. 2006, 24, 152–159. [Google Scholar] [CrossRef]
- Freeman, B.B., 3rd; Hinds, P.; Iacono, L.C.; Razzouk, B.I.; Burghen, E.; Stewart, C.F. Pharmacokinetics and pharmacodynamics of intravenous epoetin alfa in children with cancer. Pediatr. Blood Cancer 2006, 47, 572–579. [Google Scholar] [CrossRef]
- Gallegos-Castorena, S.; Martínez-Avalos, A.; Mohar-Betancourt, A.; Guerrero-Avendaño, G.; Zapata-Tarrés, M.; Medina-Sansón, A. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatr. Hematol. Oncol. 2007, 24, 403–408. [Google Scholar] [CrossRef]
- Le Deley, M.-C.; Guinebretière, J.-M.; Gentet, J.-C.; Pacquement, H.; Pichon, F.; Marec-Bérard, P.; Entz-Werlé, N.; Schmitt, C.; Brugières, L.; Vanel, D.; et al. SFOP OS94: A randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur. J. Cancer 2007, 43, 752–761. [Google Scholar] [CrossRef]
- Tebbi, C.K.; London, W.B.; Friedman, D.; Villaluna, D.; De Alarcon, P.A.; Constine, L.S.; Mendenhall, N.P.; Sposto, R.; Chauvenet, A.; Schwartz, C.L. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J. Clin. Oncol. 2007, 25, 493–500. [Google Scholar] [CrossRef]
- Lewis, I.J.; Nooij, M.A.; Whelan, J.; Sydes, M.R.; Grimer, R.; Hogendoorn, P.C.W.; Memon, M.A.; Weeden, S.; Uscinska, B.M.; van Glabbeke, M.; et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase III trial of the European Osteosarcoma Intergroup. J. Natl. Cancer Inst. 2007, 99, 112–128. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Krailo, M.D.; Chen, Z.; Burden, L.; Askin, F.B.; Dickman, P.S.; Grier, H.E.; Link, M.P.; Meyers, P.A.; Perlman, E.J.; et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children’s Oncology Group. Blood 2007, 109, 46–51. [Google Scholar] [CrossRef]
- Paulussen, M.; Craft, A.W.; Lewis, I.; Hackshaw, A.; Douglas, C.; Dunst, J.; Schuck, A.; Winkelmann, W.; Köhler, G.; Poremba, C.; et al. Results of the EICESS-92 Study: Two randomized trials of Ewing’s sarcoma treatment--cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J. Clin. Oncol. 2008, 26, 4385–4393. [Google Scholar] [CrossRef]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef]
