Designing Inclusive HPV Cancer Vaccines and Increasing Uptake among Native Americans—A Cultural Perspective Review
Abstract
1. Introduction
1.1. Cervical Cancer Disparities among Native American Women
1.2. HPV Prevalence in Native American Communities
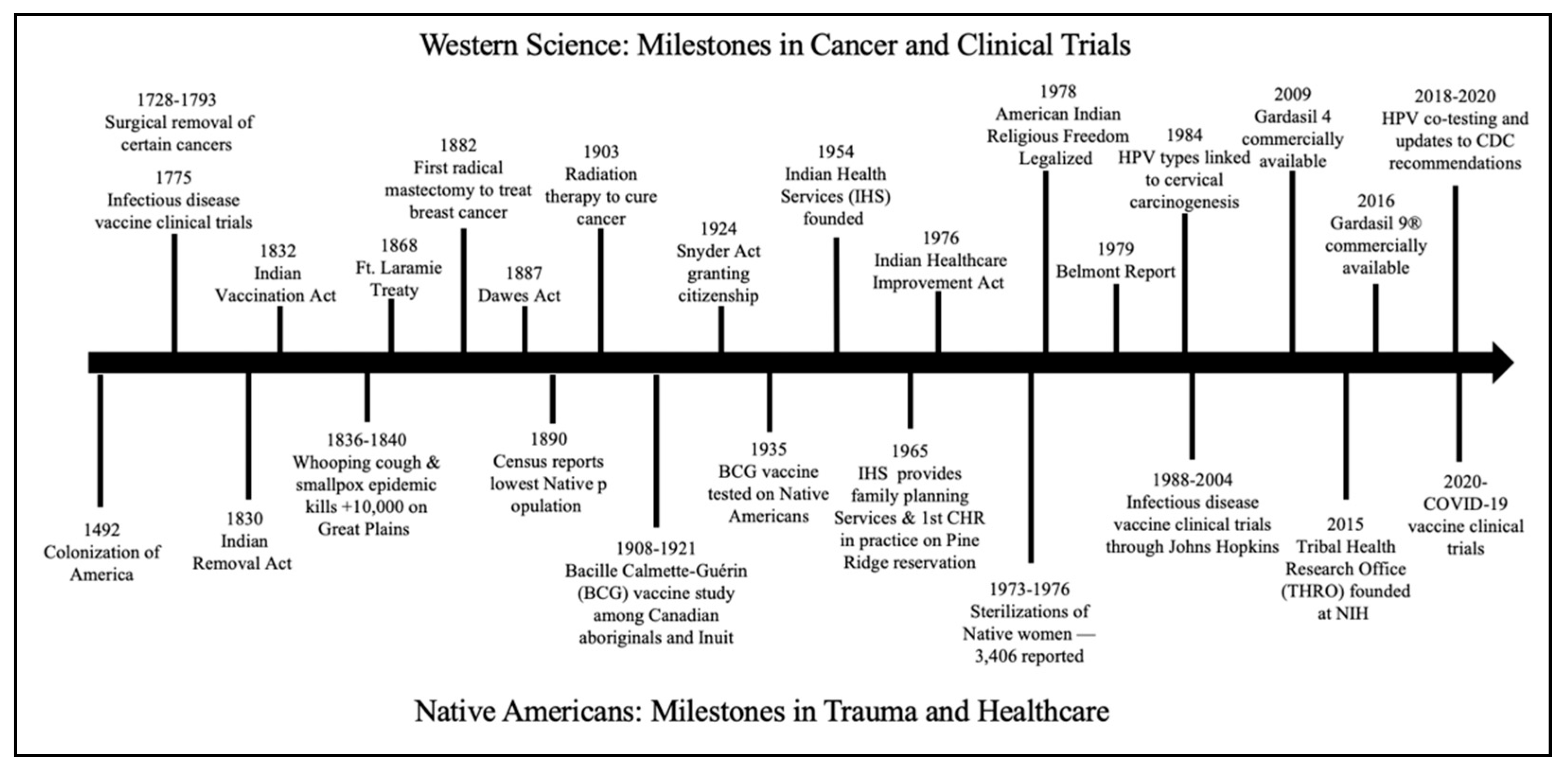
2. Historical Trauma and Medical Distrust among Native American Populations
2.1. History of Medical Distrust
2.2. Sterilization of Native American Women
2.3. Bioethical Misconduct toward Native Americans
3. Vaccine Design and Formulation
3.1. Vaccine Uptake Is High among Native American Youth
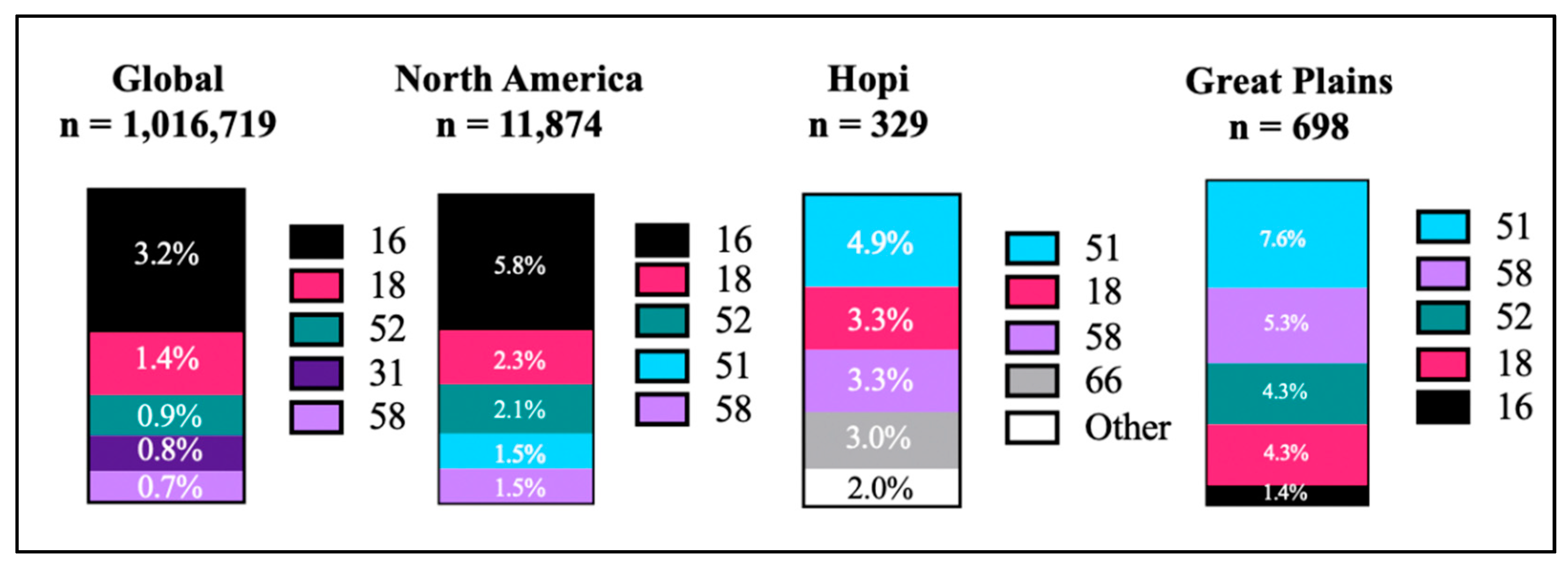
3.2. The General Population Does Not Always Represent the Whole
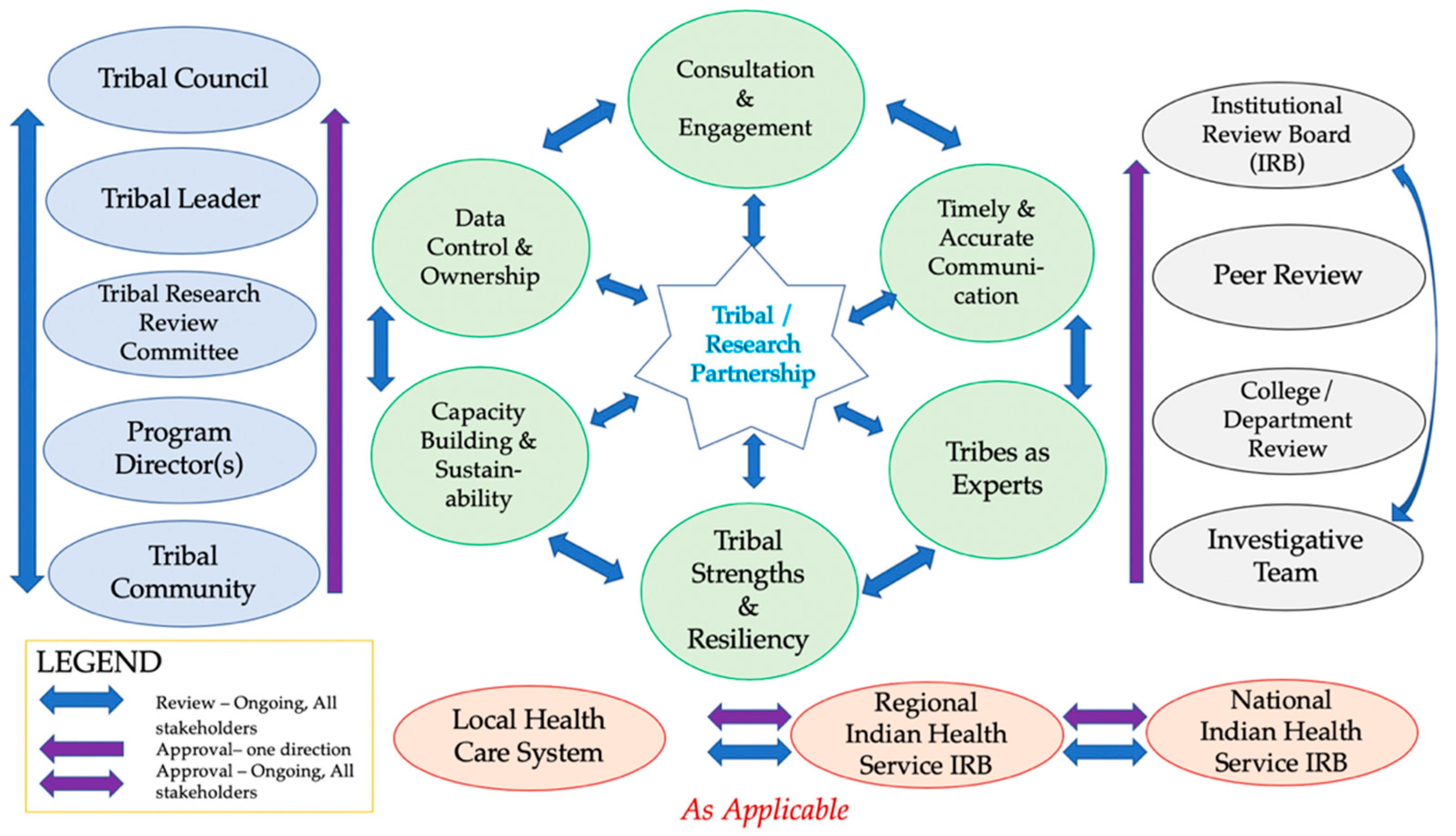
