Lung Cancer Inequalities in Stage of Diagnosis in Ontario, Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
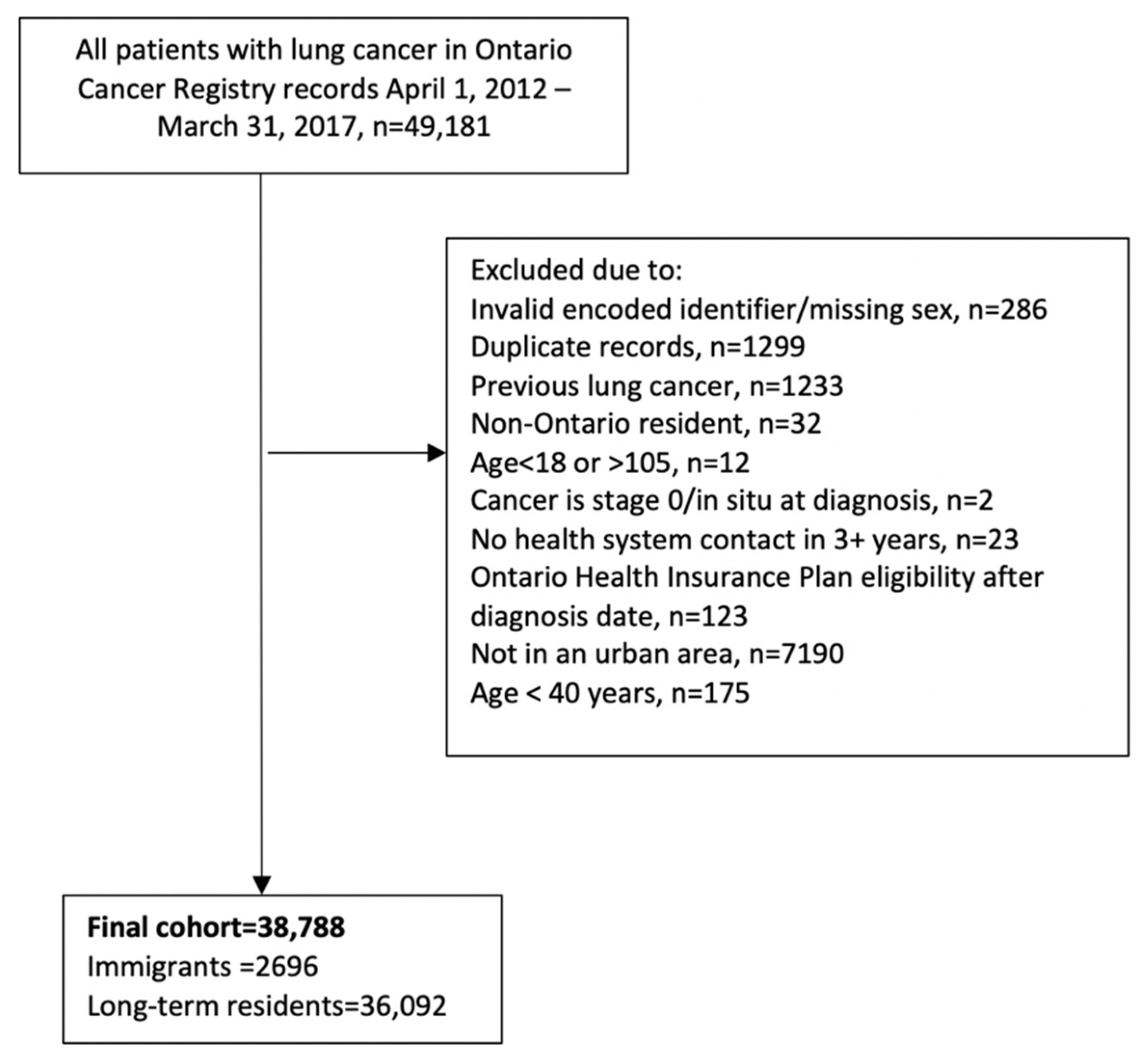
2.2. Study Cohort
2.3. Variables
2.4. Outcome
2.5. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canadian Cancer Statistics: A 2020 Special Report on Lung Cancer; Canadian Cancer Statistics Advisory Committee: Toronto, ON, Canada, 2020.
- Nadpara, P.; Madhavan, S.S.; Tworek, C. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: A population-based study. Cancer Epidemiol. 2015, 39, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Immigration and Ethnocultural Diversity in Canada: Statistics Canada. 2016. Available online: http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/99-010-x2011001-eng.cfm#a4 (accessed on 16 March 2021).