- Chou, A.J.; Kleinerman, E.S.; Krailo, M.D.; Chen, Z.; Betcher, D.L.; Healey, J.H.; Conrad, E.U., 3rd; Nieder, M.L.; Weiner, M.A.; Wells, R.J.; et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: A report from the Children’s Oncology Group. Cancer 2009, 115, 5339–5348. [Google Scholar] [CrossRef]
- Granowetter, L.; Womer, R.; Devidas, M.; Krailo, M.; Wang, C.; Bernstein, M.; Marina, N.; Leavey, P.; Gebhardt, M.; Healey, J.; et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: A Children’s Oncology Group Study. J. Clin. Oncol. 2009, 27, 2536–2541. [Google Scholar] [CrossRef] [Green Version]
- Naing, A.; Kurzrock, R.; Burger, A.; Gupta, S.; Lei, X.; Busaidy, N.; Hong, D.; Chen, H.X.; Doyle, L.A.; Heilbrun, L.K.; et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin. cancer Res. 2011, 17, 6052–6060. [Google Scholar] [CrossRef] [Green Version]
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Marcus, K.; Sailer, S.; Healey, J.H.; Dormans, J.P.; et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef] [Green Version]
- Bernthal, N.M.; Federman, N.; Eilber, F.R.; Nelson, S.D.; Eckardt, J.J.; Eilber, F.C.; Tap, W.D. Long-term results (>25 years) of a randomized, prospective clinical trial evaluating chemotherapy in patients with high-grade, operable osteosarcoma. Cancer 2012, 118, 5888–5893. [Google Scholar] [CrossRef]
- Ferrari, S.; Ruggieri, P.; Cefalo, G.; Tamburini, A.; Capanna, R.; Fagioli, F.; Comandone, A.; Bertulli, R.; Bisogno, G.; Palmerini, E.; et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: An Italian sarcoma group trial ISG/OS-1. J. Clin. Oncol. 2012, 30, 2112–2118. [Google Scholar] [CrossRef] [Green Version]
- Le Deley, M.-C.; Paulussen, M.; Lewis, I.; Brennan, B.; Ranft, A.; Whelan, J.; Le Teuff, G.; Michon, J.; Ladenstein, R.; Marec-Bérard, P.; et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: Results of the randomized noninferiority Euro-EWING99-R1 trial. J. Clin. Oncol. 2014, 32, 2440–2448. [Google Scholar] [CrossRef]
- Puri, A.; Gulia, A.; Hawaldar, R.; Ranganathan, P.; Badwe, R.A. Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. Clin. Orthop. Relat. Res. 2014, 472, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Bielack, S.S.; Smeland, S.; Whelan, J.S.; Marina, N.; Jovic, G.; Hook, J.M.; Krailo, M.D.; Gebhardt, M.; Pápai, Z.; Meyer, J.; et al. Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 2279–2287. [Google Scholar] [CrossRef]
