Discrimination of Cancer Stem Cell Markers ALDH1A1, BCL11B, BMI-1, and CD44 in Different Tissues of HNSCC Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histological Samples and Immuno-Staining
2.3. Statistical Analysis
3. Results
3.1. Study Cohort
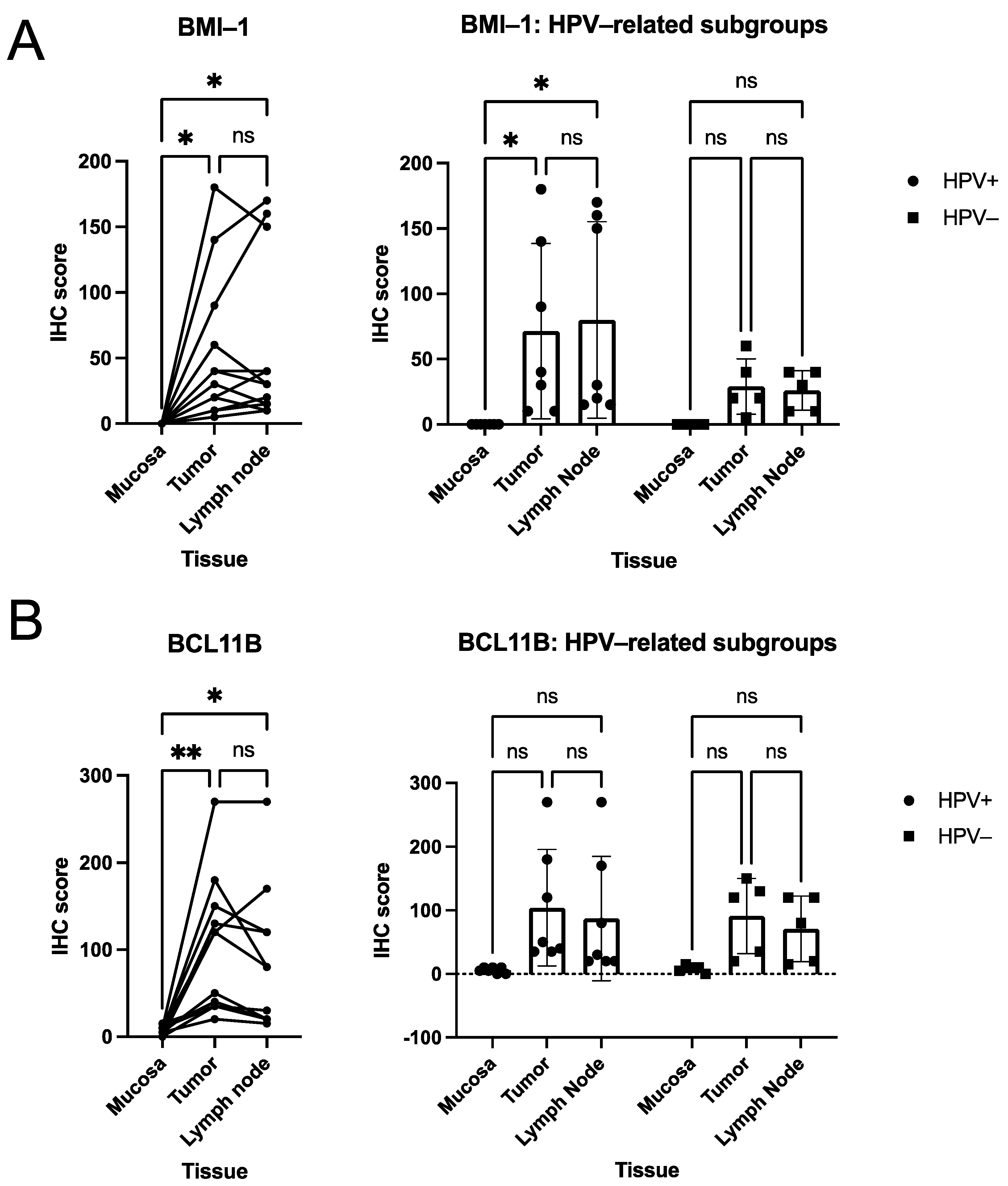
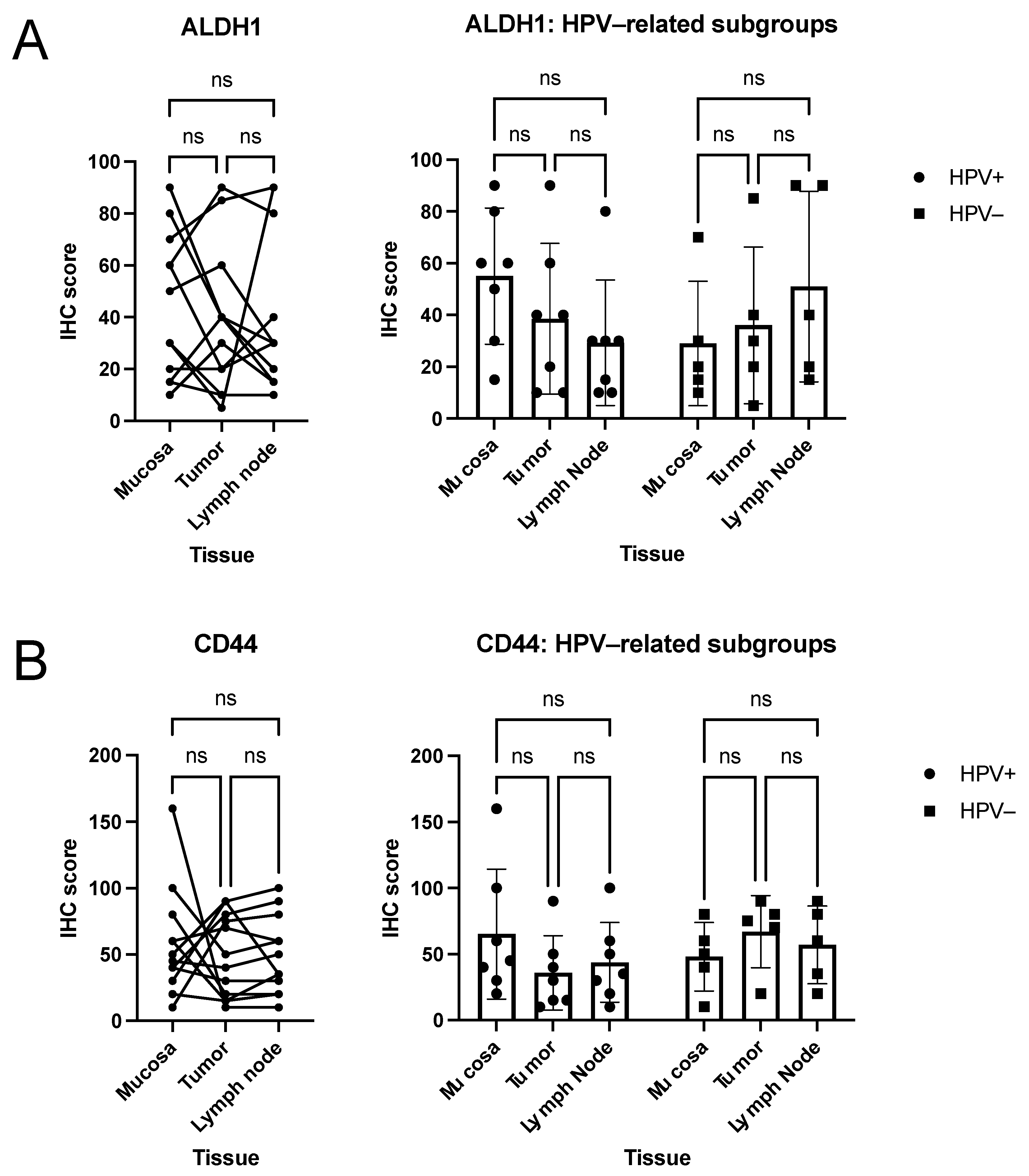
3.2. Quantitative Analysis of Immunohistochemical Scorings
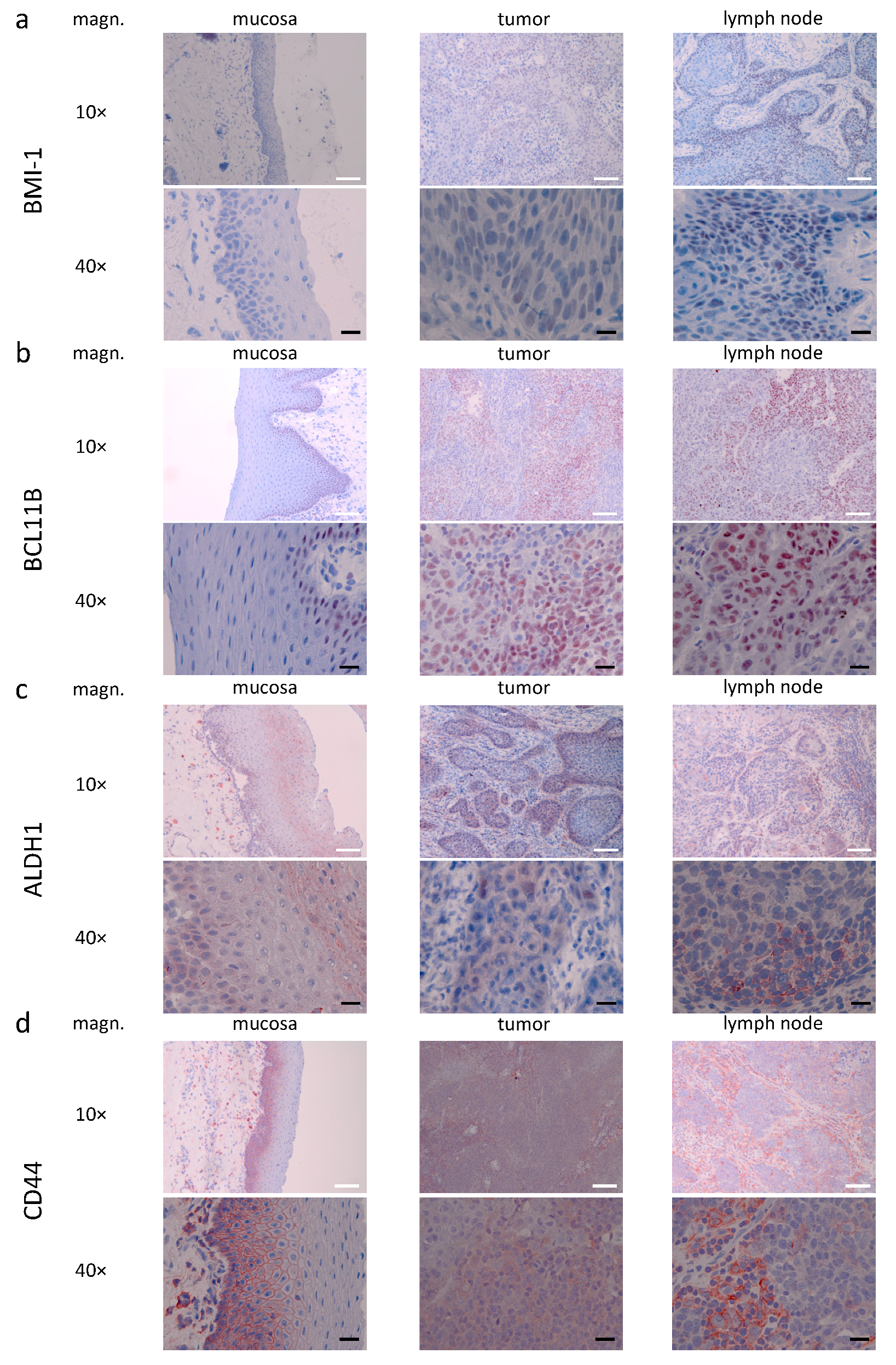
3.3. Descriptive (Microscopical) Findings in the Staining for Molecular Markers of Interest
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Spitz, M.R. Epidemiology and risk factors for head and neck cancer. Semin. Oncol. 1994, 21, 281–288. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Eggersmann, T.K.; Baumeister, P.; Kumbrink, J.; Mayr, D.; Schmoeckel, E.; Thaler, C.J.; Dannecker, C.; Jeschke, U.; Nagler, T.; Mahner, S.; et al. Oropharyngeal HPV Detection Techniques in HPV-associated Head and Neck Cancer Patients. Anticancer Res. 2020, 40, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Reuschenbach, M.; Tinhofer, I.; Wittekindt, C.; Wagner, S.; Klussmann, J.P. A systematic review of the HPV-attributable fraction of oropharyngeal squamous cell carcinomas in Germany. Cancer Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Hess, J. Predictive Factors for Outcome and Quality of Life in HPV-Positive and HPV-Negative HNSCC. Recent Results Cancer Res. Fortschr. Der Krebsforsch. Prog. Dans Les Rech. Sur Le Cancer 2017, 206, 233–242. [Google Scholar] [CrossRef]
- Keck, M.K.; Zuo, Z.; Khattri, A.; Stricker, T.P.; Brown, C.D.; Imanguli, M.; Rieke, D.; Endhardt, K.; Fang, P.; Bragelmann, J.; et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 870–881. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Huang, S.H.; Su, J.; Garden, A.S.; Sturgis, E.M.; Dahlstrom, K.; Lee, N.; Riaz, N.; Pei, X.; Koyfman, S.A.; et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol. 2016, 17, 440–451. [Google Scholar] [CrossRef]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. New Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Zhang, Z.; Filho, M.S.; Nor, J.E. The biology of head and neck cancer stem cells. Oral Oncol. 