Abstract
Surgery is the only potential curative option of CRLM if resectable. The curative approach in patients over 70 years old is challenging mainly because of comorbidities and other geriatric syndromes. Herein, we report outcomes of older patients with resectable CRLM in our center. We retrospectively analyzed characteristics and outcomes of older patients with CRLM operated at “Centre Hospitalier de l’Université de Montréal” (CHUM) between 2010 and 2019. We identified 210 patients aged ≥70 years with a median age of 76 (range: 70–85). CRLM were synchronous in 56% of patients. Median disease-free survival (DFS) was 41.3 months. Median overall survival (OS) was 62.2 months and estimated 5-year survival rate was 51.5% similar to those of younger counterparts. Patients with metachronous CRLM had a trend to a higher OS compared to those with synchronous disease (67.2 vs. 58.7 months; p = 0.42). Factors associated with lower survival in the multivariate analysis were right-sided tumors and increased Charlson Comorbidity index (CCI). Survival outcomes of patients aged ≥70 years were comparable to those of younger patients and those reported in the literature. Age should not be a limiting factor in the curative management of older patients with resectable CRLM.
1. Introduction
Colorectal cancer is the fourth most common cancer worldwide and represents about 6% of all new cancer cases [1]. In Canada, colorectal cancer is the third leading cause of cancer deaths with a five-year survival rate of 65% [2]. Age remains an important risk factor, as incidence of colorectal cancers increase in patients more than 70 years old [3]. With the increasing life span of the population, the number of older patients with CRLM is rising, leading to required optimal management [4].
Surgery remains the only potentially curative treatment for localized disease or for tumors with limited liver metastases [5,6]. Adjuvant chemotherapy in Stage II is usually restricted to patients with high risk, although controversial, and is widely recommended for stage III disease. 5-fluorouracil (5-FU)/leucovorin (LV) has shown to improve disease-free survival (DFS) and overall survival (OS) in all age groups, along with older patients [7]. MOSAIC trial demonstrated a three-year DFS of 78.2% when oxaliplatin was added to 5-FU/LV compared to 72.9% for 5-FU/LV alone; p = 0.002 [8]. However, the benefit of oxaliplatin-based chemotherapy in older patients is debatable [9,10]. Moreover, the role and the benefit of perioperative chemotherapy for resectable CRLM is not yet well determined.
On the other hand, resection of CRLM shows a survival benefit. Age has been identified as a negative prognostic factor in some population-based trials that assessed the mortality of surgical resection of colorectal tumors [11]. Many physicians are reluctant to carry a curative approach in older patients, taking into consideration the associated morbidities and early mortality. Our group previously reported that hepatectomies in older patients did not have a higher 90-days mortality rate when compared to younger patients, though it carried higher risk of complications [12]. We aimed to report our center’s experience and survival analysis of older patients with CRLM who underwent curative liver resection.
2. Materials and Methods
We conducted a retrospective analysis of our 210 older patients who underwent hepatectomies for resectable CRLM between 2010 and 2019 after the approval of the Institutional Review Board (IRB). In the weekly multidisciplinary tumor board, CT scans were reviewed by local radiologists at diagnosis and after neoadjuvant chemotherapy, if indicated. Surgical approach and chemotherapy regimen were also discussed and determined for every patient.
Descriptive statistics were used to calculate proportions, percentages and ratios. The Charlson Comorbidity Index (CCI), a widely accepted tool for risk assessment and post-operative survival predictor, was modified and calculated with the exclusion of cancer as a weighted factor [13]. This modification was done to accurately identify the influence of comorbidities on outcomes in a cohort of patients with cancer. NLR (the absolute neutrophils count divided by the absolute lymphocytes count) was calculated based on counts collected from preoperative laboratory analyses done in our center, just before resection of liver metastases. A cut-off value of 3.0 was adopted to discriminate patients with low (NLR < 3) versus high NLR (≥3), as used in a previous retrospective trial of metastatic colorectal cancer [14].
