Abstract
Chemotherapy-associated steatosis is poorly understood in the context of colorectal cancer. In this study, Stage II–III colorectal cancer patients were retrospectively selected to evaluate the frequency of chemotherapy-associated steatosis and to determine whether patients on statins throughout adjuvant chemotherapy develop chemotherapy-associated steatosis at a lower frequency. Baseline and incident steatosis for up to one year from chemotherapy start date was assessed based on radiology. Of 269 patients, 76 (28.3%) had steatosis at baseline. Of the remaining 193 cases, patients receiving adjuvant chemotherapy (n = 135) had 1.57 (95% confidence interval [CI], 0.89 to 2.79) times the adjusted risk of developing steatosis compared to patients not receiving chemotherapy (n = 58). Among patients who underwent chemotherapy, those using statins for pre-existing hyperlipidemia (n = 37) had 0.71 (95% CI, 0.10 to 2.75) times the risk of developing steatosis compared to patients who were not prevalent users of statins (n = 98). Chemotherapeutic treatment of Stage II–III colorectal cancer appears to be consistent with a moderately increased risk of steatosis, although larger studies are necessary to assess the significance of this observation. Prospective trials should be considered to further explore the potential for protective use of statins in this curative patient population.
1. Introduction
Hepatic steatosis is characterized by the infiltration and accumulation of triglyceride within the liver parenchyma [1]. Steatosis is the most common liver disease in Canada, affecting approximately 20% of Canadians without obvious risk factors such as alcohol abuse [2]. Progression of simple steatosis results in steatohepatitis, in which inflammation, hepatocyte damage and fibrosis ensue. More ominous complications of the spectrum of fatty liver diseases include cirrhosis and hepatocellular carcinoma [3,4]. While fatty liver is commonly associated with excessive alcohol consumption, hepatic steatosis has various other etiologies that implicate a decreased capacity of the liver to regulate lipid storage and mobilization within the body. Metabolic syndrome, a collection of conditions including abdominal obesity, insulin resistance, hypertension and hyperlipidemia, is the strongest risk factor for non-alcoholic fatty liver disease (NAFLD) [5]. Steatosis has also been attributed to hepatotoxicity induced by drugs, such as anti-tumour agents [6]. Fatty liver resulting from chemotherapy is known as chemotherapy-induced acute steatohepatitis (CASH) [7]. Both metabolic syndrome- and drug-induced fatty liver are thought to result from a pathological decrease in beta-oxidation due to the toxic accumulation of reactive oxygen species (ROS) in hepatocytes [5,7].
There is currently no approved pharmacologic treatment for hepatic steatosis, whether metabolic syndrome- or drug-induced. Among various candidate drugs under investigation, statins have been considered as a potential therapeutic option, and were recently shown to effectively reduce the risk of NAFLD development in a large population-based study [8].
Colorectal cancer (CRC) consistently ranks as one of the leading causes of cancer-related deaths in Canada [9]. The current standard guidelines for adjuvant chemotherapy in stage II and III CRC consists of the combined use of fluorouracil and oxaliplatin (FOLFOX), capecitabine and oxaliplatin (XELOX) or fluoropyridine alone [10,11,12]. The use of 5-fluorouracil (5-FU) has previously been associated with the development of steatosis in CRC patients [13,14]. However, the effects of the current chemotherapy regimen on the incidence of steatosis in the curative CRC patient population remain largely unexplored.
Medical oncologists at St. Michael’s Hospital in Toronto, Canada, anecdotally observed that CRC patients receiving adjuvant chemotherapy appeared to develop fatty liver at a higher rate than expected when seen in follow-up, based on imaging. In the absence of studies exploring the role of statin therapy in chemotherapy-associated steatosis (CAS), we speculated that the protective benefit of statins in fatty liver at large may translate to prevention of CAS among patients who are receiving statins at the time of their chemotherapy.
Thus, in this study, we sought to describe the frequency of CAS in stage II–III CRC patients and to determine the incidence of CAS in patients who were prevalent users of statins during their adjuvant chemotherapy.