4. Recommendations for Increasing Native American Representation in Vaccine Trials and Uptake
4.1. Consultation and Engagement
Tribal Review, Approval & Dissemination
4.2. Tribal Data Control and Ownership
4.3. Timely and Accurate Communications
4.4. Capacity Building and Sustainability
4.5. Acknowledgement of Tribes as Experts
4.6. Contribution to Tribal Strengths and Resiliency
4.7. Importance of Vaccination and Cancer Prevention Engagement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horse, P.G. Reflections on American Indian identity. In New Perspectives on Racial Identity Development: A Theoretical and Practical Anthology; Wijeyesinghe, C.L., Jackson, B.W., III, Eds.; University Press: New York, NY, USA, 2001; pp. 91–107. [Google Scholar]
- Garrison, N.A.; Hudson, M.; Ballantyne, L.L.; Garba, I.; Martinez, A.; Taualii, M.; Arbour, L.; Caron, N.R.; Rainie, S.C. Genomic Research Through an Indigenous Lens: Understanding the Expectations. Annu. Rev. Genom. Hum. Genet. 2019, 20, 495–517. [Google Scholar] [CrossRef]
- Bell, M.C.; Schmidt-Grimminger, D.; Patrick, S.; Ryschon, T.; Linz, L.; Chauhan, S.C. There is a high prevalence of human papillomavirus infection in American Indian women of the Northern Plains. Gynecol. Oncol. 2007, 107, 236–241. [Google Scholar] [CrossRef][Green Version]
- National Cancer Institute. Cancer Stat Fact Sheets: Cervical Cancer. Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 21 October 2020).