2012, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.M.; Jordan, C.T. The increasing complexity of the cancer stem cell paradigm. Science 2009, 324, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Curtarelli, R.B.; Goncalves, J.M.; Dos Santos, L.G.P.; Savi, M.G.; Nor, J.E.; Mezzomo, L.A.M.; Rodriguez Cordeiro, M.M. Expression of Cancer Stem Cell Biomarkers in Human Head and Neck Carcinomas: A Systematic Review. Stem Cell Rev. 2018, 14, 769–784. [Google Scholar] [CrossRef]
- Ganguli-Indra, G.; Wasylyk, C.; Liang, X.; Millon, R.; Leid, M.; Wasylyk, B.; Abecassis, J.; Indra, A.K. CTIP2 expression in human head and neck squamous cell carcinoma is linked to poorly differentiated tumor status. PLoS ONE 2009, 4, e5367. [Google Scholar] [CrossRef]
- Major, A.G.; Pitty, L.P.; Farah, C.S. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013, 2013, 319489. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.W.; Palle, K. Aldehyde dehydrogenases in cancer stem cells: Potential as therapeutic targets. Ann. Transl. Med. 2016, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Isobe, T.; Yamamoto, G.; Tanaka, Y.; Katakura, A.; Tachikawa, T.; Nomura, T. Correlation of BMI1 and ZEB1 expression with epithelial–mesenchymal transition in gingiva squamous cell carcinoma. J. Oral Maxillofac. Surg. Med. Pathol. 2016, 28, 462–469. [Google Scholar] [CrossRef]
- Orian-Rousseau, V. CD44 Acts as a Signaling Platform Controlling Tumor Progression and Metastasis. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef]
- Mascolo, M.; Ilardi, G.; Romano, M.F.; Celetti, A.; Siano, M.; Romano, S.; Luise, C.; Merolla, F.; Rocco, A.; Vecchione, M.L.; et al. Overexpression of chromatin assembly factor-1 p60, poly(ADP-ribose) polymerase 1 and nestin predicts metastasizing behaviour of oral cancer. Histopathology 2012, 61, 1089–1105. [Google Scholar] [CrossRef]