- Review: What Is Collaborative Stage?: National Cancer Institute. Available online: https://training.seer.cancer.gov/collaborative/intro/review.html (accessed on 16 March 2021).
- Technical Appendix: Ontario Cancer Statistics Toronto: Cancer Care Ontario. 2018. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/OCS2018TechDataAppendix.pdf (accessed on 16 June 2020).
- Kiran, T.; Victor, J.C.; Kopp, A.; Shah, B.R.; Glazier, R.H. The relationship between primary care models and processes of diabetes care in Ontario. Can. J. Diabetes 2014, 38, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Dean, J.; Edge, S.; Wilson, K.; Ghassemi, E. Double Burden of Rural Migration in Canada? Considering the Social Determinants of Health Related to Immigrant Settlement Outside the Cosmopolis. Int. J. Environ. Res. Public Health 2019, 16, 678. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins ACG System: The Johns Hopkins University. Available online: https://www.hopkinsacg.org (accessed on 21 September 2017).
- Jiang, L.; Lofters, A.; Moineddin, R.; Decker, K.; Groome, P.; Kendell, C.; Krzyzanowska, M.; Li, D.; McBride, M.L.; Mittmann, N.; et al. Primary care physician use across the breast cancer care continuum: CanIMPACT study using Canadian administrative data. Can. Fam. Physician 2016, 62, e589–e598. [Google Scholar] [PubMed]
- Singh, J.; Dahrouge, S.; Green, M.E. The impact of the adoption of a patient rostering model on primary care access and continuity of care in urban family practices in Ontario, Canada. BMC Fam. Pract. 2019, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Lofters, A.K.; Hwang, S.W.; Moineddin, R.; Glazier, R.H. Cervical cancer screening among urban immigrants by region of origin: A population-based cohort study. Prev. Med. 2010, 51, 509–516. [Google Scholar] [CrossRef]
- Austin, P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 2009, 38, 1228–1234. [Google Scholar] [CrossRef]
- Ellis, P.M.; Vandermeer, R. Delays in the diagnosis of lung cancer. J. Thorac. Dis. 2011, 3, 183–188. [Google Scholar]
- Siddiqui, F.; Siddiqui, A.H. Lung Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Darling, G.E.; Tammemägi, M.C.; Schmidt, H.; Buchanan, D.N.; Leung, Y.; McGarry, C.; Rabeneck, L. Organized Lung Cancer Screening Pilot: Informing a Province-Wide Program in Ontario, Canada. Ann Thorac Surg. 2020, 111, 1805–1811. [Google Scholar] [CrossRef]
- B.C. to Launch First lung Cancer Screening Program in Canada. Global News. 2020. Available online: https://globalnews.ca/news/7333789/bc-lung-cancer-screening-program/ (accessed on 22 October 2020).
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Lofters, A.; Gatov, E.; Lu, H.; Baxter, N.; Corrado, A.; Guilcher, S. Stage of colorectal cancer diagnosis for immigrants: A population-based retrospective cohort study in Ontario, Canada. Cancer Causes Control 2020. submitted. [Google Scholar]
- Lofters, A.K.; Guilcher, S.J.; Webster, L.; Glazier, R.H.; Jaglal, S.B.; Bayoumi, A.M. Cholesterol testing among men and women with disability: The role of morbidity. Clin. Epidemiol. 2016, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lofters, A.K.; Kopp, A.; Vahabi, M.; Glazier, R.H. Understanding those overdue for cancer screening by five years or more: A retrospective cohort study in Ontario, Canada. Prev. Med. 2019, 129, 105816. [Google Scholar] [CrossRef] [PubMed]
