Peripheral Blood Lymphocyte Percentage May Predict Chemotolerance and Survival in Patients with Advanced Pancreatic Cancer. Association between Adaptive Immunity and Nutritional State
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Materials and Procedures
2.3. Definitions
3. Results
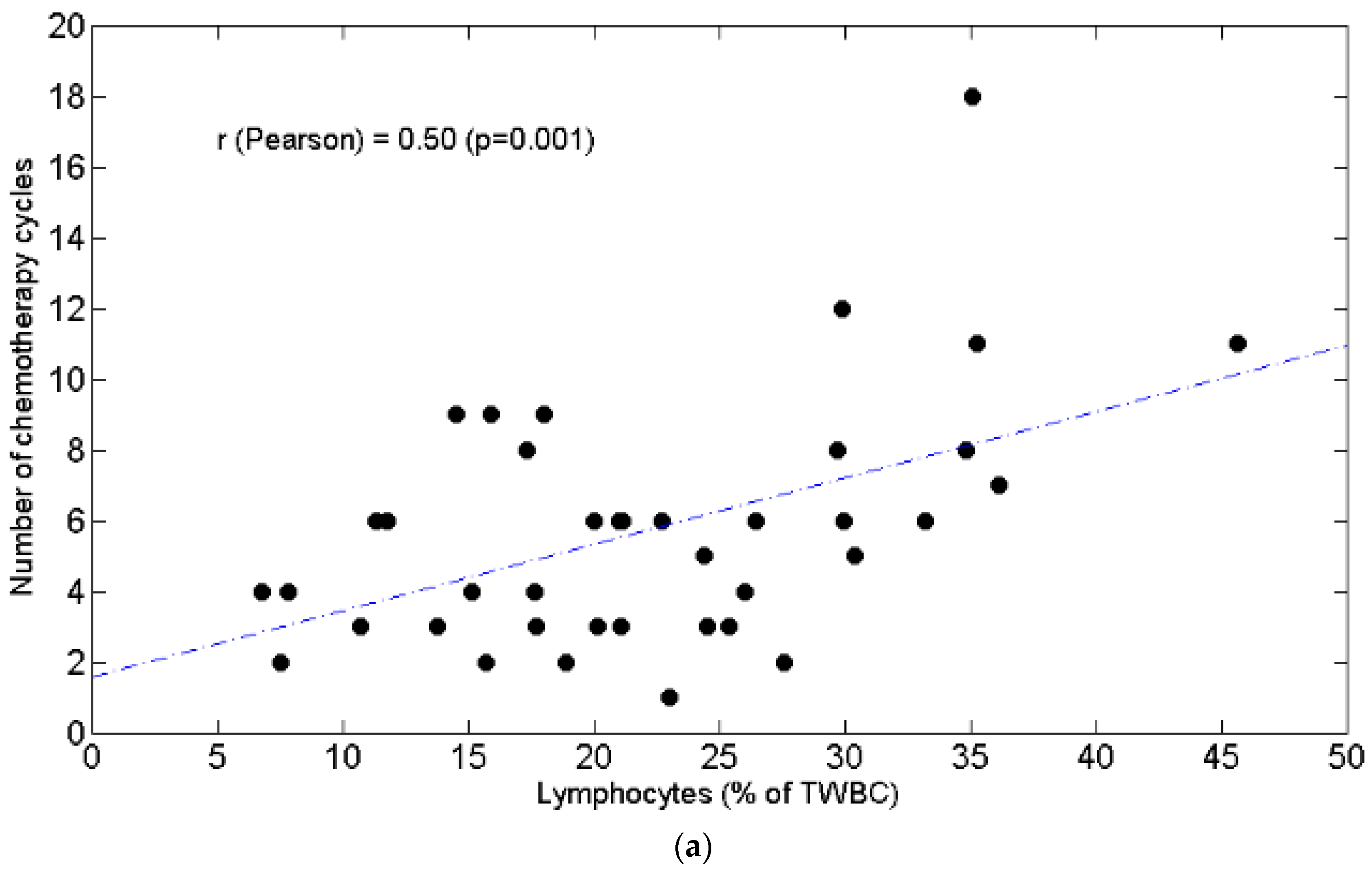