- Jakob, M.; Sharaf, K.; Schirmer, M.; Leu, M.; Küffer, S.; Bertlich, M.; Ihler, F.; Haubner, F.; Canis, M.; Kitz, J. Role of cancer stem cell markers ALDH1, BCL11B, BMI-1, and CD44 in the prognosis of advanced HNSCC. Strahlenther Onkol 2021, 197, 231–245. [Google Scholar] [CrossRef]
- Spiegelberg, D.; Nilvebrant, J. CD44v6-Targeted Imaging of Head and Neck Squamous Cell Carcinoma: Antibody-Based Approaches. Contrast Media Mol. Imaging 2017, 2017, 2709547. [Google Scholar] [CrossRef]
- Riechelmann, H.; Sauter, A.; Golze, W.; Hanft, G.; Schroen, C.; Hoermann, K.; Erhardt, T.; Gronau, S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008, 44, 823–829. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Psyrri, A. The prognostic significance of p16 overexpression in oropharyngeal squamous cell carcinoma: Implications for treatment strategies and future clinical studies. Ann. Oncol. 2010, 21, 1931–1934. [Google Scholar] [CrossRef]
- Ragin, C.C.R.; Taioli, E.; Weissfeld, J.L.; White, J.S.; Rossie, K.M.; Modugno, F.; Gollin, S.M. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br. J. Cancer 2006, 95, 1432. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.E.P.; Ailles, L.E. Cancer Stem Cells in Head and Neck Squamous Cell Cancer. J. Clin. Oncol. 2008, 26, 2871–2875. [Google Scholar] [CrossRef]
- Yu, S.S.; Cirillo, N. The molecular markers of cancer stem cells in head and neck tumors. J. Cell. Physiol. 2020, 235, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sepiashvili, L.; Bruce, J.P.; Huang, S.H.; O’Sullivan, B.; Liu, F.F.; Kislinger, T. Novel insights into head and neck cancer using next-generation “omic” technologies. Cancer Res. 2015, 75, 480–486. [Google Scholar] [CrossRef]
- Haider, S.P.; Zeevi, T.; Baumeister, P.; Reichel, C.; Sharaf, K.; Forghani, R.; Kann, B.H.; Judson, B.L.; Prasad, M.L.; Burtness, B.; et al. Potential Added Value of PET/CT Radiomics for Survival Prognostication beyond AJCC 8th Edition Staging in Oropharyngeal Squamous Cell Carcinoma. Cancers 2020, 12, 1778. [Google Scholar] [CrossRef]