Patients who did not experience the event of interest during data collection were censored at the date of last available follow-up. The Kaplan-Meier method was used to obtain estimates of median survival. The log-rank test was used to compare the survival curves between groups. A Cox proportional-hazards model was used to calculate hazard ratios for both univariate and multivariate analyses. Statistical tests were two-sided and a p value < 0.05 was considered as significant. Statistical analyses and figures were performed with R v.4.0, R Foundation for Statistical Computing (Vienna, Autria) and GraphPad Prism version 7, GraphPad Software( San Diego, CA, USA)
3. Results
A total of 210 patients aged 70 years and older who underwent resection of CRLM between 2010 and 2019 were identified. The median age of patients was 76 years old (range: 70–85). Mean carcinoembryonic antigen (CEA) was 82 µg/L. Some clinical and surgical characteristics are presented in Table 1.

Table 1.
Clinical and Surgical characteristics of the 210 patients of the cohort.
The majority of patients had only liver metastases, and about 73% of patients had no more than two liver lesions. Localization of liver metastases and surgical approach are also summarized in Table 1. Of the 204 patients with available data, 173 (84.8%) patients had received neoadjuvant systemic chemotherapy.
Median disease-free survival (DFS) and overall survival (OS) of all older patients who underwent surgical resection of CRLM were 41.3 and 62.2 months, respectively. The estimated five-year survival rate was 51.5%. There was no difference in overall survival between older patients when compared to patients < 70 years of age; hazard ratio (HR) = 0.99; p = 0.95 (Figure 1).

Figure 1.
Survival Curves of older patients with resectable CRLM. (a): Disease-free survival (DFS). (b): Overall survival (OS) of older patients compared to that of younger patients.
Patients with left-sided colon cancer had a better overall survival compared to right-sided disease, with a median OS of 67.2 months compared to 46.8 months respectively, HR = 0.69; p = 0.017 (Figure 2a).

Figure 2.
Survival curves of older patients with CRLM according to some identified factors. (a): overall survival (OS) of patients with right-sided primary compared to left-sided, Rt: Right; Lt: Left. (b): overall survival (OS) according to the timing of CRLM, synchronous versus metachronous; *: significant p-value.
Patients with metachronous CRLM did not show a statistically significant improved survival compared to those with synchronous disease (67.2 vs. 58.7 months; p = 0.42) (Figure 2b).
High Neutrophil/Lymphocyte Ratio (NLR ≥ 3 versus <3) showed a trend toward a lower survival outcome. Median DFS was 36 vs. 76 months, HR was 0.68, p = 0.07 and median OS = 58.7 months vs. not reached, and HR was 0.63, p = 0.09, for high and low NLR, respectively (Figure 3).

Figure 3.
Survival curves of older patients with resectable CRLM according to NLR. (a): disease-free survival (DFS) of patients with NLR < 3 compared to patients with NLR ≥ 3. (b): overall survival (OS) of patients with NLR < 3 compared to those with NLR ≥ 3. NLR: Neutrophil/Lymphocyte Ratio.
Comorbidities had shown a major impact on survival. Patients with low CCI showed the highest survival. To simplify the curve, patients with CCI of five, six and seven were combined as their survival was similar. The median OS was not reached for patients with CCI of 4, compared to 62 months for patients with CCI of five, six and seven and 40.2 months for those with a CCI of 8 (Figure 4).

Figure 4.
Overall survival for patients with resectable CRLM according to CCI groups. Patients were divided to three groups: CCI of 4 vs. CCI of 5, 6 and 7 vs. CCI of 8. CCI: Charlson comorbidity Score; *: significant p-value.
To control for potential confounding factors; a multivariate analysis for overall survival was performed according to certain baseline characteristics and other stratification factors. It showed that age and sex have no impact on the risk of death (p = 0.32 and p = 0.92 respectively) (Figure 5).
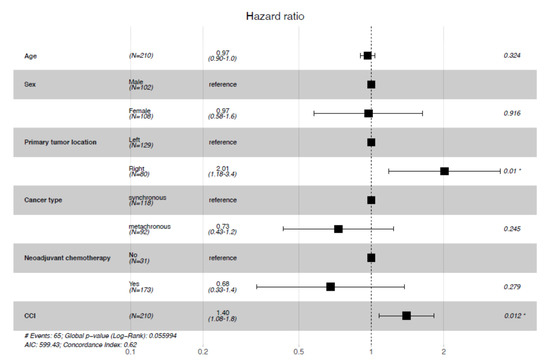
Figure 5.
