The Role of PET in Supratentorial and Infratentorial Pediatric Brain Tumors
Abstract
1. Introduction
2. Materials and Methods
Study Selection and Data Extraction
3. Results
3.1. Diagnosis, Grading and Delineation of the Target for Stereotactic Biopsy
3.1.1. 18F-FDG
3.1.2. Non-FDG Tracers
| Author | Journal | Year | Tacer | N | Major Findings |
|---|---|---|---|---|---|
| Pirotte, B.J. [19] | Journal of neurosurgery. Pediatrics | 2010 | 11C-MET | 85 | 11C-MET PET allowed the diagnosis of a higher grading than MRI |
| Laser, B.S. [23] | Neuro-oncology | 2013 | 11C-MET | 10 | (11)C MET PET uptake is significantly greater within the tumor compared with non-involved background white matter, making it more useful than FDG PET in identifying active tumor in patients with craniopharyngioma. |
| Laukam, K.R. [27] | Mol Imaging | 2017 | 11C-MET | 65 | Combined PET and MRI improve the evaluation of tumor activity, extent, type/grade prediction, and therapy-induced changes in patients with glioma and serve information highly relevant for diagnosis and management. |
| Phi, J.H. [17] | J Nucl Med | 2010 | 11C-MET AND 18F-FDG | 30 | (18)F-FDG does not contribute to the differential diagnosis and that another tracer such as (11)C-methinine is required. |
| Rheims, S. [28] | Neuro-oncology | 2014 | 11C-MET | 77 | Normal MET-PET findings in patients with an epileptogenic non-rapidly progressing brain tumor are highly suggestive of DNT, whereas a markedly increased tumor methionine uptake makes this diagnosis unlikely. |
| Misch, M. [30] | ChNS: official journal of the International Society for Pediatric Neurosurgery | 2015 | 18F-FET | 26 | (18)F-FET-PET imaging is helpful for target selection and can be integrated in surgical guidance. (18)F-FET-PET image-guided surgical targeting yielded histological diagnosis in pediatric brain tumor patients. |
| Morana, G. [31] | J Nucl Med | 2014 | 18F-DOPA | 13 | (18)F-DOPA PET/MR image fusion may be a reliable imaging biomarker of pediatric IAs. Information gathered by this combined imaging approach can be readily transferred to the everyday practice and may help clinicians to better stratify patients with IAs, especially diffuse astrocytomas and gliomatosis cerebri, for diagnostic, therapeutic, and prognostic purposes. |