- Haider, S.P.; Sharaf, K.; Zeevi, T.; Baumeister, P.; Reichel, C.; Forghani, R.; Kann, B.H.; Petukhova, A.; Judson, B.L.; Prasad, M.L.; et al. Prediction of post-radiotherapy locoregional progression in HPV-associated oropharyngeal squamous cell carcinoma using machine-learning analysis of baseline PET/CT radiomics. Transl. Oncol. 2020, 14, 100906. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Yuan, C.; Zhu, Y.; Qiu, J.; Zhang, W.; Qi, B.; Wu, H.; Ye, J.; Jiang, H.; et al. Oncogenic roles of Bmi1 and its therapeutic inhibition by histone deacetylase inhibitor in tongue cancer. Lab. Investig. A J. Tech. Methods Pathol. 2014, 94, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, M.; Chen, X.; Wang, J.; Liang, X.; Wang, H.; Wang, Z.; Cheng, B.; Xia, J. Prognostic Value of Cancer Stem Cell Markers in Head and Neck Squamous Cell Carcinoma: A Meta-analysis. Sci. Rep. 2017, 7, 43008. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Renkonen, S.; Haglund, C.; Mattila, P.S.; Leivo, I.; Hagstrom, J.; Makitie, A.A. Association of BMI-1 and p16 as prognostic factors for head and neck carcinomas. Acta Oto-Laryngol. 2016, 136, 501–505. [Google Scholar] [CrossRef]
- Yu, C.C.; Lo, W.L.; Chen, Y.W.; Huang, P.I.; Hsu, H.S.; Tseng, L.M.; Hung, S.C.; Kao, S.Y.; Chang, C.J.; Chiou, S.H. Bmi-1 Regulates Snail Expression and Promotes Metastasis Ability in Head and Neck Squamous Cancer-Derived ALDH1 Positive Cells. J. Oncol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, L.C.; Carvalho, A.L.; Lauxen, I.S.; Nor, J.E.; Cerski, C.T.; Sant’Ana Filho, M. Spatial distribution of cancer stem cells in head and neck squamous cell carcinomas. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2014, 43, 499–506. [Google Scholar] [CrossRef]
- Ma, S.R.; Wang, W.M.; Huang, C.F.; Zhang, W.F.; Sun, Z.J. Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget 2015, 6, 8807–8821. [Google Scholar] [CrossRef]
- Dong, Y.; Ochsenreither, S.; Cai, C.; Kaufmann, A.M.; Albers, A.E.; Qian, X. Aldehyde dehydrogenase 1 isoenzyme expression as a marker of cancer stem cells correlates to histopathological features in head and neck cancer: A meta-analysis. PLoS ONE 2017, 12, e0187615. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, B. The prognostic role of the cancer stem cell marker aldehyde dehydrogenase 1 in head and neck squamous cell carcinomas: A meta-analysis. Oral Oncol. 2014, 50, 1144–1148. [Google Scholar] [CrossRef]
