Successful Alectinib Treatment for Carcinoma of Unknown Primary with EML4-ALK Fusion Gene: A Case Report
Abstract
1. Introduction
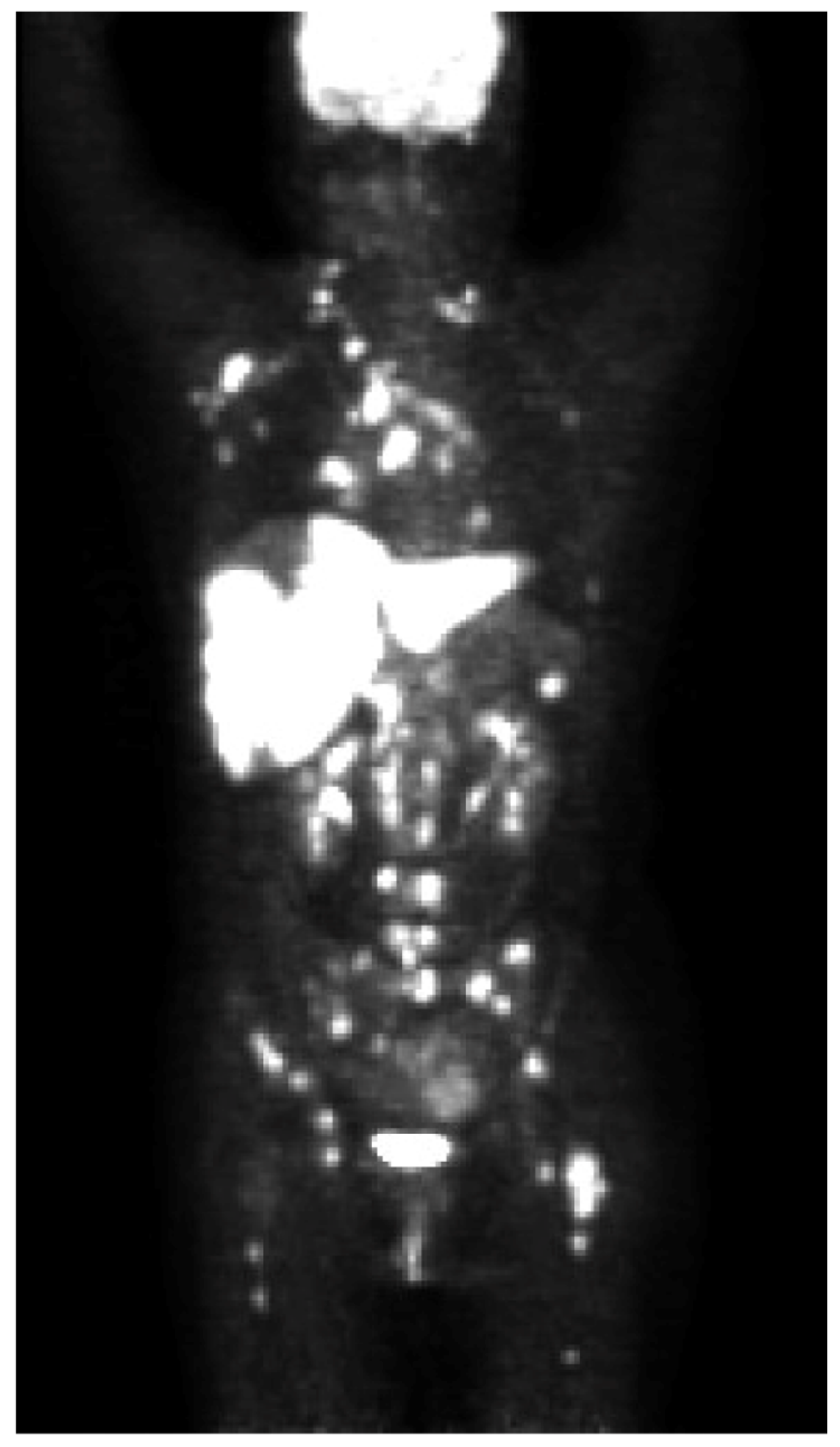
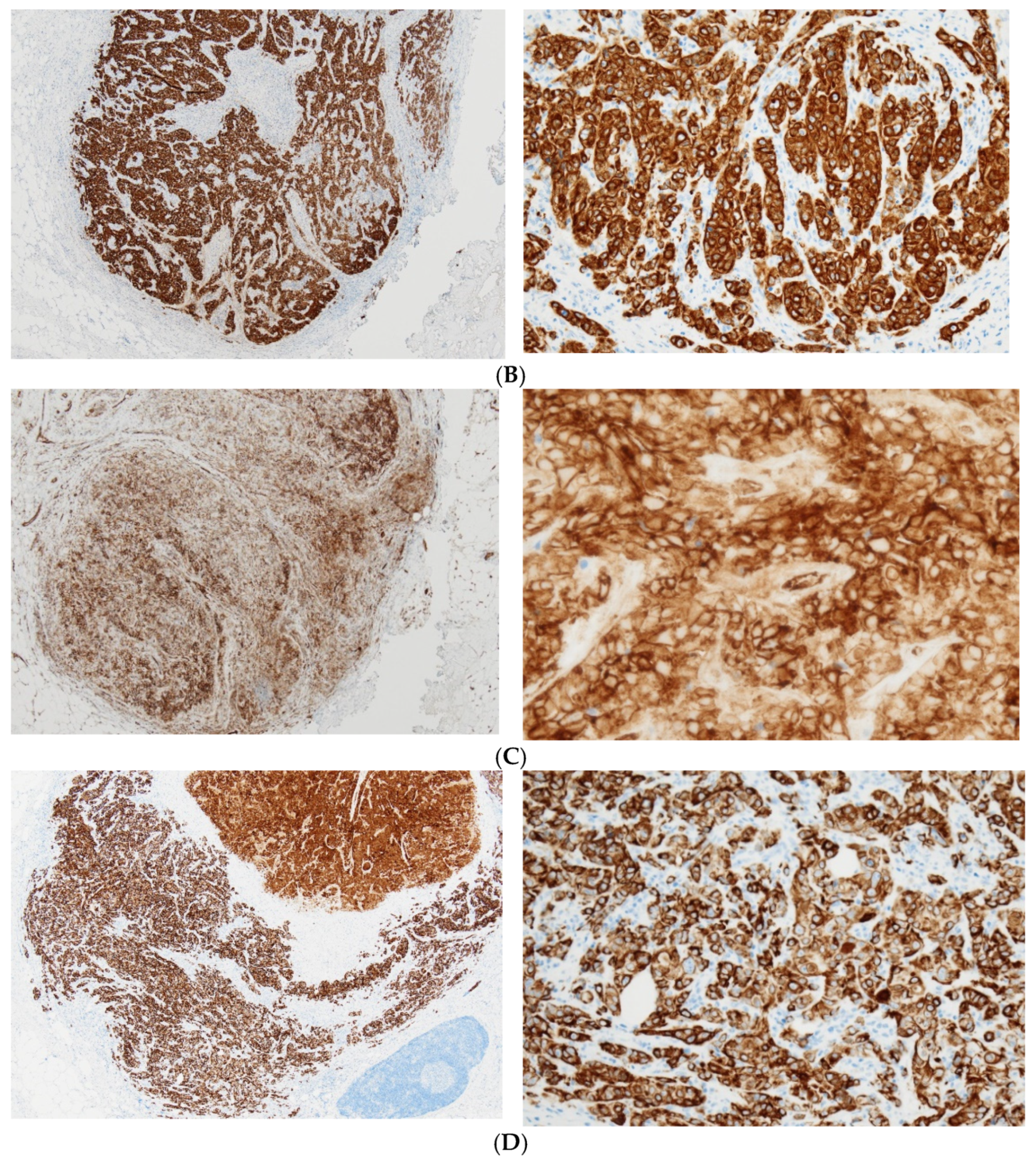
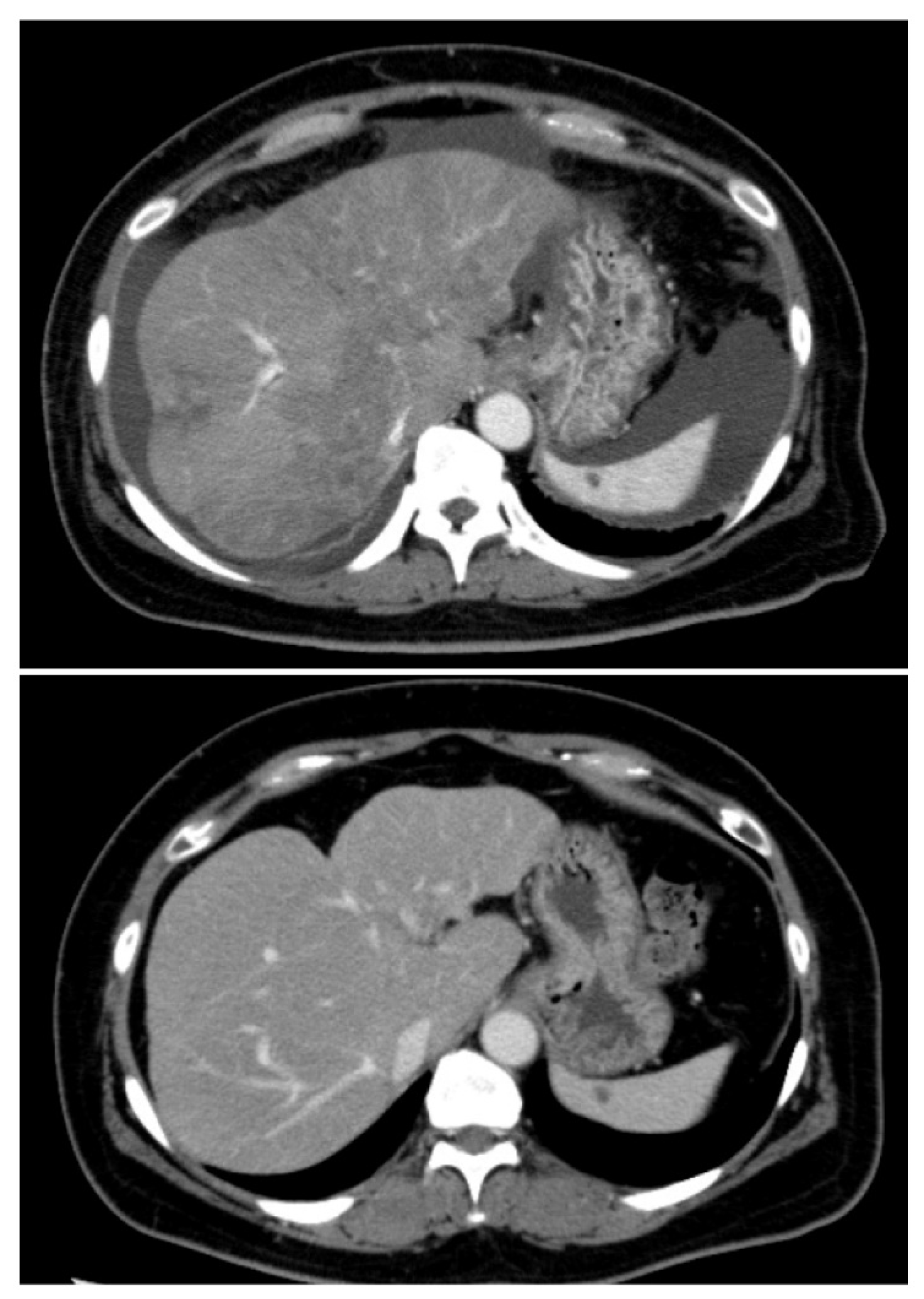
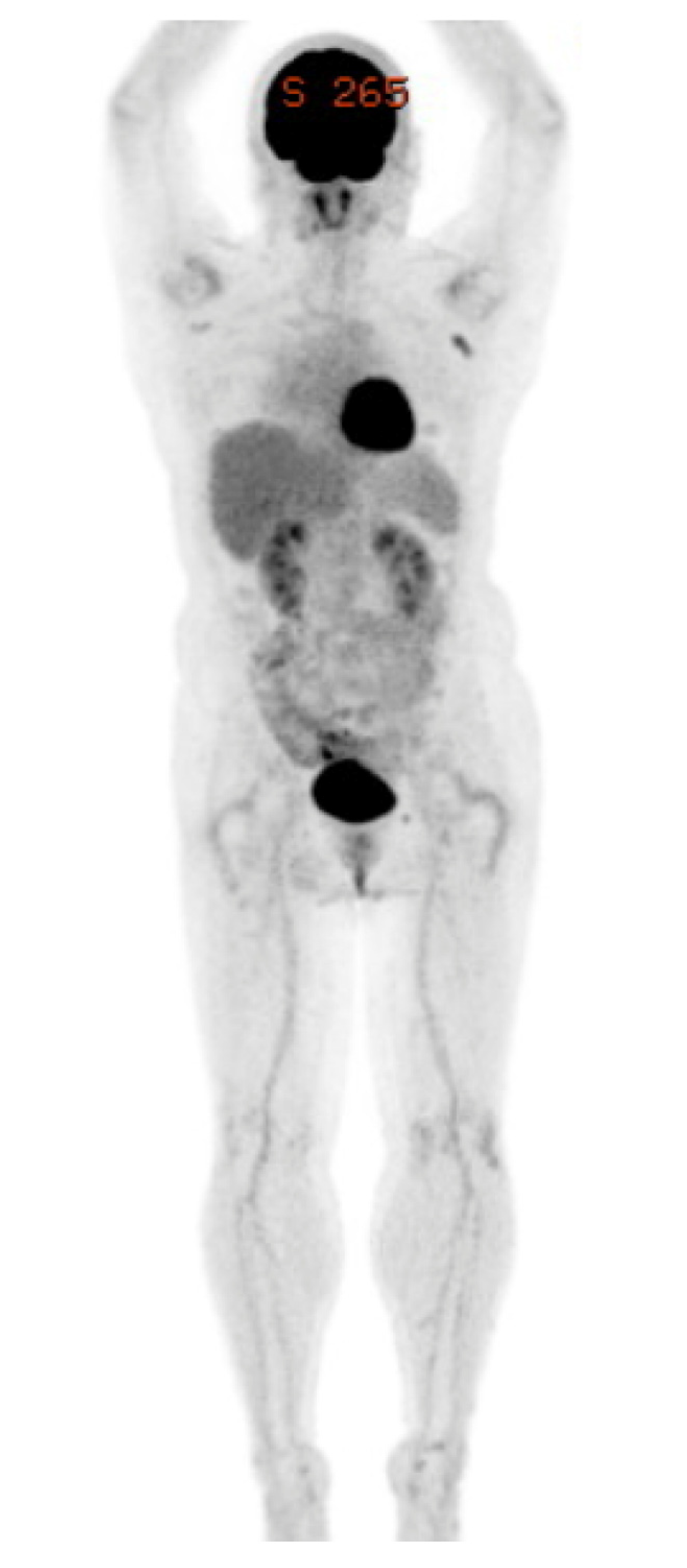
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hainsworth, J.D.; Greco, F.A. Treatment of patients with cancer of an unknown primary site. N. Engl. J. Med. 1993, 329, 257–263. [Google Scholar] [PubMed]
- Greco, F.A.; Pavlidis, N. Treatment for patients with unknown primary carcinoma and unfavorable prognostic factors. Semin. Oncol. 2009, 36, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.R.; Hornick, J.L. Metastatic carcinoma of unknown primary: Diagnostic approach using immunohistochemistry. Adv. Anat. Pathol. 2015, 22, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Massard, C.; Loriot, Y.; Fizazi, K. Carcinomas of an unknown primary origin—Diagnosis and treatment. Nat. Rev. Clin. Oncol. 