Multivariate analysis for overall survival. CCI: Charlson Comorbidity Index, *: significant p-value.
With respect to CCI, this analysis confirmed what was observed in a univariate setting, as a higher CCI leads to a higher risk of death (HR = 1.40, 95% Confidence Interval (CI)= [1.08–1.8].). Similarly, patients with right-sided colon cancer had a worse prognosis (HR = 2.01, 95% CI= [1.18–3.4].). No significant influence on overall survival was observed between patients who received neoadjuvant chemotherapy and those who did not, and between synchronous and metachronous metastases.
4. Discussion
This retrospective study showed that patients with CRLM who underwent surgical resection of the liver lesions and were aged 70 years and older had an overall survival comparable to patients less than 70 years old. It is well known that the liver is a common site of spread and metastases of tumors of the colon and high rectum, as their venous drainage is through the portal vein to the liver. Despite advances in biological targeted agents, combined with chemotherapy, survival of patients with stage IV disease remains limited, with a median of about 26–29 months [15,16]. Complete resection of liver metastases improves survival, with a median reaching up to 40 months and a five-year survival rate between 28–48% [6]. Our patients had a five-year survival rate of 51.5%, which is consistent with survival of patients enrolled in the EORTC prospective phase III trial [17]. Our study demonstrated that older patients had similar outcomes to younger counterparts, confirmed by the multivariate analysis of survival. In a systemic review, Manceau et al. demonstrated that cancer-specific survival did not decrease with age in patients undergoing surgical resection of rectal tumors [18]. In addition, age was not an independent factor for survival in older patients with limited CRLM who underwent liver resection, as shown by a propensity score matching analysis [19]. Similar survival outcomes between old and young patients were also observed in the Asian population [20]. Figueras et al. reported in a retrospective analysis that included 160 older patients, that despite higher perioperative mortality, the OS and DFS were similar to those of younger patients in recent years [21]. In a French large cohort of patients who underwent liver resection of CRLM, 20% of patients were aged more than 70 years. Although three-year survival rate was inferior to that of younger patients, 57.1% vs. 60.2%, respectively; p < 0.001, the group concluded that resection of CRLM in older patients can achieve reasonable survival with acceptable morbidity rates [22].
We previously reported that hepatectomies are safe in older patients and don’t cause higher postoperative mortality when compared to younger patients. However, older patients were prone to have one or more postoperative complications, especially infectious, when compared to younger patients [12]. Other reports showed that first and repeated resection of liver metastases can be safely performed in older patients [22,23,24]. Nevertheless, our study demonstrated that an increase in comorbidities leads to a decrease in survival. A systemic review and meta-analysis of 11 studies by Van Tuil et al., demonstrated that patients ≥ 70 years old had higher rates of postoperative morbidity and mortality and a lower five-year survival rate compared to younger patients. The same review included four other studies of patients ≥ 75 years old, that showed a comparable five-year survival rate to younger patients (42 vs. 32%; p = 0.06) [25]. There was an association observed between CCI and postoperative complications in older patients undergoing local resection of gastric neoplasms [26]. Age-adjusted Charlson comorbidity index score was an independent predictor of survival in patients with gastrointestinal tumors who underwent surgical resection in a retrospective study of 315,464 patients in China [27]. Thus, identification of comorbidities and patient assessment using screening tools and scores are to be considered rather than age. A cross-sectional study of 418 older patients with solid or hematological tumors showed that 16.7% of these patients had their initial plan of treatment changed after a comprehensive geriatric assessment (CGA) performed by a multidisciplinary team [28]. A review of evidence in 2017 concluded that CGA is feasible and can identify patients at a higher risk of adverse events such as mortality, functional decline, surgical complications and chemotherapy toxicity [29]. On the other hand, a systemic review of 178 articles, including six studies among geriatric patients undergoing major oncologic surgery, identified some predictors of postoperative complications. This review showed that no CGA predictors were identified for postoperative mortality [30]. However, the proper screening tool to predict which patients will truly benefit from a CGA is yet to be identified, as a phase II trial evaluating three different screening tools distinguished discordance in outcomes [31]. Further trials are warranted to determine the CGA effectiveness in different surgical and oncological situations.