2. Materials and Methods
2.1. Patients and Data Collection
We investigated the potential association between adjuvant chemotherapy and the development of steatosis among patients with stage II–III CRC. Following initial conception of the study, the authors retrospectively identified patients who were treated and followed up by a medical oncologist at St. Michael’s Hospital, Toronto, Canada, between 1 January 2006 and 1 January 2017. The electronic medical records of these patients were reviewed. Patient characteristics including sex, age at diagnosis, and Body Mass Index (BMI), as well as baseline comorbidities including type 2 diabetes mellitus, hyperlipidemia, and hypertension, were collected. Other variables included clinical data pertaining to their cancer and variables that may influence steatosis development, such as tumour location, whether primary surgical resection was performed, pelvic radiation status, steroid use, statin use, alcohol consumption, and duration and type of adjuvant chemotherapy received. This study was approved by the St. Michael’s Hospital Research Ethics Board (approval number: 18-166).
2.2. Determination of CAS Status
CAS was determined through a review of radiology reports, and images were reviewed by a single radiologist to maximize inter-rater reliability. Imaging modalities included contrast-enhanced abdominal CT, abdominal ultrasound, and liver or abdominal MRI. Areas of focal fatty sparing of the liver adjacent to the gallbladder and porta hepatis, absolute value of liver density less than 40 HU or a density difference greater than 25 HU between the spleen and liver on contrast-enhanced CT, increased echogenicity of the liver, attenuation of the ultrasound wave, loss of definition of the diaphragm, and poor delineation of the intrahepatic architecture on ultrasound and signal drop of liver parenchyma on the T1 weighted out of phase imaging on MRI was considered fatty liver [15,16,17,18]. Positive findings on any of the modalities qualified as presence of fatty liver. Designation of fatty liver status was made regardless of the pattern or geography of lipid distribution. Liver biochemical and function tests were not recorded and thus could not be considered in the analysis.
2.3. Statistical Analysis
A log binomial regression model was used to calculate adjusted relative risks. Variables found to be associated with both the exposure and outcome and thus, probably confounders, were selected as covariates based on a review of relevant literature. Directed acyclic graphs were used to identify a minimally sufficient set of covariates to control potential confounding in the final adjusted model (Supplementary Figures S1 and S2). Covariates included sex, BMI, type 2 diabetes mellitus status, hyperlipidemia status, and steroid use in the analysis of the association between adjuvant chemotherapy exposure and steatosis outcome, while sex, BMI, type 2 diabetes mellitus status and hyperlipidemia status were adjusted for in the analysis of the association between statin use and steatosis outcome [5,19,20,21,22]. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
3. Results
Overall, 329 patients who were diagnosed with stage II–III CRC at St. Michael’s Hospital from 1 January 2006 to 1 January 2017 were assessed for eligibility. Out of the 269 patients deemed eligible for analysis, 76 (28.3%) had fatty liver at baseline imaging, prior to treatment with adjuvant chemotherapy. The remaining 193 patients who did not have fatty liver at baseline were further divided by whether they received curative chemotherapy (Figure 1).
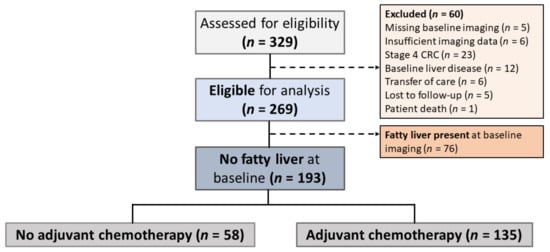
Figure 1.
Study Participant Selection. Stage II–III colorectal cancer (CRC) patients were screened for eligibility prior to data collection, and those in whom fatty liver was present at baseline were excluded from further analyses. The 187 patients who did not have signs of hepatic steatosis at baseline were further divided into two groups based on their receipt of adjuvant chemotherapy.