- Michifuri, Y.; Hirohashi, Y.; Torigoe, T.; Miyazaki, A.; Kobayashi, J.; Sasaki, T.; Fujino, J.; Asanuma, H.; Tamura, Y.; Nakamori, K.; et al. High expression of ALDH1 and SOX2 diffuse staining pattern of oral squamous cell carcinomas correlates to lymph node metastasis. Pathol. Int. 2012, 62, 684–689. [Google Scholar] [CrossRef]
- Tsai, M.S.; Chen, W.C.; Lai, C.H.; Chen, Y.Y.; Chen, M.F. Epigenetic therapy regulates the expression of ALDH1 and immunologic response: Relevance to the prognosis of oral cancer. Oral Oncol. 2017, 73, 88–96. [Google Scholar] [CrossRef]
- Ortiz, R.C.; Lopes, N.M.; Amor, N.G.; Ponce, J.B.; Schmerling, C.K.; Lara, V.S.; Moyses, R.A.; Rodini, C.O. CD44 and ALDH1 immunoexpression as prognostic indicators of invasion and metastasis in oral squamous cell carcinoma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2018, 47, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.H.; Chen, C.H.; Cruz, L.; Farnebo, L.; Yang, J.; Borges, P.; Kang, G.; Mochly-Rosen, D.; Sunwoo, J.B. Targeting aldehyde dehydrogenase activity in head and neck squamous cell carcinoma with a novel small molecule inhibitor. Oncotarget 2017, 8, 52345–52356. [Google Scholar] [CrossRef]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef]
- Ailles, L.; Prince, M. Cancer stem cells in head and neck squamous cell carcinoma. Methods Mol. Biol. 2009, 568, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Joshua, B.; Kaplan, M.J.; Doweck, I.; Pai, R.; Weissman, I.L.; Prince, M.E.; Ailles, L.E. Frequency of cells expressing CD44, a head and neck cancer stem cell marker: Correlation with tumor aggressiveness. Head Neck 2012, 34, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Mack, B.; Gires, O. CD44s and CD44v6 expression in head and neck epithelia. PLoS ONE 2008, 3, e3360. [Google Scholar] [CrossRef]