Perioperative chemotherapy used in our center was mostly FOLFOX (Folinic Acid-Fluorouracil-oxaliplatin). Patients could receive FOLFIRI (Folinic Acid-Fluorouracil-irinotecan), also. Choice of regimen was determined according to previous oxaliplatin exposure and clinician choice. Bevacizumab was added to chemotherapy regimen according to the physician’s choice, as well as standard clinical indication and contraindications. In our study, we showed that there was no difference in survival between patients who received perioperative chemotherapy and those who did not. In the phase III EORTC 40983 prospective trial of 364 patients, perioperative chemotherapy with FOLFOX did not increase survival compared to surgery of CRLM alone [17]. On the other hand, a pooled analysis of 278 patients randomized in two phase III trials, i.e., an FFCD 9002 and an ENG trial, showed a trend to better PFS and OS with adjuvant chemotherapy compared to surgery alone after resection of liver or lung colorectal metastases [32]. It should be noted that only 20% of patients in this analysis were aged 70 years and more, whereas in the EORTC 40983 trial, older patients were not specified. Absence of postoperative chemotherapy was an independent predictor of survival in the French cohort where older patients accounted for about 20% [22]. Thus, perioperative chemotherapy is to be considered cautiously in older patients after weighing the risks and benefits.
We also observed that patients with right-sided cancer had worse prognoses. Sidedness is considered a prognostic factor, as many observational studies and clinical trials have shown. Benedix et al., and other authors, reported better overall survival in left sided colorectal tumors [33,34]. The same observation was seen in clinical trials assessing the addition of biological agents like cetuximab and panitumumab, where right-sided tumors showed a lower median overall survival across treatment arms [35,36]. These results are in contrast to a large analysis that showed no difference in survival except in stage III, where right-sided tumors carried a worse prognosis [37]. Thus, sidedness matters, especially in the choice of which biological agent to use [35,36].
In addition, a trend towards better survival with metachronous metastases was observed in our cohort. Engstrand et al. showed that the time of detection of liver metastases lacked prognostic value [38]. There was no difference in the overall survival for synchronous versus metachronous detection in their retrospective analysis. No clear pattern can be concluded regarding the significance of timing of detection in operated liver metastases [38,39,40].
There is increasing evidence that a complex interaction between tumor and host systemic inflammatory response exists and determines outcomes [41]. Elevated preoperative NLR was associated with a decrease in the disease-free survival of colorectal tumors undergoing curative resection [42,43,44]. The same results were observed in metastatic colorectal cancers receiving palliative chemotherapy, even at lower thresholds [14]. In our cohort, high NLR showed nonstatistically significant unfavorable outcomes.
Our study has several limitations, including the retrospective analysis of small number of patients, which can lead to potential bias in the conclusions drawn. As our center is a referral center for hepatic surgeries, some patients continued the management after discharge at their regional hospitals; thus we sometimes lacked detailed follow-up data, including adjuvant therapy. We also lacked proper stratification of patients, as we used mainly comorbidities. Differences in the health and function of patients can be accurately assessed using a frailty score. This was difficult to apply in this retrospective study. In addition, the results observed in our study support the evidence that older patients have similar long-term survival as younger patients with some prognostic indicators. Some of these prognostic factors were considered to have an impact on survival, based on trends observed in Kaplan-Meier curves. Preoperative NLR was evaluated retrospectively and a cut-off of 3 was selected to be consistent with other studies. The prognostic role, and its clinical implication, need to be validated in prospective trials. However, the proper patient selection method and multimodality treatments would be only established by a randomized prospective trial.
5. Conclusions
Resection of CRLM in older patients is feasible and demonstrated similar outcomes to younger counterparts. Chronological age should not be a contraindication to curative treatments. Therefore, older patients should be offered the same curative treatment as younger ones, with individualized management according to shared decision making. Potential comorbidities should be carefully identified, and the benefit of perioperative chemotherapy should be properly assessed to minimize perioperative complications and mortality. Consequently, multimodality treatment of CRLM in older patients should be selectively considered. Comprehensive geriatric assessment and other screening tools are to be used to properly identify these patients. Recruiting older patients in clinical trials should be encouraged.