The characteristics of the two patient groups are outlined in Table 1. There was a greater proportion of males among patients who received adjuvant chemotherapy (81/135, 60.0%) compared to the no adjuvant chemotherapy group (24/58, 41.4%). The mean age of diagnosis was higher in the no adjuvant chemotherapy group (68.0 ± 14.1 vs. 59.9 ± 11.4). The proportion of patients whose BMI was ≥ 25 was comparable between the two groups. A higher proportion of individuals who did not receive chemotherapy had other risk factors for NAFLD present, including type 2 diabetes mellitus (19.0% vs. 13.3%), hypertension (51.7% vs. 38.5%), and hyperlipidemia (43.1% vs. 28.9%); therefore, statin use was also higher in this group (36.2% vs. 27.4%). A small number of patients in each group was using steroids, with a slightly greater proportion seen in the no adjuvant chemotherapy group (13.8% vs. 6.7%). More individuals in the adjuvant chemotherapy group received pelvic radiation (10.3% vs. 38.5%). Both groups exhibited similar rates of reported alcohol use.

Table 1.
Patient demographics.
We found that 52 of 135 patients (38.5%) who received adjuvant chemotherapy developed steatosis within one year of follow-up, compared to 14 of 58 patients (24.1%) who did not receive chemotherapy (Relative Risk [RR] 1.57, 95% confidence interval [CI] 0.89 to 2.79) after adjustment for sex, BMI, type 2 diabetes mellitus, hyperlipidemia, and steroid use. The point estimate is consistent with moderately increased risk of steatosis, but with wide confidence intervals (Table 2).

Table 2.
Association between adjuvant chemotherapy exposure and outcome of steatosis in patients with colorectal cancer.
We then examined the 135 patients who received adjuvant chemotherapy. Of these patients, 103 individuals were treated with an oxaliplatin-containing regimen, which is FOLFOX. In our study cohort, we did not observe a significant difference in the risk of developing steatosis when comparing patients receiving oxaliplatin-containing chemotherapy compared to those on a 5-FU based regimen (RR 0.64, 95% CI 0.30 to 1.38) after adjusting for sex, BMI, type 2 diabetes mellitus, hyperlipidemia, and steroid use (Table 3). A comparison of the effects of oral capecitabine versus intravenous 5-FU (Table 4) did not suggest the existence of a significant association between route of chemotherapy administration and risk of steatosis in the same group of patients (RR 1.56, 95% CI 0.65 to 3.73).

Table 3.
Oxaliplatin compared to no oxaliplatin regimens and development of steatosis.

Table 4.
Capecitabine compared to 5-FU-containing intravenous regimens and development of steatosis.
The demographics of 135 patients who were treated with chemotherapy were summarized based on statin administration status, shown in Table 5. Patients were considered to have positive statin administration if they were found to be prevalent users of a statin at the time of chemotherapy initiation. There was a greater proportion of males in the group of patients on statin therapy (70.3% vs. 56.1%). This group also exhibited a mildly higher proportion of overweight or obese individuals compared to the no statin group (54.0% vs. 43.9%). Notably, all patients who were administered statins had pre-existing hyperlipidemia, and a higher proportion of patients in this group had hypertension (73.0% vs. 25.5%).

Table 5.
Statin use in patients who received adjuvant chemotherapy for their colorectal cancer.
Among patients who were on statins at the time of their adjuvant chemotherapy, 11 of 37 (29.7%) patients developed steatosis within one year; however, 41 of 98 (41.8%) patients who did not receive statin treatment developed steatosis. The relative risk of developing steatosis was not significantly different regardless of statin therapy status at the time of adjuvant chemotherapy (RR 0.45, 95% CI 0.10 to 2.75) after adjusting for sex, BMI, type 2 diabetes mellitus, and hyperlipidemia (Table 6).

Table 6.
Steatosis in patients who received adjuvant chemotherapy for their colorectal cancer based on statin administration status.
4. Discussion
In the present study, the adjusted relative risk of adjuvant chemotherapy reflected a moderately increased risk of steatosis, although the confidence intervals were wide. According to existing literature, the frequency of hepatic steatosis development in CRC patients receiving 5-FU ranges from 30–47% [14,23,24]. Miyake et al. also retrospectively reported that 34.9% of CRC patients treated with 5-FU developed hepatic steatosis, a rate significantly higher than that seen in the no treatment group [13]. Some researchers have suggested that hepatic steatosis induced by 5-FU-containing therapy is reversible, although treatment regimens used in these studies consisted of additional agents such as levamisole and interferon alpha-2a [23,24]. In contrast, Vigano and colleagues examined the impact of oxaliplatin- and irinotecan-based chemotherapy in metastatic CRC patients and reported that while other types of chemotherapy-induced liver injury such as sinusoidal dilatation and nodular regenerative hyperplasia regressed in the majority of cases, steatosis and steatohepatitis persisted [25]. Thus, the extent of chemotherapy-associated steatosis and steatohepatitis reversibility and the implications of these findings on patients treated using the current Canadian chemotherapy guidelines need to be further elucidated.