- Wang, S.J.; Wong, G.; de Heer, A.M.; Xia, W.; Bourguignon, L.Y. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope 2009, 119, 1518–1530. [Google Scholar] [CrossRef]
- Linge, A.; Lock, S.; Gudziol, V.; Nowak, A.; Lohaus, F.; von Neubeck, C.; Jutz, M.; Abdollahi, A.; Debus, J.; Tinhofer, I.; et al. Low Cancer Stem Cell Marker Expression and Low Hypoxia Identify Good Prognosis Subgroups in HPV(-) HNSCC after Postoperative Radiochemotherapy: A Multicenter Study of the DKTK-ROG. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2639–2649. [Google Scholar] [CrossRef]
- Linge, A.; Lock, S.; Krenn, C.; Appold, S.; Lohaus, F.; Nowak, A.; Gudziol, V.; Baretton, G.B.; Buchholz, F.; Baumann, M.; et al. Independent validation of the prognostic value of cancer stem cell marker expression and hypoxia-induced gene expression for patients with locally advanced HNSCC after postoperative radiotherapy. Clin. Transl. Radiat. Oncol. 2016, 1, 19–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Jong, M.C.; Pramana, J.; van der Wal, J.E.; Lacko, M.; Peutz-Kootstra, C.J.; de Jong, J.M.; Takes, R.P.; Kaanders, J.H.; van der Laan, B.F.; Wachters, J.; et al. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 5329–5338. [Google Scholar] [CrossRef] [PubMed]
- Motegi, A.; Fujii, S.; Zenda, S.; Arahira, S.; Tahara, M.; Hayashi, R.; Akimoto, T. Impact of Expression of CD44, a Cancer Stem Cell Marker, on the Treatment Outcomes of Intensity Modulated Radiation Therapy in Patients With Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Nasman, A.; Nordfors, C.; Grun, N.; Munck-Wikland, E.; Ramqvist, T.; Marklund, L.; Lindquist, D.; Dalianis, T. Absent/weak CD44 intensity and positive human papillomavirus (HPV) status in oropharyngeal squamous cell carcinoma indicates a very high survival. Cancer Med. 2013, 2, 507–518. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cohort (n = 12) | HPV-neg. Subcohort (n = 5) | HPV-pos. Subcohort (n = 7) | |
|---|---|---|---|---|
| Sex | Male Female | 10 2 | 4 1 | 6 1 |
| Age at surgery [years] | 62.9 ± 6.8 | 59.3 ± 5.0 | 65.4 ± 7.1 | |
| Relapse at diagnosis | Positive | 0 | 0 | 0 |