- van den Berg, H.; Paulussen, M.; Le Teuff, G.; Judson, I.; Gelderblom, H.; Dirksen, U.; Brennan, B.; Whelan, J.; Ladenstein, R.L.; Marec-Berard, P.; et al. Impact of gender on efficacy and acute toxicity of alkylating agent -based chemotherapy in Ewing sarcoma: Secondary analysis of the Euro-Ewing99-R1 trial. Eur. J. Cancer 2015, 51, 2453–2464. [Google Scholar] [CrossRef]
- Bedetti, B.; Wiebe, K.; Ranft, A.; Aebert, H.; Schmidt, J.; Jürgens, H.; Dirksen, U. Local control in Ewing sarcoma of the chest wall: Results of the EURO-EWING 99 trial. Ann. Surg. Oncol. 2015, 22, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet. Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef] [Green Version]
- Piperno-Neumann, S.; Le Deley, M.-C.; Rédini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brisse, H.; Lervat, C.; Gentet, J.-C.; Entz-Werlé, N.; et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Sarem, Z.; Bumke-Vogt, C.; Mahmoud, A.M.; Assefa, B.; Weickert, M.O.; Adamidou, A.; Bähr, V.; Frystyk, J.; Möhlig, M.; Spranger, J.; et al. Glucagon Decreases IGF-1 Bioactivity in Humans, Independently of Insulin, by Modulating Its Binding Proteins. J. Clin. Endocrinol. Metab. 2017, 102, 3480–3490. [Google Scholar] [CrossRef]
- Pramanik, R.; Agarwala, S.; Gupta, Y.K.; Thulkar, S.; Vishnubhatla, S.; Batra, A.; Dhawan, D.; Bakhshi, S. Metronomic Chemotherapy vs Best Supportive Care in Progressive Pediatric Solid Malignant Tumors: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Senerchia, A.A.; Macedo, C.R.; Ferman, S.; Scopinaro, M.; Cacciavillano, W.; Boldrini, E.; Lins de Moraes, V.L.; Rey, G.; de Oliveira, C.T.; Castillo, L.; et al. Results of a randomized, prospective clinical trial evaluating metronomic chemotherapy in nonmetastatic patients with high-grade, operable osteosarcomas of the extremities: A report from the Latin American Group of Osteosarcoma Treatment. Cancer 2017, 123, 1003–1010. [Google Scholar] [CrossRef]