The use of oxaliplatin as part of the chemotherapy regimen did not appear to affect the risk of steatosis in our analysis, barring perhaps a non-significant, mildly lower rate of steatosis development in those receiving oxaliplatin compared to those on treatment regimens not containing oxaliplatin. Despite its frequent association with liver injury, oxaliplatin is classically known to damage the liver via sinusoidal dilation and is thought to have a limited role in inducing steatohepatitis [26]. However, there has yet to be a clear consensus regarding the effect of oxaliplatin on steatosis, especially in light of findings such as that of Lu and colleagues, who showed that oxaliplatin aggravates existing steatosis in a non-alcoholic fatty liver disease mouse model [27]. More widely accepted is the association of CASH with irinotecan-based chemotherapies, which were not explored in the present study but would be highly pertinent for future investigations [28].
Since the FDA approval of capecitabine in 2001 as a chemotherapeutic agent, the oral route of 5-FU delivery became a mainstay of colorectal cancer treatment, alongside intravenous infusion. While liver enzymes play a central role in the three-step enzymatic cascade of capecitabine activation to its active metabolite, hepatotoxicity is considered a relatively rare side effect of capecitabine due to its selective activation within the tumor tissue [29,30]. There are however reports of capecitabine-induced liver injury, including cases of acute liver injury and hepatic steatosis [31,32]. In our study, the adjusted risk of steatosis development was 1.56 when compared with intravenous regimens of 5-FU administration, but this point estimate was not statistically significant.
Considering the low survival rates of CRC patients in the absence of treatment, adverse effects of CRC chemotherapy have been regarded as inevitable consequences of a necessary life-saving measure. Chemotherapy-associated steatosis is pathologically indistinguishable from NAFLD, which has a benign onset as simple hepatic steatosis, but can asymptomatically progress to steatohepatitis [7]. NAFLD is associated with an increased risk of cirrhosis, and hepatocellular carcinomas that arise in NAFLD patients are associated with a higher morbidity than those with other etiologies [3,33].
Despite these risks, current treatment for hepatic steatosis is limited to changes in lifestyle to mitigate cardiovascular risk factors [34]. The development of pharmacological therapeutic agents is in progress, with promising candidates including obeticholic acid, firsocostat, and elafibranor [35]. Statins have been implicated as a potential therapeutic agent for steatosis, but their efficacy has been somewhat inconclusive according to existing literature. Statins competitively inhibit HMG-CoA reductase, a key enzyme in cholesterol biosynthesis, to exert potent LDL-cholesterol lowering effects. Hyperlipidemia is highly prevalent in the population commonly affected by NAFLD, thus patients with hepatic steatosis tend to be aggressively treated using statins [36,37,38]. There have been indications of histologically determined benefit from statin use in animal models of NASH, as well as post hoc analyses of randomized controlled trials, which revealed that atorvastatin may alleviate NAFLD [39]. Other studies showed a lack of a statistically significant difference between statin users and the control group in this regard, highlighting the need for randomized controlled trials [40,41]. Nevertheless, the recent population-based study by Lee and colleagues demonstrated a significant reduction in the development of NAFLD in patients who received statin therapy, supporting the potential for statins to confer protective benefits in preventing steatosis [8]. In our study, all patients receiving statins had pre-existing dyslipidemia. We have shown that patients who were administered statins at the time of their chemotherapy may be at a lower risk of developing CAS than those treated with chemotherapy alone, although larger studies are necessary to validate the statistical significance of these results. To our knowledge, this is the first study to examine the potential protective benefit of statins in the context of hepatic steatosis driven by chemotherapy. Given that hyperlipidemia is strongly associated with hepatic steatosis, it is possible that the effect of statin treatment was underestimated in our patient cohort.