| Morana, G. [32] | European journal of nuclear medicine and molecular imaging | 2016 | 18F-DOPA | 28 | (18)F-DOPA PET/CT correctly detected involvement of the dorsal striatum in lesions with a T/S ratio >1, but appeared to be less suitable for evaluation of the ventral striatum. The use of fused (18)F-DOPA PET/MRI further improves the accuracy for evaluation of the ventral striatum. |
| Morana, G. [33] | European journal of nuclear medicine and molecular imaging | 2017 | 18F-DOPA | 26 | 18F-DOPA PET provide useful complementary information for pediatric DAT grading. 18F-DOPA uptake better correlates with PFS prediction. Combining MRI and PET data provides the highest predictive power for prognosticating tumor progression |
| Morana, G. [34] | Neuro-oncology | 2015 | 18F-DOPA | 27 | (18)F-DOPA uptake better discriminates low-grade from high-grade gliomas and is an independent predictor of outcome vs H-MRS |
| Marner, L. [35] | Clin Transl Imaging | 2017 | 18F-FET | 300 | PET/MRI scan may increase accuracy in discriminating recurrence from treatment changes, although sequential same-day imaging on separate systems will often constitute a reliable and cost-effective alternative. |
3.2. Prognosis
3.2.1. 18F-FDG
3.2.2. Non-FDG Tracers
3.3. Evaluation of Recurrence
3.3.1. 18F-FDG
3.3.2. Non-FDG Tracers
3.4. Treatment Planning and Assessment of Treatment Response
3.4.1. 18F-FDG
3.4.2. Non-FDG Tracers
4. Discussion/Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, K.J.; Cullen, J.; Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Langer, C.E.; Turner, M.C.; McKean-Cowdin, R.; Fisher, J.L.; Lupo, P.J.; Partap, S.; et al. Childhood brain tumor epidemiology: A brain tumor epidemiology consortium review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2716–2736. [Google Scholar] [CrossRef]
- Bauchet, L.; Rigau, V.; Mathieu-Daudé, H.; Fabbro-Peray, P.; Palenzuela, G.; Figarella-Branger, D.; Moritz, J.; Puget, S.; Bauchet, F.; Pallusseau, L.; et al. Clinical epidemiology for childhood primary central nervous system tumors. J. Neuro Oncol. 2009, 92, 87–98. [Google Scholar] [CrossRef]
- Rai, R.; Iwanaga, J.; Shokouhi, G.; Oskouian, R.J.; Tubbs, R.S. The Tentorium Cerebelli: A Comprehensive Review Including Its Anatomy, Embryology, and Surgical Techniques. Cureus 2018, 10, e3079. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, L.M.; Vázquez-Moreno, J.; Heredia-Delgado, J.A.; Sevilla-Castillo, R. Presentación clínica de tumores intracraneales supratentoriales (ST) e infratentoriales (IT) en pacientes pediátricos. Gac. Med. Mex. 2016, 152, 158–162. [Google Scholar] [PubMed]