- Guilcher, S.J.; Lofters, A.; Glazier, R.H.; Jaglal, S.B.; Voth, J.; Bayoumi, A.M. Level of disability, multi-morbidity and breast cancer screening: Does severity matter? Prev. Med. 2014, 67, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Cramb, S.M.; Baade, P.D. The International Epidemiology of Lung Cancer: Geographical distribution and secular trends. J. Thorac. Oncol. 2008, 3, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Mignotte, H.; Guillem, P.; Vesin, A.; Toffart, A.C.; Colonna, M.; Bonneterre, V.; Brichon, P.Y.; Brambilla, C.; Brambilla, E.; Lantuejoul, S.; et al. Primary lung adenocarcinoma: Characteristics by smoking habit and sex. Eur. Respir. J. 2011, 38, 1412–1419. [Google Scholar] [CrossRef]
- Newbold, K.B.; Neligan, D. Disaggregating Canadian immigrant smoking behaviour by country of birth. Soc. Sci. Med. 2012, 75, 997–1005. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Lofters, A.K.; Mark, A.; Taljaard, M.; Green, M.E.; Glazier, R.H.; Dahrouge, S. Cancer screening inequities in a time of primary care reform: A population-based longitudinal study in Ontario, Canada. BMC Fam. Pract. 2018, 19, 147. [Google Scholar] [CrossRef]
- Vahabi, M.; Lofters, A.; Kumar, M.; Glazier, R.H. Breast cancer screening disparities among immigrant women by world region of origin: A population-based study in Ontario, Canada. Cancer Med. 2016, 5, 1670–1686. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.A.; Sutradhar, R.; Borkhoff, C.M.; Baxter, N.; Lofters, A.; Rabeneck, L.; Tinmouth, J.; Paszat, L.; Network, O.C. Small-area variation in screening for cancer, glucose and cholesterol in Ontario: A cross-sectional study. CMAJ Open 2015, 3, E373–E381. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Immigrants (n = 2696) | Long-Term Residents (n = 36,092) | Standardized Difference | Total (n = 38,788) |
|---|---|---|---|---|
| Sex | ||||
| Female | 1114 (41.3%) | 18,020 (49.9%) | 0.17 | 19,134 (49.3%) |
| Male | 1582 (58.7%) | 18,072 (50.1%) | 0.17 | 19,654 (50.7%) |
| Age at diagnosis | ||||
| Mean ± SD | 67.73 ± 12.19 | 71.46 ± 10.53 | 0.33 | 71.20 ± 10.69 |
| Median (IQR) | 68 (59–77) | 72 (64–79) | 0.3 | 72 (64–79) |
| Age group (years) | ||||
| 40–64 | 1105 (41.0%) | 9479 (26.3%) | 0.32 | 10,584 (27.3%) |
| 65–74 | 718 (26.6%) | 12,056 (33.4%) | 0.15 | 12,774 (32.9%) |
| 75+ | 873 (32.4%) | 14,557 (40.3%) | 0.17 | 15,430 (39.8%) |
| Neighborhood income quintile | ||||
| Quintile 1 (lowest) | 824 (30.6%) | 8840 (24.5%) | 0.14 | 9664 (24.9%) |
| Q2 | 584 (21.7%) | 8178 (22.7%) | 0.02 | 8762 (22.6%) |
| Q3 | 488 (18.1%) | 6927 (19.2%) | 0.03 | 7415 (19.1%) |
| Q4 | 464 (17.2%) | 6261 (17.3%) | 0 | 6725 (17.3%) |
| Q5 (highest) | 333 (12.4%) | 5852 (16.2%) | 0.11 | 6185 (15.9%) |
| Number of John’s Hopkins ADG comorbidities | ||||
| Mean ± SD | 7.64 ± 3.55 | 8.16 ± 3.77 | 0.14 | 8.12 ± 3.76 |
| Median (IQR) | 7 (5–10) | 8 (5–11) | 0.14 | 8 (5–11) |
| 0–5 | 779 (28.9%) | 9179 (25.4%) | 0.08 | 9958 (25.7%) |
| 6–9 | 1156 (42.9%) | 14,246 (39.5%) | 0.07 | 15,402 (39.7%) |
| 10+ | 761 (28.2%) | 12,667 (35.1%) | 0.15 | 13,428 (34.6%) |