- Alfonsi, G.A.; Datta, S.D.; Mickiewicz, T.; Koutsky, L.A.; Ghanem, K.; Hagensee, M.; Kerndt, P.; Hsu, K.; Weinstock, H.; Shlay, J.C. Prevalence of high-risk HPV types and abnormal cervical cytology in American Indian/Alaska Native women, 2003–2005. Public Health Rep. 2011, 126, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Jeudin, P.; Liveright, E.; Del Carmen, M.G.; Perkins, R.B. Race, ethnicity, and income factors impacting human papillomavirus vaccination rates. Clin. Ther. 2014, 36, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Winer, R.L.; Cherne, S.; Noonan, C.J.; Nelson, L.; Gonzales, A.A.; Umans, J.G.; Buchwald, D.; Canc, C.I.N. Human Papillomavirus Prevalence Among American Indian Women of the Great Plains. J. Infect. Dis. 2019, 219, 908–915. [Google Scholar] [CrossRef]
- Melkonian, S.C.; Weir, H.K.; Jim, M.A.; Preikschat, B.; Haverkamp, D.; White, M.C. Incidence of and Trends in the Leading Cancers With Elevated Incidence Among American Indian and Alaska Native Populations, 2012–2016. Am. J. Epidemiol. 2021, 190, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Duvall, J.; Buchwald, D. Human Papillomavirus Vaccine Policies Among American Indian Tribes in Washington State. J. Pediatr. Adolesc. Gynecol. 2012, 25, 131–135. [Google Scholar] [CrossRef]
- Leyden, W.A.; Manos, M.M.; Geiger, A.M.; Weinmann, S.; Mouchawar, J.; Bischoff, K.; Yood, M.U.; Gilbert, J.; Taplin, S.H. Cervical cancer in women with comprehensive health care access: Attributable factors in the screening process. J. Natl. Cancer Inst. 2005, 97, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.C.; Schmidt-Grimminger, D.; Jacobsen, C.; Chauhan, S.C.; Maher, D.M.; Buchwald, D.S. Risk factors for HPV infection among American Indian and white women in the Northern Plains. Gynecol. Oncol. 2011, 121, 532–536. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Castellsague, X.; Ferrer, E.; Bosch, F.X.; de Sanjose, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef]
- Winer, R.L.; Noonan, C.J.; Gonzales, A.A.; Cherne, S.; Buchwald, D.S. Assessing acceptability of self-sampling kits, prevalence, and risk factors for human papillomavirus infection in American Indian women. J. Community Health 2016, 41, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfstrom, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundstrom, K.; Dillner, J.; Sparen, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Fajardo-Bernal, L.; Aponte-Gonzalez, J.; Vigil, P.; Angel-Muller, E.; Rincon, C.; Gaitan, H.G.; Low, N. Home-based versus clinic-based specimen collection in the management of Chlamydia trachomatis and Neisseria gonorrhoeae infections. Cochrane Database Syst. Rev. 2015, 9, CD011317. [Google Scholar] [CrossRef]
- Hung, M.; Su, S.; Hon, E.S.; Licari, F.W.; Park, J.; Bounsanga, J.; Tuft, J.; Otrusinik, S.; Lipsky, M.S. Health Disparities Associated with Females Reporting Human Papillomavirus Infection in the United States. Womens Health Rep. 2021, 2, 245–253. [Google Scholar] [CrossRef]
- Oliver, S.E.; Unger, E.R.; Lewis, R.; McDaniel, D.; Gargano, J.W.; Steinau, M.; Markowitz, L.E. Prevalence of Human Papillomavirus Among Females After Vaccine Introduction-National Health and Nutrition Examination Survey, United States, 2003–2014. J. Infect. Dis. 2017, 216, 594–603. [Google Scholar] [CrossRef]