- Yu, J.; Shi, W.E.; Zhao, R.; Gao, X.; Li, H. Epidemiology of brain tumors in children aged two and under: A 10-year single-institute study. Oncol Lett 2015, 9, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Nejat, F.; El Khashab, M.; Rutka, J.T. Initial management of childhood brain tumors: Neurosurgical considerations. J. Child Neurol. 2008, 23, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Alexander, G.S.; Bakas, S.; Nikam, R.; Talekar, K.; Palmer, J.D.; Shi, W. Advanced magnetic resonance imaging in glioblastoma: A review. Chin. Clin. Oncol. 2017, 6, 40. [Google Scholar] [CrossRef]
- Vézina, L.G. Imaging of central nervous system tumors in children: Advances and limitations. J. Child Neurol. 2008, 23, 1128–1135. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef]
- Upadhyay, N.; Waldman, A.D. Conventional MRI evaluation of gliomas. Br. J. Radiol. 2011, 84 Spec No 2, S107–S111. [Google Scholar] [CrossRef]
- Goo, H.W.; Ra, Y.S. Advanced MRI for Pediatric Brain Tumors with Emphasis on Clinical Benefits. Korean J. Radiol. 2017, 18, 194–207. [Google Scholar] [CrossRef][Green Version]
- Fangusaro, J.; Witt, O.; Hernáiz Driever, P.; Bag, A.K.; de Blank, P.; Kadom, N.; Kilburn, L.; Lober, R.M.; Robison, N.J.; Fisher, M.J.; et al. Response assessment in paediatric low-grade glioma: Recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020, 21, e305–e316. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Chiaravalloti, A.; Filippi, L.; Ricci, M.; Cimini, A.; Schillaci, O. Molecular Imaging in Pediatric Brain Tumors. Cancers 2019, 11, 1853. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, N.; Laudicella, R.; Vento, A.; Pignata, S.; Mattoli, M.V.; Filice, R.; Comis, A.D.; Arnone, A.; Baldari, S.; Cabria, M.; et al. The Additional Value of (18)F-FDG PET and MRI in Patients with Glioma: A Review of the Literature from 2015 to 2020. Diagn. Basel Switz. 2020, 10, 357. [Google Scholar] [CrossRef]
- Phi, J.H.; Paeng, J.C.; Lee, H.S.; Wang, K.C.; Cho, B.K.; Lee, J.Y.; Park, S.H.; Lee, J.; Lee, D.S.; Kim, S.K. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18F-FDG and 11C-methionine pet. J. Nucl. Med. 2010, 51, 728–734. [Google Scholar] [CrossRef]