In this study, the primary mode of determining steatosis status in patients included a review of the medical records and the abdominal images (CT, ultrasound and MRI) by a single radiologist. MRI exhibits the highest sensitivity for detecting hepatic lipid infiltration and can detect as little as 5% steatosis in the liver at a sensitivity of 76.7–90.0% and a specificity of 87.1–91% [42]. Ultrasound can detect ≥20% steatosis with a sensitivity of 79.7% and specificity of 86.2% [42]. Contrast-enhanced CT has a detection rate comparable to that of ultrasound; its sensitivity ranges from 54% to 93% and specificity from 87% to 93% [42]. While some patients underwent ultrasound and MRI in addition to CT, since the primary goal of follow-up imaging was to determine whether the patient developed cancer metastasis, contrast-enhanced CT was the most regularly performed imaging modality among the largest number of patients in our study. Since not every patient’s steatosis status was verified using a second imaging modality in addition to a CT, there may have been inconsistencies in the reported steatosis status; this is a major limitation of the present study. However, there have been other studies that used contrast-enhanced CT as the sole imaging modality to assess hepatic steatosis [43].
Another limitation of this study is the relatively small sample size, particularly for the cohort of patients receiving statins. The sample size of 37 in the statin group meant that the power of the statistical analysis was smaller than the widely accepted threshold of 80%. This study was also limited in that many potentially useful clinical and demographic data such as duration of statin administration and lifestyle factors contributory to steatosis could not be collected due to the retrospective nature of the investigation. Other data that may have further enriched our findings include trending of lipid profiles as well as liver biochemical and function tests. However, while elevated levels of liver biochemical tests often correlate with a diagnosis of fatty liver, a high proportion of patients with NAFLD exhibit normal liver profiles, making these tests inappropriate diagnostic markers that may not have drastically impacted the findings presented in our study [44,45]. Finally, reporting bias inherent to this retrospective study may have affected our results in the process of analyzing self-reported variables such as alcohol consumption and family history of hyperlipidemia.
5. Conclusions
This study sought to examine hepatic steatosis, an increasingly recognized health concern worldwide. Since drug-induced hepatotoxicity was described by Grieco et al. in 2005, there has been some research on the association between steatosis and anti-tumour drugs, although the precise impacts of CRC chemotherapy have largely been unexplored. Furthermore, there is currently a lack of medical treatment for any population affected by steatosis, regardless of etiology, although a recently published population-based study suggests that statins may confer protective benefits against the development of steatosis. Here, we observed that there is a trend towards a higher rate of CAS development within one year of follow-up among stage II–III CRC patients who received chemotherapy compared to the no treatment group. Moreover, there is evidence to believe that larger, higher power studies should be conducted to further investigate the protective benefits of statins in reducing the risk of CAS, owing to the mild reduction in the adjusted relative risk of steatosis in statin users observed in the present study. These conclusions have critical implications on the quality of life and hepatic function of patients not only in the curative setting, but may also be applicable in the setting of treatment of metastatic disease, in particular in context of patients requiring liver resections for metastases in addition to indefinite metastatic treatment which may require up to 60 cycles of 5-FU-based chemotherapy.
Based on the results of our study, it is necessary to conduct prospective studies that involve a larger cohort of patients, who are controlled for comorbidities that may confound the association between CRC, statin use and the incidence of steatosis. An assessment of the safety and efficacy of statins in a randomized controlled cohort will allow for an accurate investigation into this phenomenon that contributes to a secondary health burden for the curative CRC patient population.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/curroncol28040265/s1, Figure S1: Directed Acyclic Graph (DAG) for the potential association between adjuvant chemotherapy and development of non-alcoholic fatty liver disease (NAFLD), Figure S2: Directed Acyclic Graph (DAG) for the potential association between statin administration and development of non-alcoholic fatty liver disease (NAFLD).
Author Contributions
M.C.M.L. designed the study and conducted data collection. J.J.K. analyzed and interpreted the data. P.A.V. acquired and interpreted radiology image data. R.D., A.D.T., H.S., N.B. and C.B.-M. contributed to the conception and design of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the St. Michael’s Hospital Research Ethics Board (approval number: 18-166).
Informed Consent Statement
Patient consent was waived due to retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient privacy.