3.1. Lymphopenia and Relationship with Chemotolerance and Clinical Outcomes
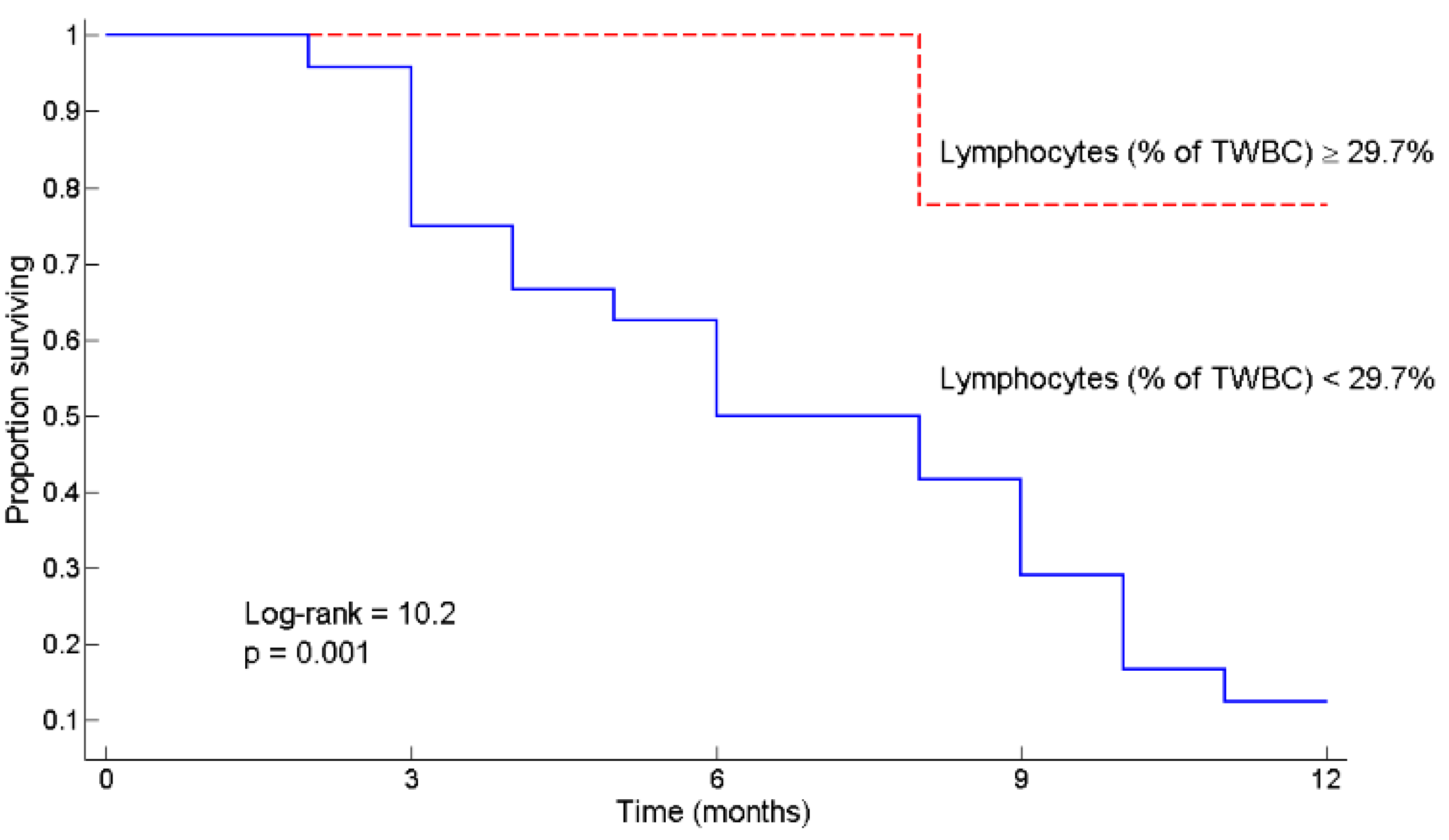
3.2. L ≥ 29.7% as a Predictor of Survival
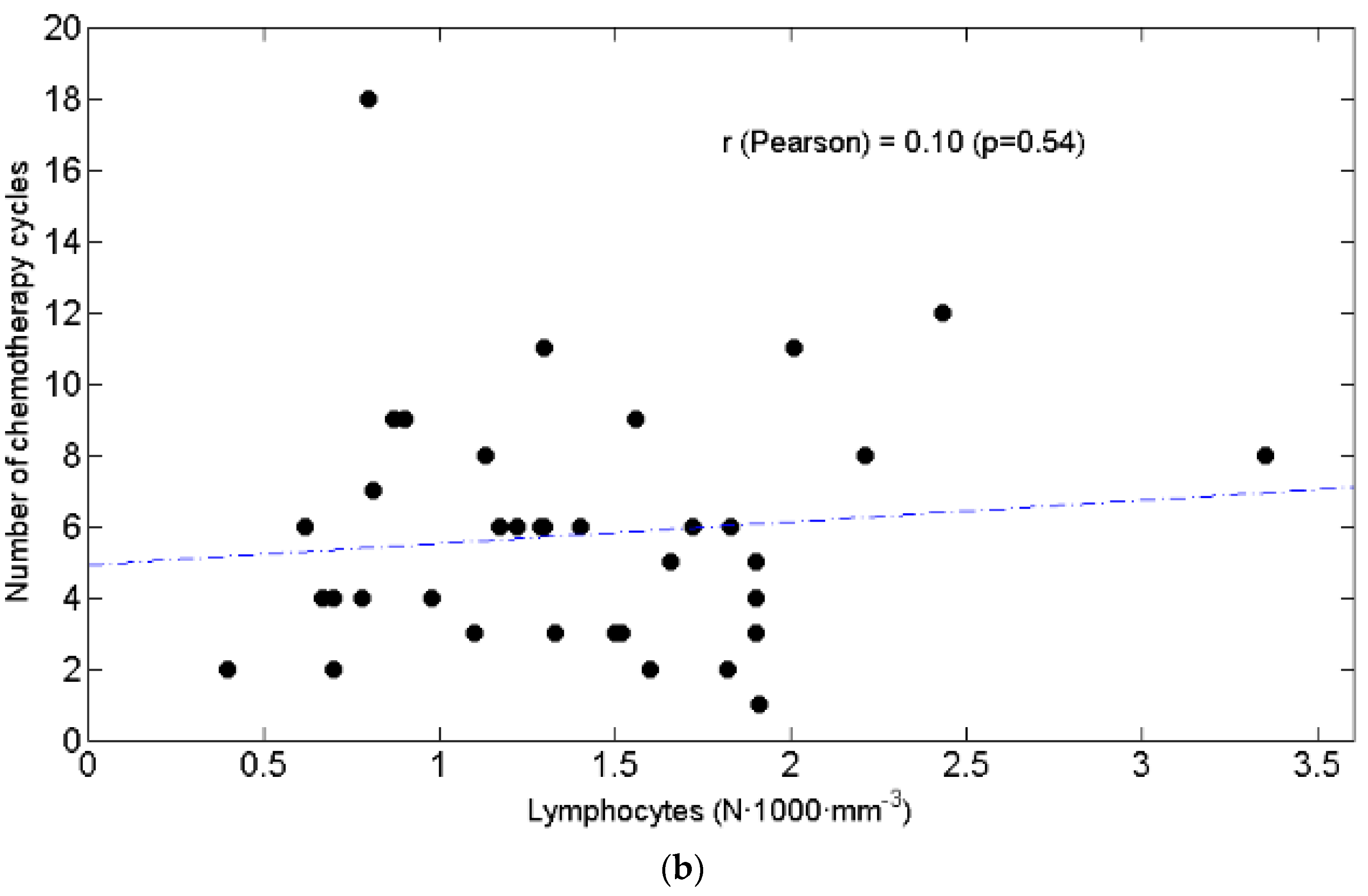
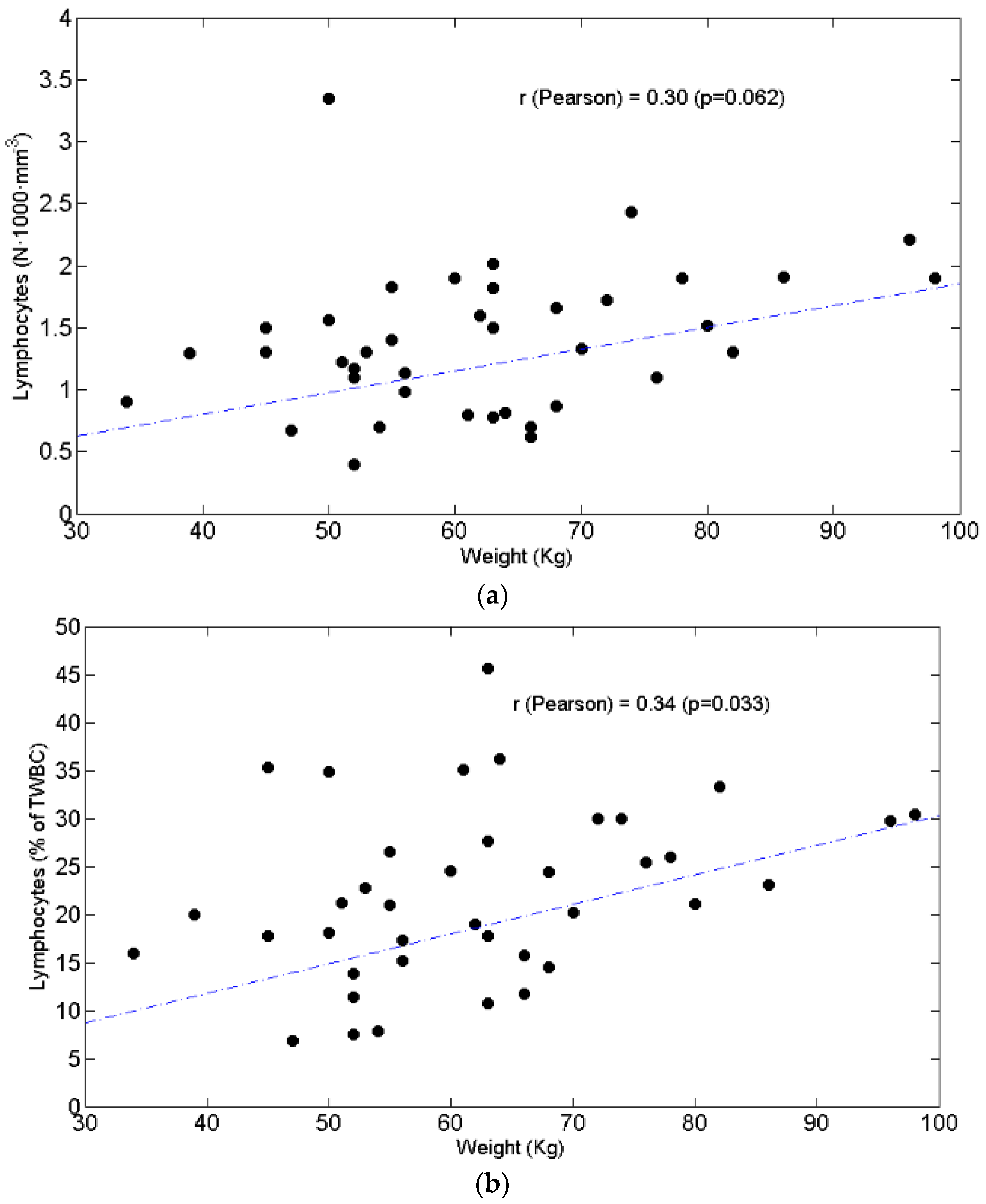
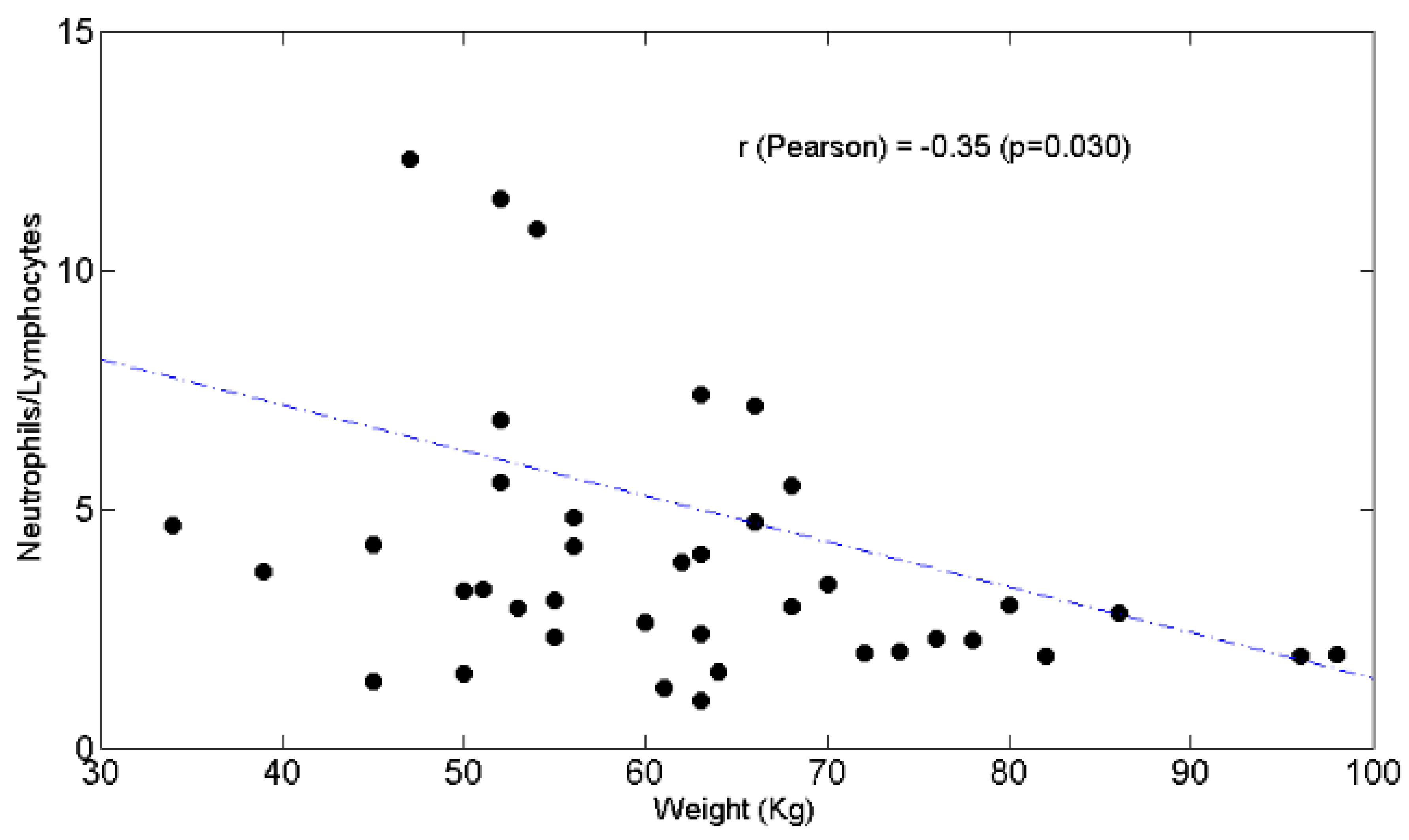
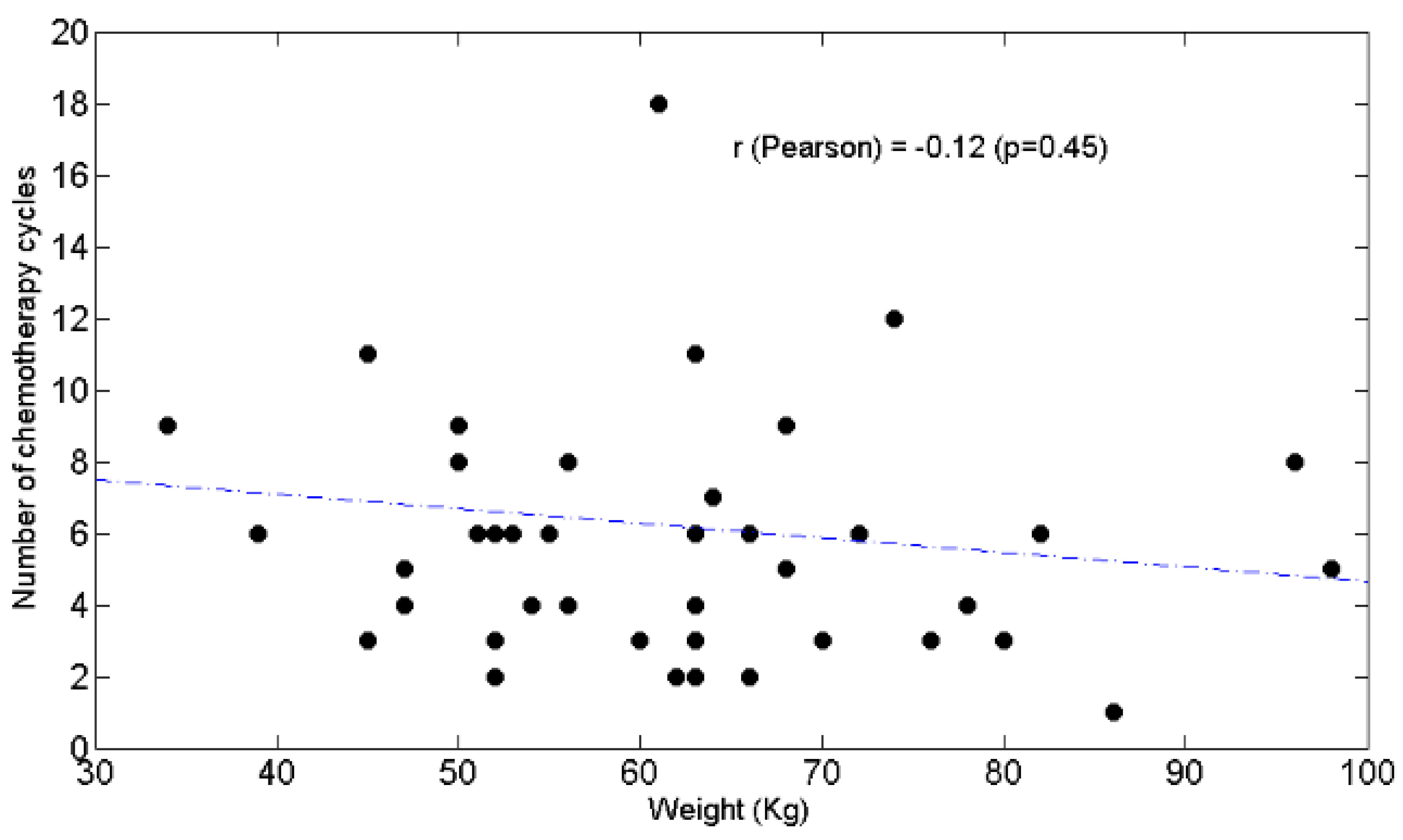