- Moharir, M.; London, K.; Howman-Giles, R.; North, K. Utility of positron emission tomography for tumour surveillance in children with neurofibromatosis type 1. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Pirotte, B.J.; Lubansu, A.; Massager, N.; Wikler, D.; Van Bogaert, P.; Levivier, M.; Brotchi, J.; Goldman, S. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J. Neurosurg. Pediatr. 2010, 5, 486–499. [Google Scholar] [CrossRef]
- Zukotynski, K.A.; Fahey, F.H.; Kocak, M.; Alavi, A.; Wong, T.Z.; Treves, S.T.; Shulkin, B.L.; Haas-Kogan, D.A.; Geyer, J.R.; Vajapeyam, S.; et al. Evaluation of 18F-FDG PET and MRI associations in pediatric diffuse intrinsic brain stem glioma: A report from the Pediatric Brain Tumor Consortium. J. Nucl. Med. 2011, 52, 188–195. [Google Scholar] [CrossRef][Green Version]
- Goda, J.S.; Dutta, D.; Raut, N.; Juvekar, S.L.; Purandare, N.; Rangarajan, V.; Arora, B.; Gupta, T.; Kurkure, P.; Jalali, R. Can multiparametric MRI and FDG-PET predict outcome in diffuse brainstem glioma? A report from a prospective phase-II study. Pediatr. Neurosurg. 2013, 49, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Zukotynski, K.A.; Fahey, F.H.; Vajapeyam, S.; Ng, S.S.; Kocak, M.; Gururangan, S.; Kun, L.E.; Poussaint, T.Y. Exploratory evaluation of MR permeability with 18F-FDG PET mapping in pediatric brain tumors: A report from the Pediatric Brain Tumor Consortium. J. Nucl. Med. 2013, 54, 1237–1243. [Google Scholar] [CrossRef]
- Laser, B.S.; Merchant, T.E.; Indelicato, D.J.; Hua, C.H.; Shulkin, B.L.; Snyder, S.E. Evaluation of children with craniopharyngioma using carbon-11 methionine PET prior to proton therapy. Neuro Oncol. 2013, 15, 506–510. [Google Scholar] [CrossRef]
- Zukotynski, K.; Fahey, F.; Kocak, M.; Kun, L.; Boyett, J.; Fouladi, M.; Vajapeyam, S.; Treves, T.; Poussaint, T.Y. 18F-FDG PET and MR imaging associations across a spectrum of pediatric brain tumors: A report from the pediatric brain tumor consortium. J. Nucl. Med. 2014, 55, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Shulkin, B.L.; Indelicato, D.J.; Li, Y.; Li, X.; Boop, F.A.; Merchant, T.E. Postoperative cerebral glucose metabolism in pediatric patients receiving proton therapy for craniopharyngioma. J. Neurosurg. Pediatr. 2015, 16, 567–573. [Google Scholar] [CrossRef]