Acknowledgments
We thank Ayesha Taqi and Aftab Malik for their generous help in the data collection process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haas, J.T.; Francque, S.; Staels, B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu. Rev. Physiol. 2016, 78, 181–205. [Google Scholar] [CrossRef]
- Canadian Liver Foundation 2017 [cited 2020 May 24]. Fatty Liver Disease. Available online: https://www.liver.ca/patients-caregivers/liver-diseases/fatty-liver-disease/ (accessed on 24 May 2020).
- Goldberg, D.; Ditah, I.C.; Saeian, K.; Lalehzari, M.; Aronsohn, A.; Gorospe, E.C.; Charlton, M. Changes in the Prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017, 152, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular carcinoma in the absence of cirrhosis in united states veterans is associated with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131.e1. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Grieco, A.; Forgione, A.; Miele, L.; Greco, A.V.; Gasbarrini, A.; Gasbarrini, G. Fatty liver and drugs. Eur. Rev. Med. Pharmacol. Sci. 2005, 9, 261–263. [Google Scholar]
- Meunier, L.; Larrey, D. Chemotherapy-associated steatohepatitis. Ann. Hepatol. 2020, 19, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Lee, H.W.; Lee, K.S.; Lee, H.S.; Park, J.Y. Effects of Statin Use on the Development and Progression of Nonalcoholic Fatty Liver Disease: A Nationwide Nested Case-Control Study. Am. J. Gastroenterol. 2021, 116, 116–124. [Google Scholar] [CrossRef]
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2017; Canadian Cancer Society: Toronto, ON, Canada, 2017; Available online: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2017-EN.pdf (accessed on 24 May 2020).
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin and adjuvant treatment in stage II and III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef]
- Twelves, C.; Scheithauer, W.; McKendrick, J.; Seitz, J.F.; Van Hazel, G.; Wong, A.; Diaz-Rubio, E.; Gilberg, F.; Cassidy, J. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: Final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann. Oncol. 2012, 23, 1190–1197. [Google Scholar] [CrossRef]
- Miyake, K.; Hayakawa, K.; Nishino, M.; Morimoto, T.; Mukaihara, S. Effects of oral 5-fluorouracil drugs on hepatic fat content in patients with colon cancer. Acad. Radiol. 2005, 12, 722–727. [Google Scholar] [CrossRef]
- Peppercorn, P.; Reznek, R.; Wilzon, P.; Slevin, M.L.; Gupta, R.K. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br. J. Cancer 1998, 77, 2008–2011. [Google Scholar] [CrossRef]
- Alpern, M.B.; Lawson, T.L.; Foley, W.D.; Perlman, S.J.; Reif, L.J.; Arevalos, E.; Rimm, A.A. Focal hepatic masses and fatty infiltration detected by enhanced dynamic CT. Radiology 1986, 158, 45–49. [Google Scholar] [CrossRef]
- Rofsky, N.M.; Fleishaker, H. CT and MRI of diffuse liver disease. Semin. Ultrasound CT MR 1995, 16, 16–33. [Google Scholar] [CrossRef]
- Joy, D.; Thava, V.R.; Scott, B.B. Diagnosis of fatty liver disease: Is biopsy necessary? Eur. J. Gastroenterol. Hepatol. 2003, 15, 539–543. [Google Scholar]
- Kreft, B.P.; Tanimoto, A.; Baba, Y.; Zhao, L.; Chen, J.; Middleton, M.S.; Compton, C.C.; Finn, J.P.; Stark, D.D. Diagnosis of fatty liver with MR imaging. J. Magn. Reson. Imaging 1992, 2, 463–471. [Google Scholar] [CrossRef]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef]
- Fan, R.; Wang, J.; Du, J. Association between body mass index and fatty liver risk: A dose-response analysis. Sci. Rep. 2018, 8, 15273. [Google Scholar] [CrossRef]