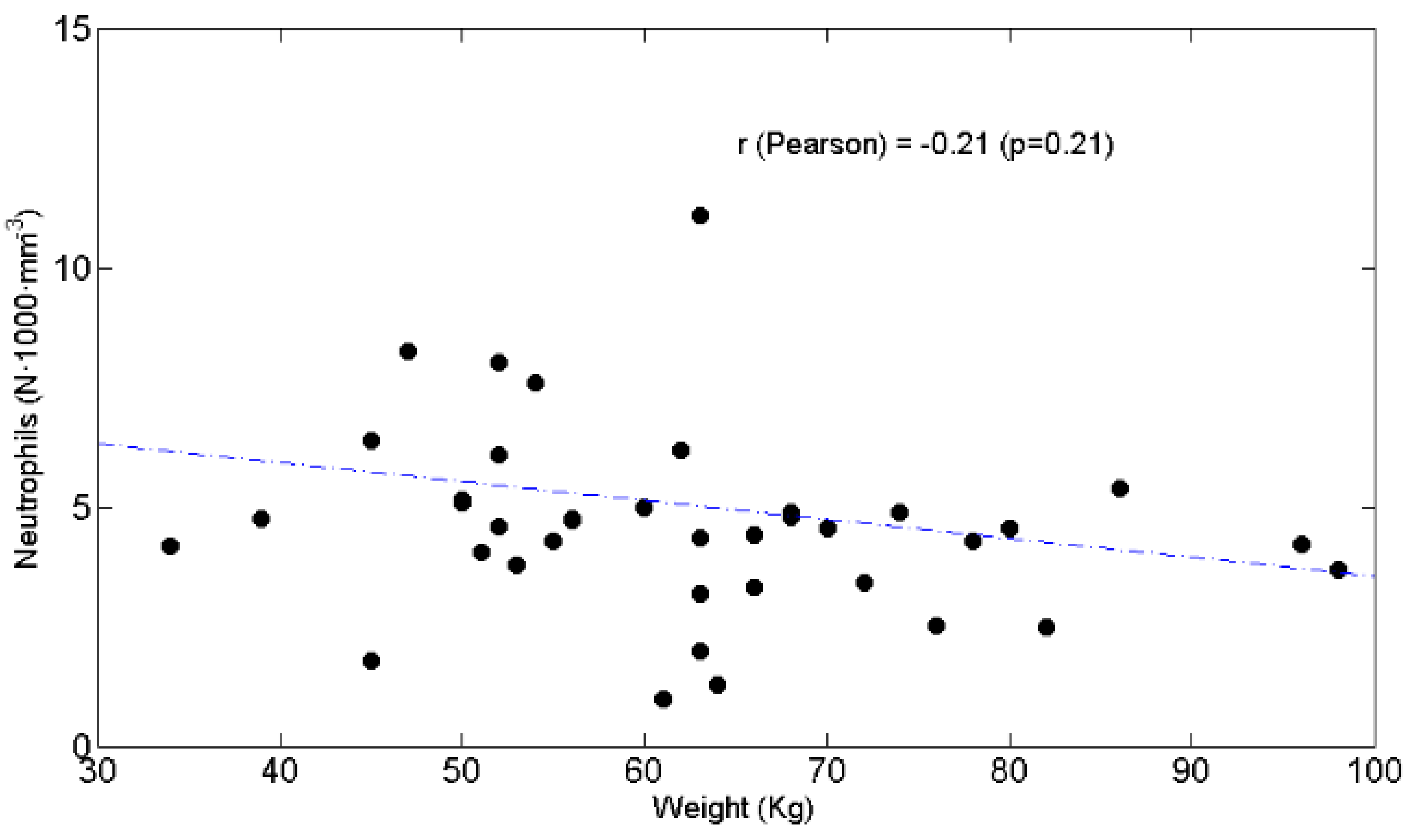
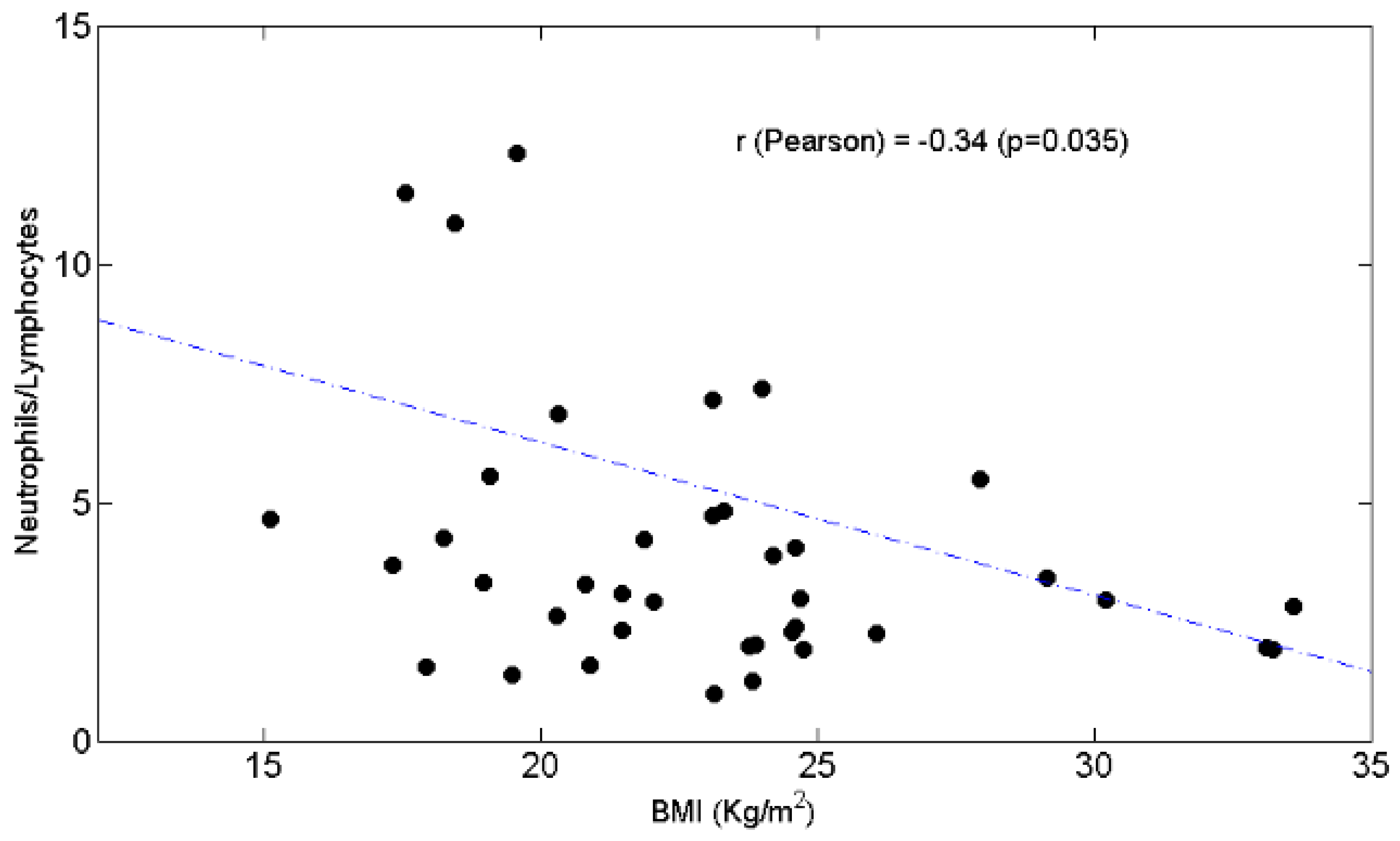
3.3. Relationships between BW and Lymphocytes, Neutrophils, N/L Ratio, Chemotolerance and Clinical Outcomes
4. Discussion
4.1. Patients’ Characteristics
4.2. Lymphopenia, Chemotolerance, Clinical Outcomes and BW
4.3. Potential Mechanisms Linking L ≥ 29.7% to Chemotolerance and Clinical Outcomes
4.4. Strengths and Limitations
4.5. Generation of Work Hypotheses
- (1)
- Does nutrition improve L%? If so, what types of nutrient intakes?
- (2)
- Does the improvement of skeletal muscle mass—the main body protein/amino acid store—support lymphocyte proliferation? Lymphocytes are avid consumers of amino acids for exerting their metabolic activities [29];
- (3)
- May plasma total and/or individual amino acid levels influence lymphocyte proliferation?
- (4)
- Do micronutrients shape the immune response? [46].
- (5)
- May an anti-inflammatory diet contribute to enhancing the prevalence of adaptive immunity? [47].