Author Contributions
M.T. conceived the original idea and supervised the project and the analysis. A database of patients who underwent resection of liver metastases was provided by F.V.-M. and L.M. did the data collection and submitted the project to the IRB for approval. R.N. with the help of C.R. contributed in performing the analysis. R.N. wrote the manuscript. All other authors, F.A., J.-P.A., M.D., R.L. (Real Lapointe), R.L. (Richard Letourneau), M.P., A.R. and S.T. participated in editing, critical review and approval of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Centre Hospitalier de l’Université de Montréal (ethics approval project number: 19.093; 18,000) on 13 June 2019.
Informed Consent Statement
Patient consent was waived as this is a retrospective study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Society. Cancer-Specific-Stats 2020. 2020. Available online: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics%20supplementary%20information/2020/2020_cancer-specific-stats.pdf?la=en (accessed on 30 November 2020).
- Edwards, B.K.; Howe, H.L.; Ries, L.A.; Thun, M.J.; Rosenberg, H.M.; Yancik, R.; Wingo, P.A.; Jemal, A.; Feigal, E.G. An-nual Report to the Nation on the Status of Cancer, 1973-1999, Featuring Implications of Age and Aging on U.S. Cancer Bur-den. Cancer 2002, 94, 2766–2792. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.J.; D’Onofrio, A.; Møller, B.; Black, R.; Martinez-Garcia, C.; Møller, H.; Rahu, M.; Robertson, C.; Schouten, L.J.; La Vecchia, C.; et al. Cancer mortality trends in the EU and acceding countries up to 2015. Ann. Oncol. 2003, 14, 1148–1152. [Google Scholar] [CrossRef]
- Scheele, J.; Stang, R.; Altendorf-Hofmann, A.; Paul, M. Resection of colorectal liver metastases. World J. Surg. 1995, 19, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Govindarajan, A.; Ito, H.; Fong, Y. Surgical Treatment of Hepatic Colorectal Metastasis: Evolving Role in the Setting of Improving Systemic Therapies and Ablative Treatments in the 21st Century. Cancer J. 2010, 16, 103–110. [Google Scholar] [CrossRef]
- Fata, F.; Mirza, A.; Craig Wood, G.; Nair, S.; Law, A.; Gallagher, J.; Ellison, N.; Bernath, A. Efficacy and Toxicity of Adjuvant Chemotherapy in Elderly Patients with Colon Carcinoma: A 10-Year Experience of the Geisinger Medical Center. Cancer 2002, 94, 1931–1938. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Sargent, D.J.; Goldberg, R.M.; Jacobson, S.D.; Macdonald, J.S.; Labianca, R.; Haller, D.G.; Shepherd, L.E.; Seitz, J.F.; Francini, G. A Pooled Analysis of Adjuvant Chemotherapy for Resected Colon Cancer in Elderly Patients. N. Engl. J. Med. 2001, 345, 1091–1097. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Bonnetain, F.; Chibaudel, B.; Lledo, G.; Hickish, T.; Tabernero, J.; Boni, C.; Bachet, J.B.; Teixeira, L.; et al. Adjuvant Therapy with Fluorouracil and Oxaliplatin in Stage Ii and Elderly Patients (between Ages 70 and 75 Years) with Colon Cancer: Subgroup Analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer Trial. J. Clin. Oncol. 2012, 30, 3353–3360. [Google Scholar]
- Duron, J.J.; Duron, E.; Dugue, T.; Pujol, J.; Muscari, F.; Collet, D.; Pessaux, P.; Hay, J.M. Risk Factors for Mortality in Major Digestive Surgery in the Elderly: A Multicenter Prospective Study. Ann. Surg. 2011, 254, 375–382. [Google Scholar] [CrossRef]