- Woods, C.P.; Hazlehurst, J.M.; Tomlinson, J.W. Glucocorticoids and non-alcoholic fatty liver disease. J. Steroid Biochem. Mol. Biol. 2015, 154, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22 (Suppl. 3), 1–203. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.; Fleming, T.; Macdonald, J.; Haller, D.G.; Laurie, J.A. Hepatic toxicity associated with fluorouracil plus levamisole adjuvant therapy. J. Clin. Oncol. 1993, 11, 2386–2390. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.; Edal, A.; Madsen, E.; Fenger, C.; Poulsen, M.R.; Petersen, O.F. Reversible hepatic steatosis in patients treated with interferon alfa-2A and 5-fluorouracil. Cancer 1995, 75, 2592–2596. [Google Scholar] [CrossRef]
- Vigano, L.; De Rosa, G.; Toso, C.; Andres, A.; Ferrero, A.; Roth, A.; Sperti, E.; Majno, P.; Rubbia-Brandt, L. Reversibility of chemotherapy-related liver injury. J. Hepatol. 2017, 67, 10–11. [Google Scholar] [CrossRef]
- Schumacher, J.D.; Guo, G.L. Mechanistic review of drug-induced steatohepatitis. Toxicol. Appl. Pharmacol. 2015, 289, 40–47. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Huang, X.; Wu, S.; Wei, J.; Yang, C. Oxaliplatin aggravates hepatic oxidative stress, inflammation and fibrosis in a non-alcoholic fatty liver disease mouse model. Int. J. Mol. Med. 2019, 43, 2398–2408. [Google Scholar] [CrossRef]
- Gangi, A.; Lu, S.C. Chemotherapy-associated liver injury in colorectal cancer. Therap. Adv. Gastroenterol. 2020, 13, 1756284820924194. [Google Scholar] [CrossRef]
- Miwa, M.; Ura, M.; Nishida, M.; Sawada, N.; Ishikawa, T.; Mori, K.; Shimma, N.; Umeda, I.; Ishitsuka, H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur. J. Cancer 1998, 34, 1274–1281. [Google Scholar] [CrossRef]
- Saif, M.W.; Katirtzoglou, N.A.; Syrigos, K.N. Capecitabine: An overview of the side effects and their management. Anticancer Drugs 2008, 19, 447–464. [Google Scholar] [CrossRef]
- Habib, M.B.; Hanafi, I.; Al Zoubi, M.; Bdeir, Z.; Yassin, M.A. Severe and Late Acute Liver Injury Induced by Capecitabine. Cureus 2021, 13, e12477. [Google Scholar] [PubMed]
- Chin, S.N.; Kim, T.K.; Siu, L.L. Hepatic steatosis secondary to capecitabine: A case report. J. Med. Case Rep. 2010, 4, 227. [Google Scholar] [CrossRef][Green Version]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S. HCC-NADFL Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N. NASH and NAFLD: Emerging drugs, therapeutic targets and translational and clinical challenges. Expert Opin. Investig. Drugs 2020, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.; Mantzoros, C. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Cobbe, S.; Ford, I.; Isles, C.G.; Lorimer, A.R.; MacFarlane, P.W.; McKillop, J.H.; Packard, C.J. Prevention of coronary heart diseases with pravastatin in men with hypercholesterolemia: West of Scotland coronary prevention study group. N. Engl. J. Med. 1995, 333, 1301–1307. [Google Scholar] [CrossRef]
- Doumas, M.; Imprialos, K.; Dimakopoulou, A.; Stavropoulos, K.; Binas, A.; Athyros, V.G. The role of statins in the management of nonalcoholic fatty liver disease. Curr. Pharm. Des. 2018, 24, 4587–4592. [Google Scholar] [CrossRef] [PubMed]
- Sigler, M.A.; Congdon, L.; Edwards, K.L. An evidence-based review of statin use in patients with nonalcoholic fatty liver disease. Clin. Med. Insights Gastroenterol. 2018, 11, 1179552218787502. [Google Scholar] [CrossRef]
- Pastori, D.; Polimeni, L.; Baratta, F.; Pani, A.; Del Ben, M.; Angelico, F. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig. Liver Dis. 2015, 47, 4–11. [Google Scholar] [CrossRef]
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.A.; Oliva, I.B.; Israel, G.M. Detection of hepatic steatosis on contrast-enhanced CT images: Diagnostic accuracy of identification of areas of presumed focal fatty sparing. AJR Am. J. Roentgenol. 2012, 199, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Obika, M.; Noguchi, H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp. Diabetes Res. 2012, 2012, 145754. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).