- (6)
- Is there a BW value(s) (or BMI) at which an interruption of its association with plasma L% may occur?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Torres, C.; Grippo, P.J. Pancreatic cancer subtypes: A roadmap for precision medicine. Ann. Med. 2018, 50, 277–287. [Google Scholar] [CrossRef]
- Reni, M.; Passoni, P.; Panucci, M.G.; Nicoletti, R.; Galli, L.; Balzano, G.; Zerbi, A.; Di Carlo, V.; Villa, E. Definitive results of a phase II trial of cisplatin, epirubicin, continuous-infusion fluorouracil, and gemcitabine in stage IV pancreatic adenocarcinoma. J. Clin. Oncol. 2001, 19, 2679–2686. [Google Scholar] [CrossRef]
- El Kamar, F.G.; Grossbard, M.L.; Kozuch, P.S. Metastatic pancreatic cancer: Emerging strategies in chemotherapy and palliative care. Oncology 2003, 8, 18–34. [Google Scholar] [CrossRef]
- Takai, S.; Satoi, S.; Toyokawa, H.; Yanagimoto, H.; Sugimoto, N.; Tsuji, K.; Araki, H.; Matsui, Y.; Imamura, A.; Kwon, A.-H.; et al. Clinicopathologic evaluation after resection for ductal adenocarcinoma of the pancreas: A retrospective, single-institution experience. Pancreas 2003, 26, 243–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Crocetti, E.; Buzzoni, C.; AIRTUM Working Group. The contribution of the Italian association of cancer registries (AIRTUM). Epidemiol. Prev. 2016, 40, 28–30. [Google Scholar]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Kleeff, J.; Reiser, C.; Hinz, U.; Bachmann, J.; Debus, J.; Jaeger, D.; Friess, H.; Büchler, M.W. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann. Surg. 2007, 245, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.K.; Odhav, B.; Bhoola, K.D. Immune responses in cancer. Pharmacol. Ther. 2003, 99, 113–132. [Google Scholar] [CrossRef]
- Ahmad, M.; Rees, R.C.; Ali, S.A. Escape from immunotherapy: Possible mechanisms that influence tumor regression/progression. Cancer Immunol. Immunother 2004, 53, 844–854. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Mapara, M.Y.; Sykes, M. Tolerance and cancer: Mechanisms of tumor evasion and strategies for breaking tolerance. J. Clin. Oncol. 2004, 22, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- von Bernstorff, W.; Voss, M.; Freichel, S.; Schmid, A.; Vogel, I.; Jöhnk, C.; Henne-Bruns, D.; Kremer, B.; Kalthoff, H. Systemic and Local Immunosuppression in Pancreatic Cancer Patients. Clin. Cancer Res 2001, 7, 925s–932s. [Google Scholar]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, H.; Takai, S.; Satoi, S.; Toyokawa, H.; Takahashi, K.; Terakawa, N.; Kwon, A.-H.; Kamiyama, Y. Impaired function of circulating dendritic cells in patients with pancreatic cancer. Clin. Immunol. 2005, 114, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; Fletcher, C.D.; O’Reilly, D.J.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. A comparison of inflammation-based prognostic scores in patients with cancer. A glasgow inflammation outcome study. Eur. J. Cancer 2011, 47, 2633–2641. [Google Scholar] [CrossRef]
- Jamieson, N.B.; Glen, P.; McMillan, D.C.; McKay, C.J.; Foulis, A.K.; Carter, R.; Imrie, C.W. Systemic inflammatory response predicts outcome in patients undergoing resection for ductal adenocarcinoma head of pancreas. Br. J. Cancer 2005, 92, 21–23. [Google Scholar] [CrossRef]
- Stevens, L.; Pathak, S.; Nunes, Q.M.; Pandanaboyana, S.; Macutkiewicz, C.; Smart, N.; Smith, A.M. Prognostic significance of pre-operative C-reactive protein and the Neutrophil–Lymphocyte ratio in resectable pancreatic cancer: A systematic review. HPB 2015, 17, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Shiba, H.; Sakamoto, T.; Horiuchi, T.; Haruki, K.; Fujiwara, Y.; Futagawa, Y.; Ohashi, T.; Yanaga, K. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery 2015, 158, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tao, L.; Lu, M.; Xiu, D. Prognostic role of platelet to lymphocyte ratio in pancreatic cancers: A meta-analysis Including 3028 patients. Medicine 2018, 97, e9616. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Sideras, K.; Aziz, N.A.; Mauff, K.; Haen, R.; Roos, D.; Saida, L.; Suker, M.; van der Harst, E.; Mieog, J.S.; et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: A retrospective multicenter cohort study. Ann. Surg. 2019, 270, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sideras, K.; Braat, H.; Kwekkeboom, J.; van Eijck, C.H.; Peppelenbosch, M.P.; Sleijfer, S.; Bruno, M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat. Rev. 2014, 40, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Fogar, P.; Sperti, C.; Basso, D.; Sanzari, M.C.; Greco, E.; Davoli, C.; Navaglia, F.; Zambon, C.-F.; Pasquali, C.; Venza, E.; et al. Decreased Total Lymphocyte Counts in Pancreatic Cancer: An Index of Adverse Outcome. Pancreas 2006, 32, 22–28. [Google Scholar] [CrossRef]
- Pointer, D.T.; Roife, D.; Powers, B.D.; Murimwa, G.; Elessawy, S.; Thompson, Z.J.; Schell, M.J.; Hodul, P.J.; Pimiento, J.M.; Fleming, J.B.; et al. Neutrophil to lymphocyte ratio, not platelet to lymphocyte or lymphocyte to monocyte ratio, is predictive of patient survival after resection of early-stage pancreatic ductal adenocarcinoma. BMC Cancer 2020, 20, 750. [Google Scholar] [CrossRef]