- Zukotynski, K.A.; Vajapeyam, S.; Fahey, F.H.; Kocak, M.; Brown, D.; Ricci, K.I.; Onar-Thomas, A.; Fouladi, M.; Poussaint, T.Y. Correlation of (18)F-FDG PET and MRI Apparent Diffusion Coefficient Histogram Metrics with Survival in Diffuse Intrinsic Pontine Glioma: A Report from the Pediatric Brain Tumor Consortium. J. Nucl. Med. 2017, 58, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Lindemann, F.; Weckesser, M.; Hesselmann, V.; Ligges, S.; Wölfer, J.; Jeibmann, A.; Zinnhardt, B.; Viel, T.; Schäfers, M.; et al. Multimodal Imaging of Patients With Gliomas Confirms (11)C-MET PET as a Complementary Marker to MRI for Noninvasive Tumor Grading and Intraindividual Follow-Up After Therapy. Mol. Imaging 2017, 16, 1536012116687651. [Google Scholar] [CrossRef] [PubMed]
- Rheims, S.; Rubi, S.; Bouvard, S.; Bernard, E.; Streichenberger, N.; Guenot, M.; Le Bars, D.; Hammers, A.; Ryvlin, P. Accuracy of distinguishing between dysembryoplastic neuroepithelial tumors and other epileptogenic brain neoplasms with [11C]methionine PET. Neuro Oncol. 2014, 16, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Dunkl, V.; Cleff, C.; Stoffels, G.; Judov, N.; Sarikaya-Seiwert, S.; Law, I.; Bøgeskov, L.; Nysom, K.; Andersen, S.B.; Steiger, H.J.; et al. The usefulness of dynamic O-(2-18F-fluoroethyl)-L-tyrosine PET in the clinical evaluation of brain tumors in children and adolescents. J. Nucl. Med. 2015, 56, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Misch, M.; Guggemos, A.; Driever, P.H.; Koch, A.; Grosse, F.; Steffen, I.G.; Plotkin, M.; Thomale, U.W. (18)F-FET-PET guided surgical biopsy and resection in children and adolescence with brain tumors. Child’s Nerv. Syst. 2015, 31, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Morana, G.; Piccardo, A.; Milanaccio, C.; Puntoni, M.; Nozza, P.; Cama, A.; Zefiro, D.; Cabria, M.; Rossi, A.; Garrè, M.L. Value of 18F-3,4-dihydroxyphenylalanine PET/MR image fusion in pediatric supratentorial infiltrative astrocytomas: A prospective pilot study. J. Nucl. Med. 2014, 55, 718–723. [Google Scholar] [CrossRef][Green Version]
- Morana, G.; Puntoni, M.; Garrè, M.L.; Massollo, M.; Lopci, E.; Naseri, M.; Severino, M.; Tortora, D.; Rossi, A.; Piccardo, A. Ability of (18)F-DOPA PET/CT and fused (18)F-DOPA PET/MRI to assess striatal involvement in paediatric glioma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1664–1672. [Google Scholar] [CrossRef]
- Morana, G.; Piccardo, A.; Tortora, D.; Puntoni, M.; Severino, M.; Nozza, P.; Ravegnani, M.; Consales, A.; Mascelli, S.; Raso, A.; et al. Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F-DOPA PET. Eur. J. Nucl. Med. Mol. 2017, 44, 2084–2093. [Google Scholar] [CrossRef]