2011, 8, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Ando, M.; Yatabe, Y.; Mitani, S.; Honda, K.; Masuishi, T.; Narita, Y.; Taniguchi, H.; Kadowaki, S.; Ura, T.; et al. Site-specific chemotherapy based on predicted primary site by pathological profile for carcinoma of unknown primary site. Clin. Oncol. 2018, 30, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Takiguchi, Y.; Minami, H.; Akiyoshi, K.; Segawa, Y.; Ueda, H.; Iwamoto, Y.; Kondoh, C.; Matsumoto, K.; Takahashi, S.; et al. Site-specific and targeted therapy based on molecular profiling by next-generation sequencing for cancer of unknown primary site: A nonrandomized phase 2 clinical trial. JAMA Oncol. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Ross, J.S.; Sokol, E.S.; Moch, H.; Mileshkin, L.; Baciarello, G.; Losa, F.; Beringer, A.; Thomas, M.; Elvin, J.A.; Ngo, N.; et al. Comprehensive genomic profiling of carcinoma of unknown primary origin: Retrospective molecular classification considering the CUPISCO study design. Oncologist 2021, 26, e394–e402. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. ALEX Trial Investigators. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.W.; Mok, T.; Polli, A.; et al. CROWN Trial Investigators. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.; Han, J.Y.; Lee, J.S.; Hochmair, M.J.; Li, J.Y.; Chang, G.C.; Lee, K.H.; et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Choi, Y.H.; Lee, H.R.; Na, I.I.; Yuh, Y.J.; Kim, B.S.; Chung, I.J.; Bae, W.K.; Shim, H.J.; Song, E.K.; et al. A phase II trial of modified FOLFOX6 as first-line therapy for adenocarcinoma of an unknown primary site. Cancer Chemother. Pharmacol. 2016, 77, 163–168. [Google Scholar] [CrossRef]
- Varghese, A.M.; Arora, A.; Capanu, M.; Camacho, N.; Won, H.H.; Zehir, A.; Gao, J.; Chakravarty, D.; Schultz, N.; Klimstra, D.S.; et al. Clinical and molecular characterization of patients with cancer of unknown primary in the modern era. Ann. Oncol. 