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013, 31, 259–283. [Google Scholar] [CrossRef]
- MacIver, N.J.; Rathmell, J.C. Editorial overview: Metabolism of T cells: Integrating nutrients, signals, and cell fate. Curr. Opin. Immunol. 2017, 46, viii. [Google Scholar] [CrossRef][Green Version]
- Bhalla, M.; Aldakkak, M.; Kulkarni, N.M.; O’Connor, S.D.; Griffin, M.O.; Christians, K.K.; Evans, D.B.; Tsai, S.; Tolat, P.P. Characterizing indeterminate liver lesions in patients with localized pancreatic cancer at the time of diagnosis. Abdom. Radiol. 2018, 43, 351–363. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, T.; Ma, J.; Duan, W.; Xu, Q.; Li, X.; Han, L.; Li, W.; Wang, Z.; Zhang, D.; et al. Hypoxia-inducible factor-1α mediates hyperglycemia-induced pancreatic cancer glycolysis. ACAMC 2019, 19, 1503–1512. [Google Scholar] [CrossRef]
- Aquilani, R.; Brugnatelli, S.; Dossena, M.; Maestri, R.; Delfanti, S.; Buonocore, D.; Boschi, F.; Simeti, E.; Condino, A.M.; Verri, M. Oxaliplatin-fluoropyrimidine combination (XELOX) therapy does not affect plasma amino acid levels and plasma markers of oxidative stress in colorectal cancer surgery patients: A pilot study. Nutrients 2019, 11, 2667. [Google Scholar] [CrossRef]
- Hong, X.; Cui, B.; Wang, M.; Yang, Z.; Wang, L.; Xu, Q. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J. Exp. Med. 2015, 236, 297–304. [Google Scholar] [CrossRef]
- Zhong, J.-H.; Huang, D.-H.; Chen, Z.-Y. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 75381–75388. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Šeruga, B.; Ocaña, A.; Tannock, I.F.; Amir, E. prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef]
- Ritz, E. Intestinal-renal syndrome: Mirage or reality? Blood Purif. 2011, 31, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.W.; Grosenbach, D.W.; Aarts, W.M.; Poole, D.J.; Schlom, J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin. Cancer Res. 2003, 9, 1837–1849. [Google Scholar] [PubMed]
- Ikeda, H.; Chamoto, K.; Tsuji, T.; Suzuki, Y.; Wakita, D.; Takeshima, T.; Nishimura, T. The Critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004, 95, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chess, L. An integrated view of suppressor T cell subsets in immunoregulation. J. Clin. Investig. 2004, 114, 1198–1208. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
- Saucillo, D.C.; Gerriets, V.A.; Sheng, J.; Rathmell, J.C.; MacIver, N.J. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 2014, 192, 136–144. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef]
- Elmadfa, I.; Meyer, A.L. The role of the status of selected micronutrients in shaping the immune function. EMIDDT 2019, 19, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Ricker, M.A.; Haas, W.C. Anti-inflammatory diet in clinical practice: A review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
| Variable | n° Patients |
|---|---|
| Median Age years | |
| <65 | 7 |
| 66 ≤ x ≤ 79 | 30 |
| >80 | 6 |
| Sex | |
| Female | 14 |
| Male | 28 |
| Body mass index (kg/m2) | |
| <18.49 | 7 |
| 18.5 ≤ x ≤ 24.99 | 26 |
| 25 ≤ x ≤ 29.99 | 5 |
| >30 | 3 |
| Absolute average weight (kg) | 61.83 ± 14.4 |
| Stages of Performance Status World Health Organization | |
| 0 | 28 |
| 1 | 11 |
| 2 | 3 |
| 3 | 0 |
| Previous surgery | |
| Yes | 14 |
| No | 28 |
| Previous adjuvant/neoadjuvant chemotherapy | |
| Yes | 5/11 |
| No | 27 |
| First-line chemotherapy | |
| Abraxane-Gemcitabine | 32 |
| Gemcitabine | 3 |
| FOLFOX6 | 5 |
| FOLFIRINOX | 2 |
| Chemotolerance | |
| Yes | 28 |
| No | 14 |
| Reason for discontinuation of therapy | |
| progressive disease | 19 |
| unacceptable toxicity without—progressive disease | 9 |
| other | 14 |
| Second-line chemotherapy | |
| Yes | 26 |
| No | 16 |
| Patient clinical outcomes | |
| Response to first-line chemotherapy | |
| Complete response (CR) | 0 |
| Partial response (PR) | 18 |
| Stable disease (SD) | 5 |
| Progressive disease (PD) | 19 |
| Progression-free survival | 4 |
| Overall survival | 9.5 |
| Variable | Mean ± SD | Min–Max | |
|---|---|---|---|
| Alkaline phosphatase | (nv 40–150 mU/mL) | 203.60 ± 167.89 | 47–800 |
| Alanine transaminase | (nv 11–34 mU/mL) | 92.41 ± 145.65 | 9–716 |
| Amylase total | (nv 25–125 mU/mL) | 92.43 ± 117.15 | 23–480 |
| Aspartate transaminase | (nv 40–150 mU/mL) | 51.82 ± 68.15 | 12–359 |
| Bilirubin total | (nv 0.2–1.1 mg/dL) | 4.65 ± 9.86 | 0.28–55 |
| Calcium | (nv 8.6–10.3 mg/dL) | 9.40 ± 0.51 | 8.5–10.5 |
| Creatinine | (nv 0.55–1.02 mg/dL) | 0.69 ± 0.14 | 0.39–0.93 |
| Estimated glomerular filtration rate | (mL/min/1.73 m2) | 91.87 ± 12.12 | 58.47–112.47 |
| Gamma glutamyl transpeptidase | (nv 11–53 mU/mL) | 349.58 ± 497.93 | 9–2111 |
| Glucose | (nv 76–110 mg/dL) | 134.79 ± 53.33 | 52–297 |
| Lactate dehydrogenase | (nv 125–220 mU/mL) | 224.50 ± 51.06 | 166–324 |
| Potassium | (nv 3.50–5.30 mEq/L) | 3.79 ± 0.45 | 2.66–4.66 |
| Sodium | (nv 135–153 mEq/L) | 139.17 ± 3.39 | 132–144 |
| Urea | (nv 10–50 mg/dL) | 30.83 ± 7.12 | 13–42 |
| Hemoglobin | (nv 11.7–15.5 g/dL) | 12.51 ± 1.59 | 7.7–15.6 |
| Erythrocytes | (nv 3.80–5.20 × 106/µL) | 4.23 ± 0.60 | 2.86–5.53 |
| Hematocrit | (nv 35–45%) | 37.17 ± 5.07 | 23.1–46.2 |