- Wheeler, C.M.; Hunt, W.C.; Cuzick, J.; Langsfeld, E.; Pearse, A.; Montoya, G.D.; Robertson, M.; Shearman, C.A.; Castle, P.E. A Population-based Study of HPV Genotype Prevalence in the United States: Baseline Measures Prior to Mass HPV Vaccination. Int. J. Cancer 2013, 132, 1–19. [Google Scholar] [CrossRef]
- Petereit, D.G.; Burhansstipanov, L. Establishing trusting partnerships for successful recruitment of American Indians to clinical trials. Cancer Control 2008, 15, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Liddell, J.L.; Burnette, C.E.; Roh, S.; Lee, Y.S. Healthcare barriers and supports for American Indian women with cancer. Soc. Work Health Care 2018, 57, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Povertyusa. The Population of Poverty USE. Available online: https://www.povertyusa.org/facts (accessed on 30 July 2021).
- Cackler, C.J.; Shapiro, V.B.; Lahiff, M. Female Sterilization and Poor Mental Health: Rates and Relatedness among American Indian and Alaska Native Women. Womens Health Issues 2016, 26, 168–175. [Google Scholar] [CrossRef]
- Lawrence, J. The Indian Health Service and the sterilization of Native American women. Am. Indian Q. 2000, 24, 400–419. [Google Scholar] [CrossRef]
- Carpio, M. The Lost Generation: American Indian Women and Sterilization Abuse. Soc. Justice 2004, 31, 40–53. [Google Scholar]
- Pacheco, C.M.; Daley, S.M.; Brown, T.; Filippi, M.; Greiner, K.A.; Daley, C.M. Moving forward: Breaking the cycle of mistrust between American Indians and researchers. Am. J. Public Health 2013, 103, 2152–2159. [Google Scholar] [CrossRef]
- Garrison, N.A. Genomic Justice for Native Americans: Impact of the Havasupai Case on Genetic Research. Sci. Technol. Hum. Values 2013, 38, 201–223. [Google Scholar] [CrossRef]
- Garrison, N.A.; Cho, M.K. Awareness and Acceptable Practices: IRB and Researcher Reflections on the Havasupai Lawsuit. AJOB Prim. Res. 2013, 4, 55–63. [Google Scholar] [CrossRef]
- Walker, T.Y.; Elam-Evans, L.D.; Yankey, D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; Singleton, J.A.; Stokley, S. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 718–723. [Google Scholar] [CrossRef]
- Sevigny, M. For Native People, Coronavirus Vaccine Trial Raises Specter of Past Traumas. Available online: https://www.knau.org/post/native-people-coronavirus-vaccine-trial-raises-specter-past-traumas (accessed on 20 December 2020).
- Flores, L.E.; Frontera, W.R.; Andrasik, M.P.; Del Rio, C.; Mondriguez-Gonzalez, A.; Price, S.A.; Krantz, E.M.; Pergam, S.A.; Silver, J.K. Assessment of the Inclusion of Racial/Ethnic Minority, Female, and Older Individuals in Vaccine Clinical Trials. JAMA Netw. Open 2021, 4, e2037640. [Google Scholar] [CrossRef]
- LaVallie, D.L.; Wolf, F.M.; Jacobsen, C.; Buchwald, D. Barriers to cancer clinical trial participation among native elders. Ethn. Dis. 2008, 18, 210–217. [Google Scholar]
- Sprague, D.; Russo, J.; LaVallie, D.L.; Buchwald, D. Barriers to Cancer Clinical Trial Participation Among American Indian and Alaska Native Tribal College Students. J. Rural Health 2013, 29, 55–60. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- COVPN. COVPN Community and Stakeholder Engagement Strategic Plan. Available online: https://www.coronaviruspreventionnetwork.org/files/covpn-community-stakeholder-engagement-strategic-plan.pdf (accessed on 28 July 2021).