- Morana, G.; Piccardo, A.; Puntoni, M.; Nozza, P.; Cama, A.; Raso, A.; Mascelli, S.; Massollo, M.; Milanaccio, C.; Garrè, M.L.; et al. Diagnostic and prognostic value of 18F-DOPA PET and 1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: A comparative study. Neuro Oncol. 2015, 17, 1637–1647. [Google Scholar] [CrossRef][Green Version]
- Marner, L.; Henriksen, O.M.; Lundemann, M.; Larsen, V.A.; Law, I. Clinical PET/MRI in neurooncology: Opportunities and challenges from a single-institution perspective. Clin. Transl. Imaging 2017, 5, 135–149. [Google Scholar] [CrossRef]
- Rosenfeld, A.; Etzl, M.; Bandy, D.; Carpenteri, D.; Gieseking, A.; Dvorchik, I.; Kaplan, A. Use of positron emission tomography in the evaluation of diffuse intrinsic brainstem gliomas in children. J. Pediatr. Hematol. 2011, 33, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Strenger, V.; Lackner, H.; Mayer, R.; Sminia, P.; Sovinz, P.; Mokry, M.; Pilhatsch, A.; Benesch, M.; Schwinger, W.; Seidel, M.; et al. Incidence and clinical course of radionecrosis in children with brain tumors. A 20-year longitudinal observational study. Strahlenther. Onkol. 2013, 189, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Jethwa, K.R.; Ost, P.; Williams, S.; Kwon, E.D.; Lowe, V.J.; Davis, B.J. Prostate cancer-specific PET radiotracers: A review on the clinical utility in recurrent disease. Pr. Radiat. Oncol. 2018, 8, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Fraioli, F.; Shankar, A.; Hargrave, D.; Hyare, H.; Gaze, M.N.; Groves, A.M.; Alongi, P.; Stoneham, S.; Michopoulou, S.; Syed, R.; et al. 18F-fluoroethylcholine (18F-Cho) PET/MRI functional parameters in pediatric astrocytic brain tumors. Clin. Nucl. Med. 2015, 40, e40–e45. [Google Scholar] [CrossRef] [PubMed]
- Tsouana, E.; Stoneham, S.; Fersht, N.; Kitchen, N.; Gaze, M.; Bomanji, J.; Fraioli, F.; Hargrave, D.; Shankar, A. Evaluation of treatment response using integrated 18F-labeled choline positron emission tomography/magnetic resonance imaging in adolescents with intracranial non-germinomatous germ cell tumours. Pediatr. Blood Cancer 2015, 62, 1661–1663. [Google Scholar] [CrossRef]
- Gauvain, K.; Ponisio, M.R.; Barone, A.; Grimaldi, M.; Parent, E.; Leeds, H.; Goyal, M.; Rubin, J.; McConathy, J. (18)F-FDOPA PET/MRI for monitoring early response to bevacizumab in children with recurrent brain tumors. Neurooncol. Pract. 2018, 5, 28–36. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Y.; Li, D.; Niu, G.; Lang, L.; Li, F.; Liu, Y.; Zhu, Z.; Chen, X. (68)Ga-NOTA-Aca-BBN(7-14) PET imaging of GRPR in children with optic pathway glioma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2152–2162. [Google Scholar] [CrossRef]