| Mean corpuscular volume | (nv 82–98 fl) | 88.16 ± 7.03 | 63.4–99.4 |
| Mean cell hemoglobin | (nv 27–32 pg) | 29.78 ± 3.30 | 20.5–41.3 |
| Mean cell hemoglobin concentration | (nv 32–37 g/dL) | 33.76 ± 2.50 | 30.1–47.8 |
| Red Cell Distribution Width | (nv 11.6–16%) | 14.86 ± 1.53 | 12.9–20.3 |
| White blood cells | (nv 4–10 × 103/µL) | 6.71 ± 2.26 | 2.2–13.9 |
| Neutrophils | (nv 2–8 × 103/µL) | 4.60 ± 1.93 | 1–11.1 |
| Neutrophils% | (%) | 67.86 ± 9.45 | 47.2–84.9 |
| Lymphocytes | (nv 1.5–47 × 103/µL) | 1.39 ± 0.58 | 0.4–3.4 |
| Lymphocytes% | (%) | 22.16 ± 8.99 | 6.8–45.7 |
| Neutrophils/Lymphocytes ratio | 3.96 ± 2.74 | 0.98–12.33 | |
| Monocytes | (nv 0.1–1 × 103/µL) | 0.54 ± 0.30 | 0.05–1.7 |
| Monocytes% | (%) | 8.07 ± 3.45 | 0.89–19.52 |
| Eosinophils | (nv 0.1–0.5 × 103/µL) | 0.13 ± 0.11 | 0–0.4 |
| Eosinophils% | (%) | 1.96 ± 1.54 | 0–5.70 |
| Basophils | (nv 0–0.2 × 103/µL) | 0.04 ± 0.04 | 0–0.12 |
| Basophils% | (%) | 0.49 ± 0.43 | 0–2.19 |
| Platelets | (nv 150–450 × 103/µL) | 239.46 ± 90.52 | 81–508 |
| Prothrombin | (nv 70–120%) | 94.40 ± 21.88 | 18–130 |
| International normalized ratio | (nv 0.90–120) | 1.13 ± 0.57 | 0.87–3.77 |
| Carbohydrate antigen 19-9 | (<37 IU/mL) | 15,757.86 ± 35,367.33 | 2.5–140,000 |
| Carcinoembryonic Antigen | (no smoking:<2.5; smoking <5 ng/mL) | 60.48 ± 216.66 | 1–1095 |
| n° of tolerated chemotherapy cycles | 5.73 ± 3.32 | 1–18 |
| (a) | |||||||
| Lymphocytes ≥ 1500 | Lymphocytes < 1500 | p | |||||
| Neutrophils (N × 1000 × mm−3) | 5.00 ± 1.88 | 4.29 ± 1.95 | 0.14 | ||||
| Neutrophils% | 64.68 ± 7.05 | 70.32 ± 10.45 | 0.037 | ||||
| Lymphocytes (N × 1000 × mm−3) | 1.90 ± 0.45 | 0.99 ± 0.28 | <0.0001 | ||||
| Lymphocytes% | 25.82 ± 7.85 | 19.34 ± 8.96 | 0.017 | ||||
| Neutrophils/Lymphocytes | 2.80 ± 1.44 | 4.86 ± 3.17 | 0.017 | ||||
| Chemotherapy cycles | 5.39 ± 3.16 | 6.00 ± 3.49 | 0.53 | ||||
| (b) | |||||||
| Lymphocytes ≥ 22% | Lymphocytes < 22% | p | |||||
| Neutrophils (N × 1000 × mm−3) | 3.58 ± 1.40 | 5.47 ± 1.91 | 0.006 | ||||
| Neutrophils% | 60.81 ± 6.83 | 73.91 ± 6.85 | <0.0001 | ||||
| Lymphocytes (N × 1000 × mm−3) | 1.74 ± 0.61 | 1.09 ± 0.36 | 0.00038 | ||||
| Lymphocytes% | 30.02 ± 5.89 | 15.43 ± 4.59 | <0.0001 | ||||
| Neutrophils/Lymphocytes | 2.06 ± 0.57 | 5.59 ± 2.81 | <0.0001 | ||||
| Chemotherapy cycles | 6.78 ± 4.15 | 4.86 ± 2.37 | 0.15 | ||||
| (c) | |||||||
| Lymphocytes ≥ 29.7% | Lymphocytes < 29.7% | p | |||||
| Neutrophils (N × 1000 × mm−3) | 3.00 ± 1.50 | 5.15 ± 1.76 | 0.003 | ||||
| Neutrophils% | 58.19 ± 7.46 | 71.20 ± 7.65 | 0.00032 | ||||
| Lymphocytes (N × 1000 × mm−3) | 1.78 ± 0.78 | 1.25 ± 0.43 | 0.038 | ||||
| Lymphocytes% | 34.03 ± 4.82 | 18.07 ± 5.90 | <0.0001 | ||||
| Neutrophils/Lymphocytes | 1.65 ± 0.36 | 4.76 ± 2.75 | <0.0001 | ||||
| Chemotherapy cycles | 9.20 ± 3.91 | 4.55 ± 2.26 | 0.0005 | ||||
| (d) | |||||||
| PR 18 pts | SD 5 pts | DP 19 pts | p | ||||
| Neutrophils (N × 1000 × mm−3) | 4.48 ± 1.25 | 3.79 ± 1.5 | 4.9 ± 1.0 | 0.35 | |||
| Neutrophils% | 66.3 ± 4.5 | 68.7 ± 2.9 | 68.8 ± 3.7 | 0.49 | |||
| Lymphocytes (N × 1000 × mm−3) | 1.40 ± 0.51 | 1.36 ± 0.46 | 1.35 ± 0.56 | 0.53 | |||
| Lymphocytes% | 22.17 ± 2.10 ^ | 27.4 ± 3.50 * | 19.68 ± 1.63 | * p < 0.001 vs. PR, DP | |||
| ^ p < 0.02 vs. DP | |||||||
| Neutrophils/Lymphocytes | 3.20 ± 1.68 | 2.81 ± 0.85 ^,° | 3.62 ± 1.998 | ^ p < 0.02 vs. DP | |||
| ° p < 0.05 vs. PR | |||||||
| Chemotherapy cycles | 5.7 ± 2.8 | 8.95 ± 3.2 * | 4.78 ± 1.4 | * p < 0.001 vs. PR, DP | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquilani, R.; Brugnatelli, S.; Maestri, R.; Boschi, F.; Filippi, B.; Perrone, L.; Barbieri, A.; Buonocore, D.; Dossena, M.; Verri, M. Peripheral Blood Lymphocyte Percentage May Predict Chemotolerance and Survival in Patients with Advanced Pancreatic Cancer. Association between Adaptive Immunity and Nutritional State. Curr. Oncol. 2021, 28, 3280-3296. https://doi.org/10.3390/curroncol28050285
Aquilani R, Brugnatelli S, Maestri R, Boschi F, Filippi B, Perrone L, Barbieri A, Buonocore D, Dossena M, Verri M. Peripheral Blood Lymphocyte Percentage May Predict Chemotolerance and Survival in Patients with Advanced Pancreatic Cancer. Association between Adaptive Immunity and Nutritional State. Current Oncology. 2021; 28(5):3280-3296. https://doi.org/10.3390/curroncol28050285
Chicago/Turabian StyleAquilani, Roberto, Silvia Brugnatelli, Roberto Maestri, Federica Boschi, Beatrice Filippi, Lorenzo Perrone, Annalisa Barbieri, Daniela Buonocore, Maurizia Dossena, and Manuela Verri. 2021. "Peripheral Blood Lymphocyte Percentage May Predict Chemotolerance and Survival in Patients with Advanced Pancreatic Cancer. Association between Adaptive Immunity and Nutritional State" Current Oncology 28, no. 5: 3280-3296. https://doi.org/10.3390/curroncol28050285
APA StyleAquilani, R., Brugnatelli, S., Maestri, R., Boschi, F., Filippi, B., Perrone, L., Barbieri, A., Buonocore, D., Dossena, M., & Verri, M. (2021). Peripheral Blood Lymphocyte Percentage May Predict Chemotolerance and Survival in Patients with Advanced Pancreatic Cancer. Association between Adaptive Immunity and Nutritional State. Current Oncology, 28(5), 3280-3296. https://doi.org/10.3390/curroncol28050285