2017, 28, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Takeuchi, K.; Togashi, Y.; Hatano, S.; Ninomiya, H.; Motoi, N.; Mun, M.Y.; Sakao, Y.; Okumura, S.; Nakagawa, K.; et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod. Pathol. 2009, 22, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Li, L.; Guan, Y.; Soriano, R.; Rivers, C.S.; Mohan, S.; Pandita, A.; Tang, J.; Modrusan, Z. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol. Cancer Res. 2009, 7, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Peng, L.; Wu, W.; Zheng, Y.; Jiang, W.; Zhang, H.; Tong, Z.; Liu, L.; Ma, R.; Wang, L.; et al. Carcinoma of unknown primary with EML4-ALK fusion response to ALK inhibitors. Oncologist 2019, 24, 449–454. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiyama, K.; Izumika, A.; Iwakoshi, A.; Nishibori, R.; Sato, M.; Shiraishi, K.; Hattori, H.; Nishimura, R.; Kitagawa, C. Successful Alectinib Treatment for Carcinoma of Unknown Primary with EML4-ALK Fusion Gene: A Case Report. Curr. Oncol. 2021, 28, 1938-1945. https://doi.org/10.3390/curroncol28030180
Sugiyama K, Izumika A, Iwakoshi A, Nishibori R, Sato M, Shiraishi K, Hattori H, Nishimura R, Kitagawa C. Successful Alectinib Treatment for Carcinoma of Unknown Primary with EML4-ALK Fusion Gene: A Case Report. Current Oncology. 2021; 28(3):1938-1945. https://doi.org/10.3390/curroncol28030180
Chicago/Turabian StyleSugiyama, Keiji, Ai Izumika, Akari Iwakoshi, Riko Nishibori, Mariko Sato, Kazuhiro Shiraishi, Hiroyoshi Hattori, Rieko Nishimura, and Chiyoe Kitagawa. 2021. "Successful Alectinib Treatment for Carcinoma of Unknown Primary with EML4-ALK Fusion Gene: A Case Report" Current Oncology 28, no. 3: 1938-1945. https://doi.org/10.3390/curroncol28030180
APA StyleSugiyama, K., Izumika, A., Iwakoshi, A., Nishibori, R., Sato, M., Shiraishi, K., Hattori, H., Nishimura, R., & Kitagawa, C. (2021). Successful Alectinib Treatment for Carcinoma of Unknown Primary with EML4-ALK Fusion Gene: A Case Report. Current Oncology, 28(3), 1938-1945. https://doi.org/10.3390/curroncol28030180