- Jansen, M.H.; Veldhuijzen van Zanten, S.E.M.; van Vuurden, D.G.; Huisman, M.C.; Vugts, D.J.; Hoekstra, O.S.; van Dongen, G.A.; Kaspers, G.L. Molecular Drug Imaging: (89)Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J. Nucl. Med. 2017, 58, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Price, P.M.; Aboagye, E.O. PET for in vivo pharmacokinetic and pharmacodynamic measurements. Eur. J. Cancer 2002, 38, 2094–2107. [Google Scholar] [CrossRef]
- Jung, J.H.; Ahn, B.C. Current Radiopharmaceuticals for Positron Emission Tomography of Brain Tumors. Brain Tumor Res. Treat. 2018, 6, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Juhász, C.; Bosnyák, E. PET and SPECT studies in children with hemispheric low-grade gliomas. Child’s Nerv. Syst. 2016, 32, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
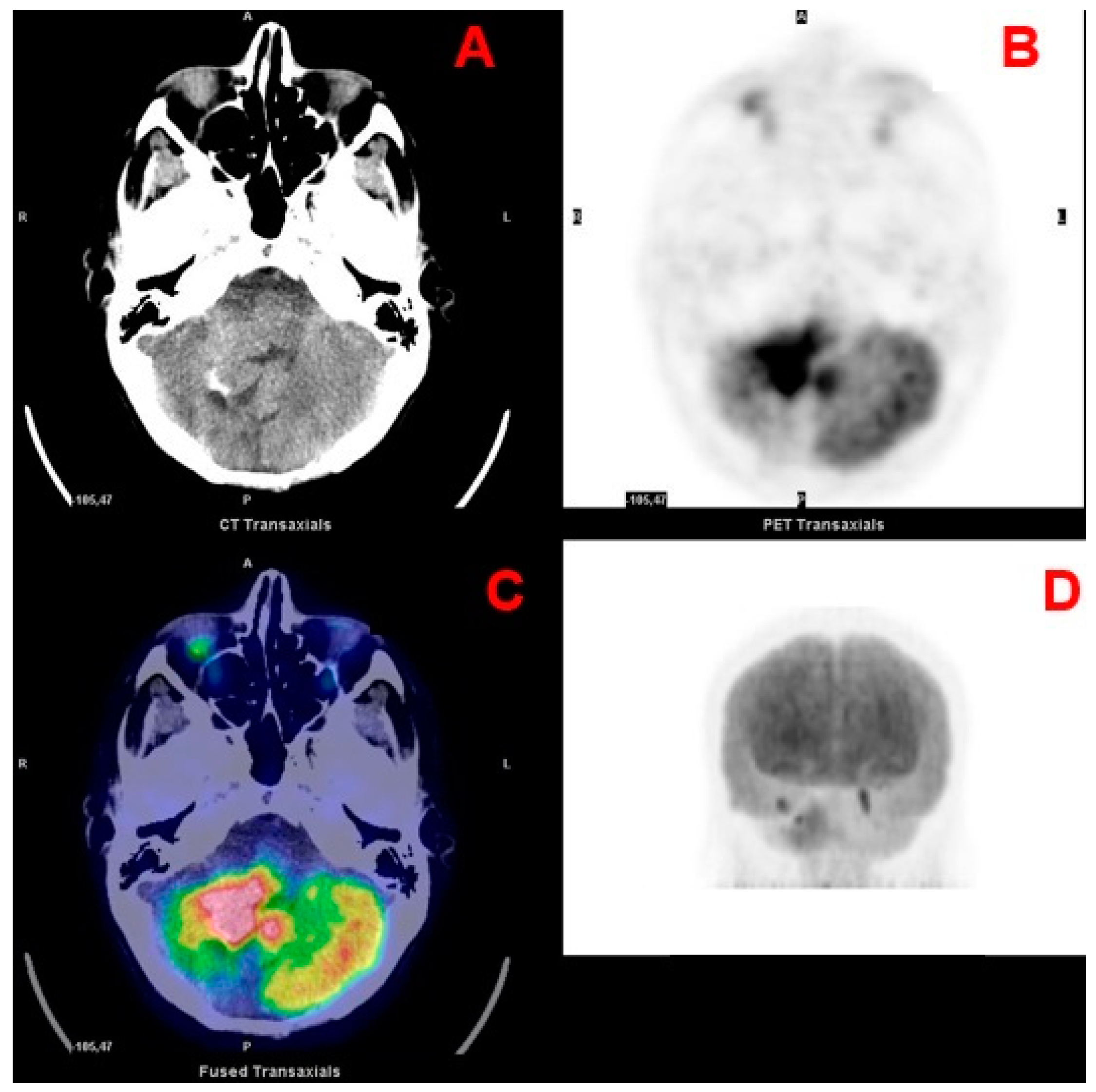
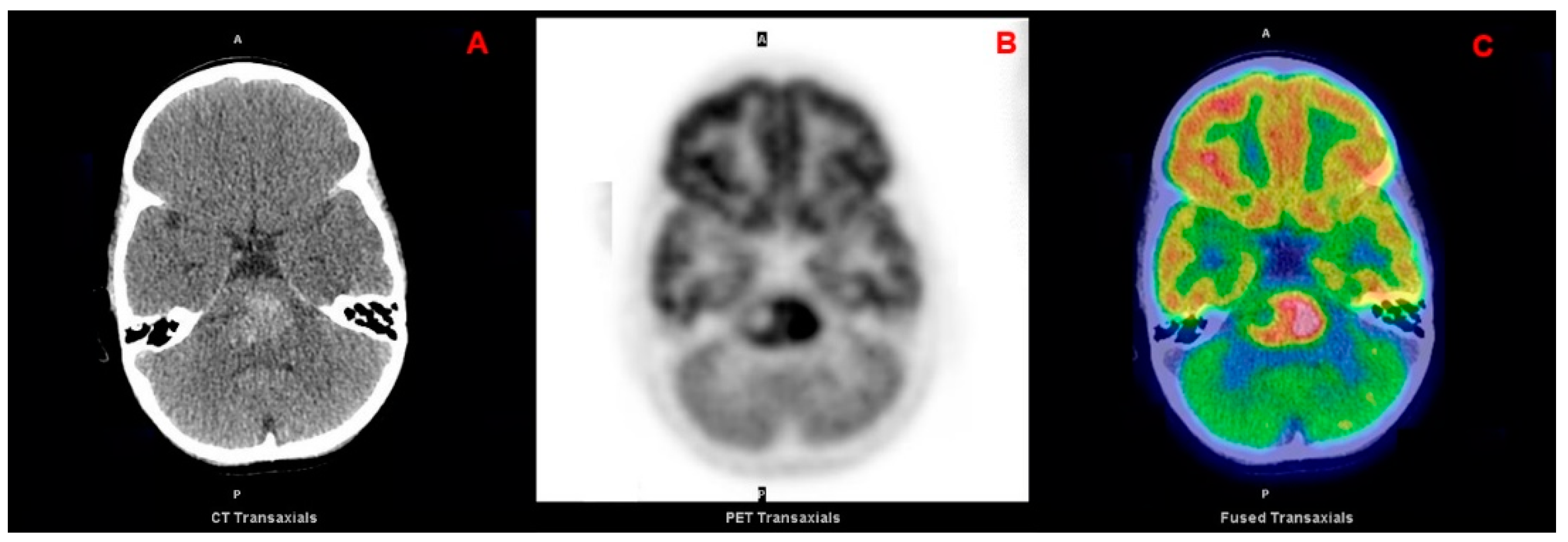
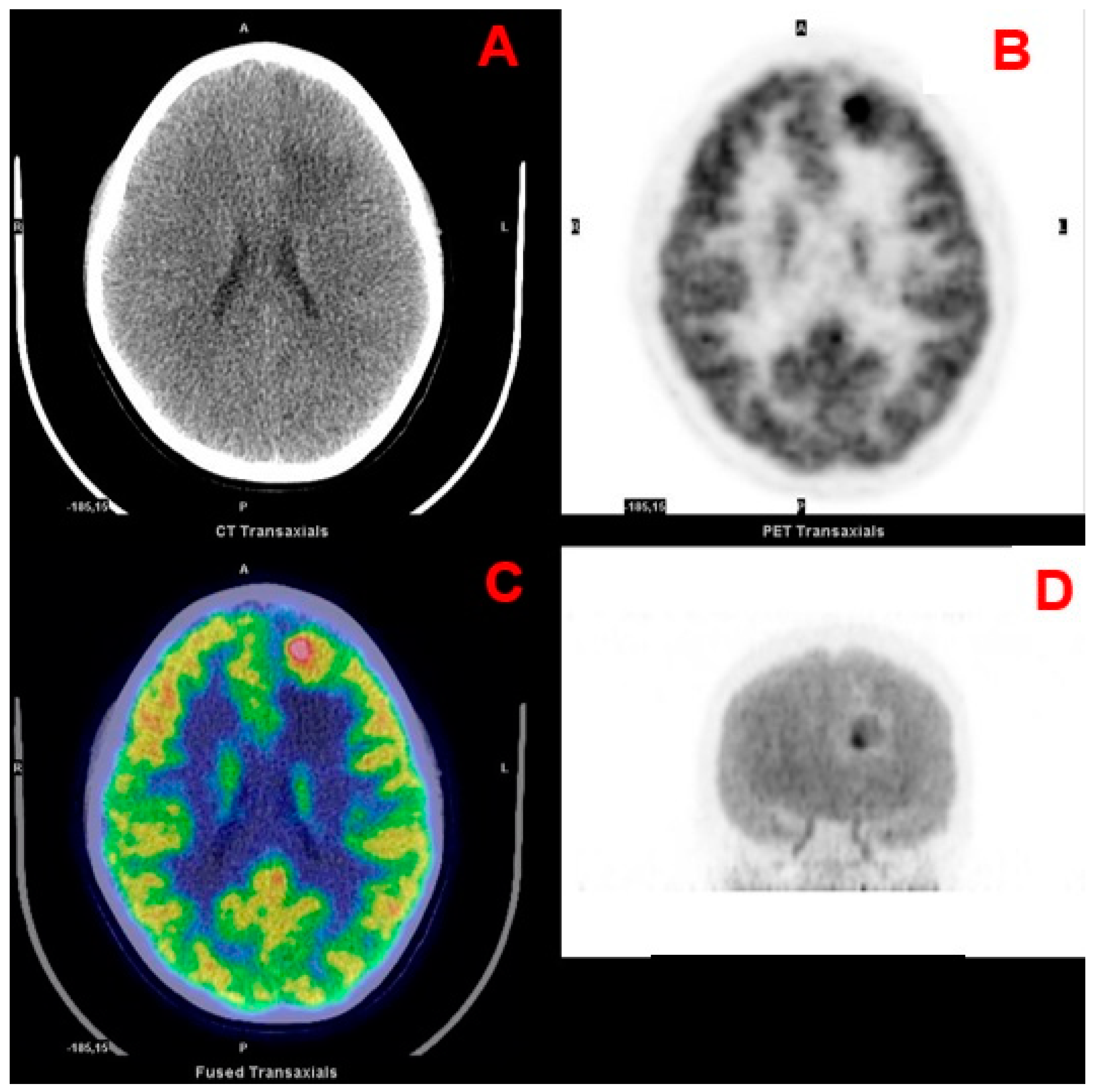
| 1st Authors | Year | Journal | Country | Study Design | N pts | Tumor Location | Histology |
|---|---|---|---|---|---|---|---|
| Phi [17] | 2010 | JNM | Korea | retrospective | 30 | supratentorial | 11 FCD; 8 DNT; 11 GG |
| Moharir [18] | 2010 | EJNMMI | Australia | retrospective | 18 | supratentorial | 7 OPG; 7 PNF; 4 OPG + PNF |
| Pirotte [19] | 2010 | J neurosurg pediatrics | Belgium | prospective | 85 | Supratentorial infratentorial | 10 GBM; 10 AA; 13 LGA; 5 PNET; 3 germ cell tumor; 14 PA; 11 ependymoma; 9 GG; 10 OD |
| Zukotynski [20] | 2011 | JNM | USA | prospective | 40 | NR | NR |
| Goda [21] | 2013 | Ped Neurosurgery | India | prospective | 20 | infratentorial | 20 DIPG |
| Zukotynski [22] | 2013 | JNM | USA | retrospective | 24 | supratentorial infratentorial | 7 HGG; 9 LGG; 4 BSG; 2 medulloblastoma; 2 ependynoma |
| Laser [23] | 2013 | Neuro-oncology | USA | prospective | 10 | supratentorial | craniopharyngioma |
| Zukotynski [24] | 2014 | JNM | USA | retrospective | 203 | supratentorial infratentorial | 71 BSG; 24 GBM; 30 AA; 23 astrocytoma; 15 ependymoma; 10 medulloblastoma; 5 pineoblastoma; 25 other |
| Hua [25] | 2015 | JNS pediatrics | USA | prospective | 50 | supratentorial | craniopharyngioma |
| Zukotynski [26] | 2017 | JNM | USA | prospective | 33 | infratentorial | 33 PG |