- Palmerini, E.; Colangeli, M.; Nanni, C.; Fanti, S.; Marchesi, E.; Paioli, A.; Picci, P.; Cambioli, S.; Donati, D.; Cevolani, L.; et al. The role of FDG PET/CT in patients treated with neoadjuvant chemotherapy for localized bone sarcomas. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-P.; Li, X.; Li, H.; Sun, X.-H.; Yan, X.-F. Significance of neoadjuvant chemotherapy (NACT) in limb salvage treatment of osteosarcoma and its effect on GLS1 expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6538–6544. [Google Scholar] [CrossRef]
- Bouaoud, J.; Temam, S.; Cozic, N.; Galmiche-Rolland, L.; Belhous, K.; Kolb, F.; Bidault, F.; Bolle, S.; Dumont, S.; Laurence, V.; et al. Ewing’s Sarcoma of the Head and Neck: Margins are not just for surgeons. Cancer Med. 2018, 7, 5879–5888. [Google Scholar] [CrossRef]
- Utyuzh, A.S.; Yumashev, A.V.; Lang, H.W.; Zekiy, A.O.; Lushkov, R.M. Comprehensive Treatment and Rehabilitation of Patients With Osteosarcoma of the Mandible. Implant Dent. 2018, 27, 332–341. [Google Scholar] [CrossRef]
- Fox, E.; Levin, K.; Zhu, Y.; Segers, B.; Balamuth, N.; Womer, R.; Bagatell, R.; Balis, F. Pantoprazole, an Inhibitor of the Organic Cation Transporter 2, Does Not Ameliorate Cisplatin-Related Ototoxicity or Nephrotoxicity in Children and Adolescents with Newly Diagnosed Osteosarcoma Treated with Methotrexate, Doxorubicin, and Cisplatin. Oncologist 2018, 23, 762-e79. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yi, Y.; Tang, D.; Chen, Y.; Jiang, Y.; Peng, J.; Xiao, J. Gabapentin as an Adjuvant Therapy for Prevention of Acute Phantom-Limb Pain in Pediatric Patients Undergoing Amputation for Malignant Bone Tumors: A Prospective Double-Blind Randomized Controlled Trial. J. Pain Symptom Manag. 2018, 55, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, N.; Occean, B.-V.; Pacquement, H.; Bompas, E.; Bouvier, C.; Brisse, H.J.; Castex, M.-P.; Cheurfa, N.; Corradini, N.; Delaye, J.; et al. Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. Eur. J. Cancer 2018, 88, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Mir, O.; Boudou-Rouquette, P.; Piperno-Neumann, S.; Penel, N.; Bompas, E.; Delcambre, C.; Kalbacher, E.; Italiano, A.; Collard, O.; et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019, 20, 120–133. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Hou, Y. Effects of mindfulness-based stress reduction combined with music therapy on pain, anxiety, and sleep quality in patients with osteosarcoma. Rev. Bras. Psiquiatr. 2019, 41, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, P.; Pei, Y.; Zheng, K.; Wang, W.; Qiu, E.; Zhang, X. Addition of Zoledronate to Chemotherapy in Patients with Osteosarcoma Treated with Limb-Sparing Surgery: A Phase III Clinical Trial. Med. Sci. Monit. 2019, 25, 1429–1438. [Google Scholar] [CrossRef]


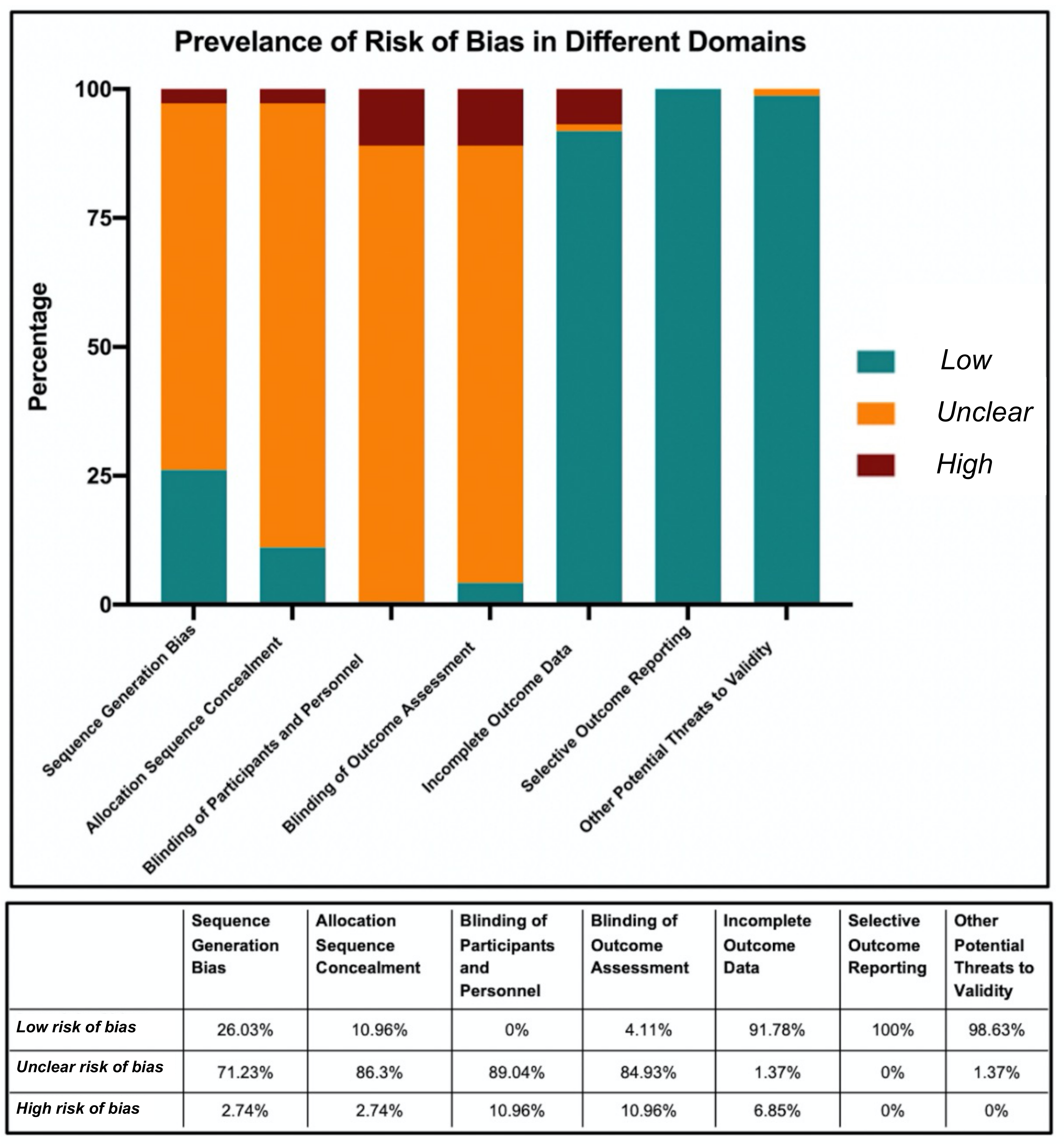

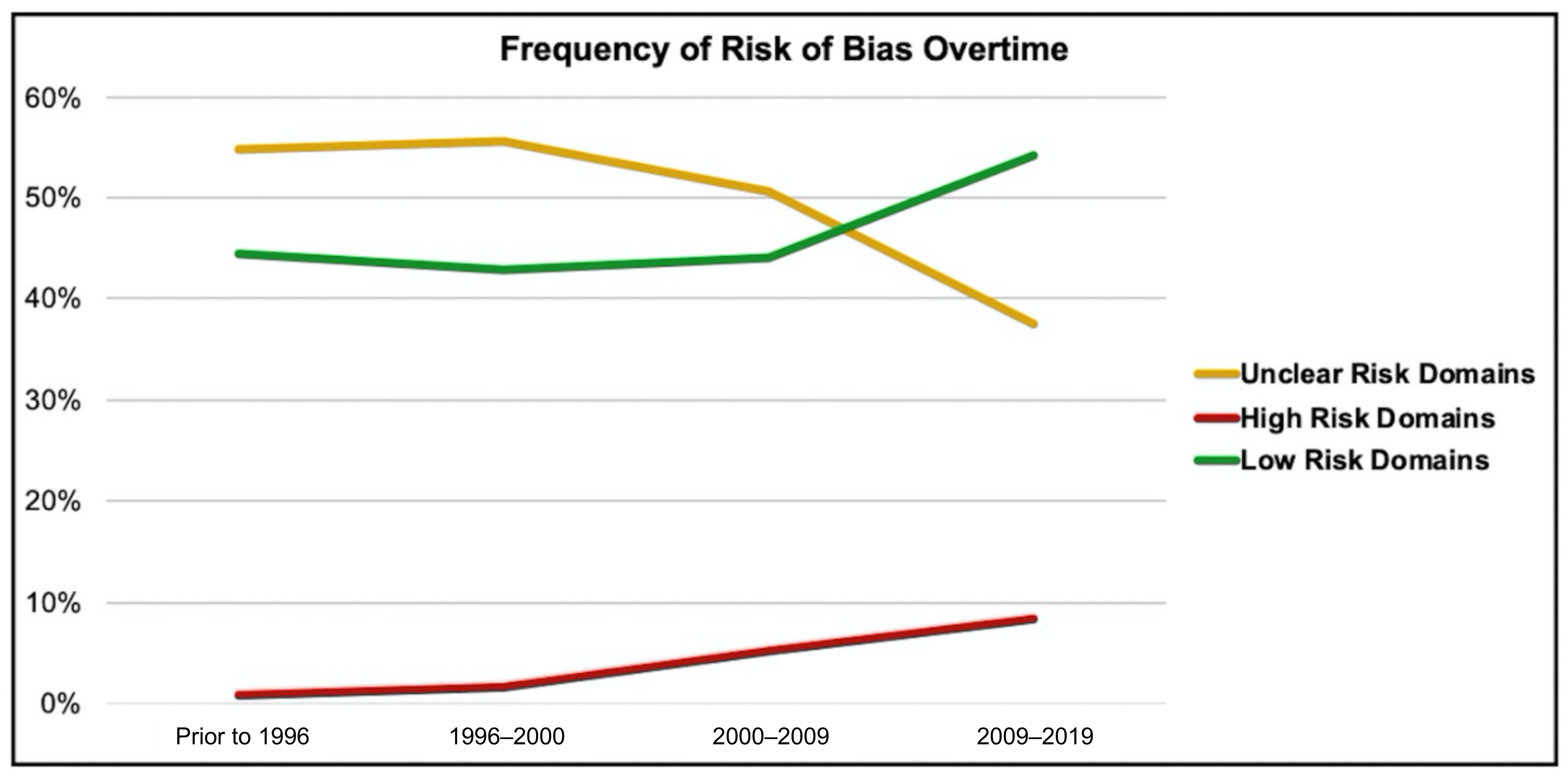
| Domain | Type of Bias Addressed | Description | Example of Low Risk Characteristics | Example of High Risk Characteristics |
|---|---|---|---|---|
| Random Sequence Generation | Selection Bias | Addresses whether there were sufficient information describing the method used by the RCT to generate the allocation sequence. |
|
|
| Allocation Sequence Concealment | Selection Bias | Addresses whether there were sufficient information describing the method used to mask the allocation sequence. |
|
|
| Blinding of Participants and Personnel | Performance Bias | Describes whether the participants and personnel were unaware of the interventions that the participants received. |
|
|
| Blinding of Outcome Assessment | Detection Bias | Describes measures used to blind outcome assessors to interventions that the participants received. |
|
|
| Incomplete Outcome Data | Attrition Bias | Describes the completeness of outcome data for each major outcome. |
|
|
| Selective Outcome Reporting | Reporting Bias | Describes reporting of all primary and secondary outcomes discussed within the introduction or methods section of the RCT. |
|
|
| Other Sources of Bias | State any important concerns about validity of the study not addressed elsewhere. | - |
|
| Risk of Bias Rating | Interpretation |
|---|---|
| Low Risk | Interpreted as potential bias unlikely to affect the results. |
| Unclear Risk | Interpreted as potential bias that raises some concerns about the results. |
| High Risk | Interpreted as potential bias that seriously reduces confidence in the results. |