- Vigil, D.; Sinaii, N.; Karp, B. American Indian and Alaska Native Enrollment in Clinical Studies in the National Institutes of Health’s Intramural Research Program. Ethics Hum. Res. 2021, 43, 2–9. [Google Scholar] [CrossRef]
- Gachupin, F.; Molina, F. How to Conduct Research in American Indian and Alaska Native Communities; University of Arizona: Tucson, AZ, USA, 2019; pp. 1–4. [Google Scholar]
- Gachupin, F.; Tracy, K.; Slowtalker, J. Clinical Trials, What Are They? University of Arizona: Tucson, AZ, USA, 2020; pp. 1–4. [Google Scholar]
- IHS. Human Subjects Research Protections. Available online: https://www.ihs.gov/dper/research/hsrp/ (accessed on 20 July 2021).
- Beans, J.A.; Saunkeah, B.; Brian Woodbury, R.; Ketchum, T.S.; Spicer, P.G.; Hiratsuka, V.Y. Community Protections in American Indian and Alaska Native Participatory Research-A Scoping Review. Soc. Sci. 2019, 8, 127. [Google Scholar] [CrossRef]
- Gachupin, F.C. Protections to consider when engaging American Indians/Alaska Natives in human subjects research. In Health and Social Issues of Native American Women; Jenny, R., Joe, J.R., Gachupin, F.C., Eds.; Praeger Publishers: Westport, CT, USA, 2012. [Google Scholar]
- Gachupin, F.C.; Freeman, W.L. Chapter 11—Ethics of Biospecimin Research. In Conducting Health Research with Native American Communities; Solomon, T.G.A., Randall, L.L., Eds.; American Public Health Association: Washington, DC, USA, 2014. [Google Scholar] [CrossRef]
- NBDC. Research for Natives, By Natives. Available online: http://www.nativebio.org/ (accessed on 21 July 2021).
- Claw, K.G.; Anderson, M.Z.; Begay, R.L.; Tsosie, K.S.; Fox, K.; Garrison, N.A.; Nanibaa’A, G.; Summer Internship for INdigenous Peoples in Genomics (SING) Consortium. A framework for enhancing ethical genomic research with Indigenous communities. Nat. Commun. 2018, 9, 2957. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordeaux, S.J.; Baca, A.W.; Begay, R.L.; Gachupin, F.C.; Caporaso, J.G.; Herbst-Kralovetz, M.M.; Lee, N.R. Designing Inclusive HPV Cancer Vaccines and Increasing Uptake among Native Americans—A Cultural Perspective Review. Curr. Oncol. 2021, 28, 3705-3716. https://doi.org/10.3390/curroncol28050316
Bordeaux SJ, Baca AW, Begay RL, Gachupin FC, Caporaso JG, Herbst-Kralovetz MM, Lee NR. Designing Inclusive HPV Cancer Vaccines and Increasing Uptake among Native Americans—A Cultural Perspective Review. Current Oncology. 2021; 28(5):3705-3716. https://doi.org/10.3390/curroncol28050316
Chicago/Turabian StyleBordeaux, Skyler J., Anthony W. Baca, Rene L. Begay, Francine C. Gachupin, J. Gregory Caporaso, Melissa M. Herbst-Kralovetz, and Naomi R. Lee. 2021. "Designing Inclusive HPV Cancer Vaccines and Increasing Uptake among Native Americans—A Cultural Perspective Review" Current Oncology 28, no. 5: 3705-3716. https://doi.org/10.3390/curroncol28050316
APA StyleBordeaux, S. J., Baca, A. W., Begay, R. L., Gachupin, F. C., Caporaso, J. G., Herbst-Kralovetz, M. M., & Lee, N. R. (2021). Designing Inclusive HPV Cancer Vaccines and Increasing Uptake among Native Americans—A Cultural Perspective Review. Current Oncology, 28(5), 3705-3716. https://doi.org/10.3390/curroncol28050316