| 1st Authors | Device | Activity Injected MBq Mean (Range) | Uptake Time Min Mean (Range) | PET Analysis | Semiquantitative Parameters | SUV Max Mean (Range) |
|---|---|---|---|---|---|---|
| Phi [17] | PET or PET/CT | 7.4 MBq/Kg | 40 | visual and semiquantitative | LGR | NR |
| Moharir [18] | PET/CT | 370 MBq | 30 | visual and semiquantitative | SUVmax | 2.89 (1.75–5.57) |
| Pirotte [19] | PET | 222–333 | 40–60 | visual | N/A | N/A |
| Zukotynski [20] | PET | 5.55 MBq/Kg (18–370) | 40–60 | visual | N/A | N/A |
| Goda [21] | PET/CT | NR | NR | visual and semiquantitative | SUVmax | NR |
| Zukotynski [22] | PET | 5.55 MBq/Kg (18–370) | 40–60 | visual | N/A | N/A |
| Laser [23] | PET/CT | 5.5 MBq/Kg | 60 | visual and semiquantitative | SUVmax | 2.65 (1.3–7.4) |
| Zukotynski [24] | PET | 5.55 MBq/Kg (18–370) | 40–60 | visual | N/A | N/A |
| Hua [25] | PET/CT | 5.55 MBq/Kg (74–444) | 60 | visual and semiquantitative | SUVmax and ratios | NR |
| Zukotynski [26] | PET | 5.55 MBq/Kg (18–370) | 40–60 | visual and semiquantitative | SUVmax | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cistaro, A.; Albano, D.; Alongi, P.; Laudicella, R.; Pizzuto, D.A.; Formica, G.; Romagnolo, C.; Stracuzzi, F.; Frantellizzi, V.; Piccardo, A.; et al. The Role of PET in Supratentorial and Infratentorial Pediatric Brain Tumors. Curr. Oncol. 2021, 28, 2481-2495. https://doi.org/10.3390/curroncol28040226
Cistaro A, Albano D, Alongi P, Laudicella R, Pizzuto DA, Formica G, Romagnolo C, Stracuzzi F, Frantellizzi V, Piccardo A, et al. The Role of PET in Supratentorial and Infratentorial Pediatric Brain Tumors. Current Oncology. 2021; 28(4):2481-2495. https://doi.org/10.3390/curroncol28040226
Chicago/Turabian StyleCistaro, Angelina, Domenico Albano, Pierpaolo Alongi, Riccardo Laudicella, Daniele Antonio Pizzuto, Giuseppe Formica, Cinzia Romagnolo, Federica Stracuzzi, Viviana Frantellizzi, Arnoldo Piccardo, and et al. 2021. "The Role of PET in Supratentorial and Infratentorial Pediatric Brain Tumors" Current Oncology 28, no. 4: 2481-2495. https://doi.org/10.3390/curroncol28040226
APA StyleCistaro, A., Albano, D., Alongi, P., Laudicella, R., Pizzuto, D. A., Formica, G., Romagnolo, C., Stracuzzi, F., Frantellizzi, V., Piccardo, A., & Quartuccio, N. (2021). The Role of PET in Supratentorial and Infratentorial Pediatric Brain Tumors. Current Oncology, 28(4), 2481-2495. https://doi.org/10.3390/curroncol28040226