| Journal | Number of RCTs | Impact in 2019 | Percentage of Domain with Unclear Risk | Percentage of Domain with High Risk |
|---|---|---|---|---|
| Journal of Clinical Oncology | 13 | 32.956 | 43.96% (40/91) | 6.59% (6/91) |
| International Journal of Radiation Oncology, Biology, Physics | 5 | 5.859 | 54.29% (19/35) | 2.86% (1/35) |
| Cancer | 4 | 5.742 | 57.14% (16/28) | 0.00% (0/28) |
| The Lancet Oncology | 3 | 33.752 | 9.52% (2/21) | 19.05% (4/21) |
| European Journal of Cancer | 3 | 7.275 | 47.62% (10/21) | 0.00% (0/21) |
| Annals of Oncology | 3 | 18.274 | 47.62% (10/21) | 9.52% (2/21) |
| The New England Journal of Medicine | 2 | 74.699 | 50.00% (7/14) | 0.00% (0/14) |
| Pediatric Blood & Cancer | 2 | 2.355 | 57.14% (8/14) | 7.14% (1/14) |
| Clinical Orthopaedics and Related Research | 2 | 4.091 | 28.57% (4/14) | 21.43% (3/14) |
| British journal of cancer | 2 | 5.791 | 57.14% (8/14) | 0.00% (0/14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koucheki, R.; Gazendam, A.M.; Perera, J.R.; Griffin, A.; Ferguson, P.; Wunder, J.; Tsoi, K. Assessment of Risk of Bias in Osteosarcoma and Ewing’s Sarcoma Randomized Controlled Trials: A Systematic Review. Curr. Oncol. 2021, 28, 3771-3794. https://doi.org/10.3390/curroncol28050322
Koucheki R, Gazendam AM, Perera JR, Griffin A, Ferguson P, Wunder J, Tsoi K. Assessment of Risk of Bias in Osteosarcoma and Ewing’s Sarcoma Randomized Controlled Trials: A Systematic Review. Current Oncology. 2021; 28(5):3771-3794. https://doi.org/10.3390/curroncol28050322
Chicago/Turabian StyleKoucheki, Robert, Aaron M. Gazendam, Jonathan R. Perera, Anthony Griffin, Peter Ferguson, Jay Wunder, and Kim Tsoi. 2021. "Assessment of Risk of Bias in Osteosarcoma and Ewing’s Sarcoma Randomized Controlled Trials: A Systematic Review" Current Oncology 28, no. 5: 3771-3794. https://doi.org/10.3390/curroncol28050322
APA StyleKoucheki, R., Gazendam, A. M., Perera, J. R., Griffin, A., Ferguson, P., Wunder, J., & Tsoi, K. (2021). Assessment of Risk of Bias in Osteosarcoma and Ewing’s Sarcoma Randomized Controlled Trials: A Systematic Review. Current Oncology, 28(5), 3771-3794. https://doi.org/10.3390/curroncol28050322