| Lung cancer type | ||||
| Adenocarcinoma | 1336 (49.6%) | 13,478 (37.3%) | 0.25 | 14,814 (38.2%) |
| Small cell | 340 (12.6%) | 6158 (17.1%) | 0.13 | 6498 (16.8%) |
| Squamous cell | 363 (13.5%) | 6169 (17.1%) | 0.1 | 6532 (16.8%) |
| Other | 657 (24.4%) | 10,287 (28.5%) | 0.09 | 10,944 (28.2%) |
| No. PCP visits 6–30 months < index—all primary care providers | ||||
| Mean ± SD | 9.41 ± 8.50 | 8.95 ± 8.67 | 0.05 | 8.98 ± 8.66 |
| Median (IQR) | 8 (4–13) | 7 (3–12) | 0.08 | 7 (3–12) |
| No. PCP visits 6–30 months < index—patient’s usual provider of care | ||||
| Mean ± SD | 6.91 ± 7.07 | 6.89 ± 7.38 | 0 | 6.89 ± 7.36 |
| Median (IQR) | 5 (1–10) | 5 (2–10) | 0.01 | 5 (2–10) |
| Usual Provider of Care (UPC) index | ||||
| 0 visits | 240 (8.9%) | 3438 (9.5%) | 0.02 | 3678 (9.5%) |
| 1–2 visits | 307 (11.4%) | 4898 (13.6%) | 0.07 | 5205 (13.4%) |
| UPC <= 75% | 593 (22.0%) | 6522 (18.1%) | 0.1 | 7115 (18.3%) |
| UPC > 75% | 1556 (57.7%) | 21,234 (58.8%) | 0.02 | 22,790 (58.8%) |
| Characteristics | Immigrants | Long-Term Residents | ||||
|---|---|---|---|---|---|---|
| Early Stage (n = 692) | Late Stage (n = 1713) | Standardized Difference | Early Stage (n = 9595) | Late Stage (n = 22,394) | Standardized Difference | |
| Sex | ||||||
| Female | 341 (49.3%) | 630 (36.8%) | 0.25 | 5141 (53.6%) | 10,759 (48.0%) | 0.11 |
| Male | 351 (50.7%) | 1083 (63.2%) | 0.25 | 4454 (46.4%) | 11,635 (52.0%) | 0.11 |
| Age at diagnosis | ||||||
| Mean ± SD | 67.77 ± 11.40 | 66.84 ± 12.05 | 0.08 | 71.25 ± 9.75 | 70.70 ± 10.43 | 0.05 |
| Median (IQR) | 68 (59–77) | 67 (58–76) | 0.07 | 72 (65–78) | 71 (63–78) | 0.06 |
| Age group (years) | ||||||
| 40–64 | 274 (39.6%) | 746 (43.5%) | 0.08 | 2359 (24.6%) | 6424 (28.7%) | 0.09 |
| 65–74 | 195 (28.2%) | 460 (26.9%) | 0.03 | 3478 (36.2%) | 7573 (33.8%) | 0.05 |
| 75+ | 223 (32.2%) | 507 (29.6%) | 0.06 | 3758 (39.2%) | 8397 (37.5%) | 0.03 |
| Neighborhood income quintile | ||||||
| Quintile 1 (lowest) | 216 (31.2%) | 527 (30.8%) | 0.01 | 2280 (23.8%) | 5511 (24.6%) | 0.02 |
| Q2 | 152 (22.0%) | 378 (22.1%) | 0 | 2117 (22.1%) | 5115 (22.8%) | 0.02 |
| Q3 | 124 (17.9%) | 289 (16.9%) | 0.03 | 1839 (19.2%) | 4301 (19.2%) | 0 |
| Q4 | 98 (14.2%) | 321 (18.7%) | 0.12 | 1735 (18.1%) | 3861 (17.2%) | 0.02 |
| Q5 (highest) | 100 (14.5%) | 197 (11.5%) | 0.09 | 1619 (16.9%) | 3583 (16.0%) | 0.02 |
| No. John’s Hopkins ADG comorbidities | ||||||
| Mean ± SD | 8.39 ± 3.43 | 7.25 ± 3.43 | 0.33 | 8.88 ± 3.60 | 7.77 ± 3.74 | 0.3 |
| Median (IQR) | 8 (6–11) | 7 (5–9) | 0.32 | 9 (6–11) | 8 (5–10) | 0.31 |
| 0–5 | 138 (19.9%) | 559 (32.6%) | 0.29 | 1706 (17.8%) | 6503 (29.0%) | 0.27 |
| 6–9 | 308 (44.5%) | 743 (43.4%) | 0.02 | 3878 (40.4%) | 8899 (39.7%) | 0.01 |
| 10+ | 246 (35.5%) | 411 (24.0%) | 0.25 | 4011 (41.8%) | 6992 (31.2%) | 0.22 |
| Lung cancer type | ||||||
| Adenocarcinoma | 380 (54.9%) | 887 (51.8%) | 0.06 | 4036 (42.1%) | 8788 (39.2%) | 0.06 |
| Small cell | 24 (3.5%) | 300 (17.5%) | 0.47 | 639 (6.7%) | 5319 (23.8%) | 0.49 |
| Squamous cell | 100 (14.5%) | 241 (14.1%) | 0.01 | 2290 (23.9%) | 3628 (16.2%) | 0.19 |
| Other | 188 (27.2%) | 285 (16.6%) | 0.26 | 2630 (27.4%) | 4659 (20.8%) | 0.15 |
| No. PCP visits 6–30 months < index—all primary care providers | ||||||