| Substance abuse | Negative | 3 | 0 | 3 |
| Nicotine (>10py) | 7 | 4 | 3 | |
| Alcohol | 0 | 0 | 0 | |
| Combined (Nicotine+Alcohol) | 2 | 1 | 1 | |
| Localization | Oropharynx | 9 | 3 | 6 |
| Hypopharynx | 2 | 1 | 1 | |
| Larynx | 1 | 1 | 0 | |
| UICC 8th edition staging | pT 1 | 2 | 0 | 2 |
| 2 | 3 | 1 | 2 | |
| 3 | 4 | 2 | 2 | |
| 4 | 3 | 2 | 1 | |
| pN positive | 12 | 5 | 7 | |
| pN 1 | 7 | 1 | 6 | |
| 2 | 1 | 0 | 1 | |
| 2a | 1 | 1 | 0 | |
| 3b | 3 | 3 | 0 | |
| ENE positive | 8 | 3 | 5 | |
| cM1 | 0 | 0 | 0 | |
| Stage (UICC, 8th edition) | I | 4 | 0 | 4 |
| II | 2 | 0 | 2 | |
| III | 1 | 1 | 0 | |
| IVa | 2 | 2 | 0 | |
| IVb | 3 | 2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharaf, K.; Lechner, A.; Haider, S.P.; Wiebringhaus, R.; Walz, C.; Kranz, G.; Canis, M.; Haubner, F.; Gires, O.; Baumeister, P. Discrimination of Cancer Stem Cell Markers ALDH1A1, BCL11B, BMI-1, and CD44 in Different Tissues of HNSCC Patients. Curr. Oncol. 2021, 28, 2763-2774. https://doi.org/10.3390/curroncol28040241
Sharaf K, Lechner A, Haider SP, Wiebringhaus R, Walz C, Kranz G, Canis M, Haubner F, Gires O, Baumeister P. Discrimination of Cancer Stem Cell Markers ALDH1A1, BCL11B, BMI-1, and CD44 in Different Tissues of HNSCC Patients. Current Oncology. 2021; 28(4):2763-2774. https://doi.org/10.3390/curroncol28040241
Chicago/Turabian StyleSharaf, Kariem, Axel Lechner, Stefan P. Haider, Robert Wiebringhaus, Christoph Walz, Gisela Kranz, Martin Canis, Frank Haubner, Olivier Gires, and Philipp Baumeister. 2021. "Discrimination of Cancer Stem Cell Markers ALDH1A1, BCL11B, BMI-1, and CD44 in Different Tissues of HNSCC Patients" Current Oncology 28, no. 4: 2763-2774. https://doi.org/10.3390/curroncol28040241
APA StyleSharaf, K., Lechner, A., Haider, S. P., Wiebringhaus, R., Walz, C., Kranz, G., Canis, M., Haubner, F., Gires, O., & Baumeister, P. (2021). Discrimination of Cancer Stem Cell Markers ALDH1A1, BCL11B, BMI-1, and CD44 in Different Tissues of HNSCC Patients. Current Oncology, 28(4), 2763-2774. https://doi.org/10.3390/curroncol28040241