| Mean ± SD | 10.47 ± 7.85 | 8.92 ± 8.54 | 0.19 | 9.89 ± 8.32 | 8.59 ± 8.74 | 0.15 |
| Median (IQR) | 9 (5–14) | 7 (3–12) | 0.27 | 8 (4–13) | 6 (3–12) | 0.22 |
| No. PCP visits 6–30 months < index—patient’s usual provider of care | ||||||
| Mean ± SD | 7.65 ± 6.57 | 6.52 ± 7.03 | 0.17 | 7.67 ± 7.34 | 6.63 ± 7.32 | 0.14 |
| Median (IQR) | 7 (3–11) | 5 (1–9) | 0.24 | 6 (2–11) | 5 (1–9) | 0.19 |
| UPC index | ||||||
| 0 visits | 45 (6.5%) | 159 (9.3%) | 0.1 | 687 (7.2%) | 2123 (9.5%) | 0.08 |
| 1–2 visits | 60 (8.7%) | 211 (12.3%) | 0.12 | 1103 (11.5%) | 3195 (14.3%) | 0.08 |
| UPC <= 75% | 167 (24.1%) | 380 (22.2%) | 0.05 | 1788 (18.6%) | 4010 (17.9%) | 0.02 |
| UPC > 75% | 420 (60.7%) | 963 (56.2%) | 0.09 | 6017 (62.7%) | 13,066 (58.3%) | 0.09 |
| Variables | Relative Risk (95% Confidence Interval) |
|---|---|
| Unadjusted | |
| Immigrant (vs. long term resident) | 1.02 (0.99–1.05) |
| Age and sex-adjusted | |
| Immigrant (vs. long term resident) | 1.00 (0.98–1.05) |
| Male (vs. female) | 1.08 (1.06–1.09) |
| Age (as a continuous variable) | 1.00 (1.00–1.00) |
| Full model | |
| Immigrant (vs. long term resident) | 1.01 (0.99–1.04) |
| Male (vs. female) | 1.07 (1.05–1.08) |
| Age (as a continuous variable) | 1.00 (1.00–1.00) |
| Neighborhood income quintile (quintile 5 as the reference group) | |
| Income quintile 1 (lowest) | 1.02 (1.00–1.04) |
| Income quintile 2 | 1.02 (0.99–1.04) |
| Income quintile 3 | 1.01 (0.99–1.03) |
| Income quintile 4 | 1.01 (0.98–1.03) |
| Comorbidities (0–5 ADGs as the reference group) | |
| 6–9 ADGs | 0.89 (0.88–0.91) |
| 10 + ADGs | 0.82 (0.80–0.84) |
| Primary care visits in the 6–30 months prior to diagnosis (as a continuous variable) | 1.00 (1.00–1.00) |
| Continuity of care (Usual Provider of Care Index of 75% or greater as the reference group) | |
| 0 visits to primary care | 1.02 (1.00–1.05) |
| 1–2 visits to primary care | 1.02 (1.00–1.04) |
| Usual Provider of Care Index less than 75% | 1.02 (1.01–1.04) |
| Lung cancer type (adenocarcinoma as the reference group) | |
| Small cell | 1.29 (1.27–1.31) |
| Squamous cell | 0.89 (0.87–0.91) |
| Other | 0.93 (0.91–0.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lofters, A.K.; Gatov, E.; Lu, H.; Baxter, N.N.; Guilcher, S.J.T.; Kopp, A.; Vahabi, M.; Datta, G.D. Lung Cancer Inequalities in Stage of Diagnosis in Ontario, Canada. Curr. Oncol. 2021, 28, 1946-1956. https://doi.org/10.3390/curroncol28030181
Lofters AK, Gatov E, Lu H, Baxter NN, Guilcher SJT, Kopp A, Vahabi M, Datta GD. Lung Cancer Inequalities in Stage of Diagnosis in Ontario, Canada. Current Oncology. 2021; 28(3):1946-1956. https://doi.org/10.3390/curroncol28030181
Chicago/Turabian StyleLofters, Aisha K., Evgenia Gatov, Hong Lu, Nancy N. Baxter, Sara J. T. Guilcher, Alexander Kopp, Mandana Vahabi, and Geetanjali D. Datta. 2021. "Lung Cancer Inequalities in Stage of Diagnosis in Ontario, Canada" Current Oncology 28, no. 3: 1946-1956. https://doi.org/10.3390/curroncol28030181
APA StyleLofters, A. K., Gatov, E., Lu, H., Baxter, N. N., Guilcher, S. J. T., Kopp, A., Vahabi, M., & Datta, G. D. (2021). Lung Cancer Inequalities in Stage of Diagnosis in Ontario, Canada. Current Oncology, 28(3), 1946-1956. https://doi.org/10.3390/curroncol28030181