- Gryspeerdt, F.; Vandenbroucke-Menu, F.; Lapointe, R.; Turcotte, S.; Dagenais, M.; Plasse, M.; Letourneau, R.; Roy, A. Hepatectomy for malignancy in the aged population: A comparative study. HPB 2019, 21, S99. [Google Scholar] [CrossRef][Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Dell’Aquila, E.; Cremolini, C.; Zeppola, T.; Lonardi, S.; Bergamo, F.; Masi, G.; Stellato, M.; Marmorino, F.; Schirripa, M.; Urbano, F.; et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: A retrospective analysis of the TRIBE study by GONO. Ann. Oncol. 2018, 29, 924–930. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab–Folfox4 Treatment and Ras Mutations in Colorectal Cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Mahoney, M.R.; O’Neil, B.H.; Shaw, J.E.; Polite, B.N.; Hochster, H.S.; Atkins, J.N.; et al. Calgb/Swog 80405: Phase Iii Trial of Irinotec-an/5-Fu/Leucovorin (Folfiri) or Oxaliplatin/5-Fu/Leucovorin (Mfolfox6) with Bevacizumab (Bv) or Cetuximab (Cet) for Pa-tients (Pts) with Kras Wild-Type (Wt) Untreated Metastatic Adenocarcinoma of the Colon or Rectum (Mcrc). J. Clin. Oncol. 2014, 32, LBA3. [Google Scholar]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; O Bechstein, W.; Primrose, J.N.; Walpole, E.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Manceau, G.; Karoui, M.; Werner, A.; Mortensen, N.J.; Hannoun, L. Comparative outcomes of rectal cancer surgery between elderly and non-elderly patients: A systematic review. Lancet Oncol. 2012, 13, e525–e536. [Google Scholar] [CrossRef]
- Jin, K.-M.; Wang, K.; Bao, Q.; Wang, H.W.; Xing, B.C. Liver Resection for Colorectal Liver-Limited Me-tastases in Elderly Patients: A Propensity Score Matching Analysis. World J. Surg. Oncol. 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Chandrasinghe, P.C.; Ediriweera, D.S.; Nazar, T.; Kumarage, S.; Hewavisenthi, J.; Deen, K.I. Overall Survival of Elderly Patients Having Surgery for Colorectal Cancer Is Comparable to Younger Patients: Results from a South Asian Population. Gastroenterol. Res. Pract. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Figueras, J.; Ramos, E.; Lopez-Ben, S.; Torras, J.; Albiol, M.; Llado, L.; González, H.D.; Rafecas, A. Surgical treatment of liver metastases from colorectal carcinoma in elderly patients. When is it worthwhile? Clin. Transl. Oncol. 2007, 9, 392–400. [Google Scholar] [CrossRef]
- Adam, R.; Frilling, A.; Elias, D.; Laurent, C.; Ramos, E.; Capussotti, L.; Poston, G.J.; Wicherts, D.A.; de Haas, R.J. Liver Re-section of Colorectal Metastases in Elderly Patients. Br. J. Surg. 2010, 97, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, T.; Jaeck, D.; Oussoultzoglou, E.; Bachellier, P.; Weber, J.-C. First and Repeat Resection of Colorectal Liver Metastases in Elderly Patients. Ann. Surg. 2004, 240, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Nachmany, I.; Pencovich, N.; Zohar, N.; Goykhman, Y.; Lubezky, N.; Nakache, R.; Klausner, J.M. Resection of colorectal liver metastases in the elderly-Is it justified? J. Surg. Oncol. 2016, 113, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Van Tuil, T.; Dhaif, A.A.; Riele, W.W.T.; van Ramshorst, B.; van Santvoort, H.C. Systematic Review and Meta-Analysis of Liver Resection for Colorectal Metastases in Elderly Patients. Dig. Surg. 2019, 36, 111–123. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.H.; Park, S.-Y.; Park, C.H.; Kim, H.S.; Choi, S.K.; Rew, J.S. Association between Charlson comorbidity index and complications of endoscopic resection of gastric neoplasms in elderly patients. BMC Gastroenterol. 2020, 20, 213. [Google Scholar] [CrossRef]
- Tian, Y.; Jian, Z.; Xu, B.; Liu, H. Age-adjusted Charlson comorbidity index score as predictor of survival of patients with digestive system cancer who have undergone surgical resection. Oncotarget 2017, 8, 79453–79461. [Google Scholar] [CrossRef]
- Sourdet, S.; Brechemier, D.; Steinmeyer, Z.; Gerard, S.; Balardy, L. Impact of the Compre-hensive Geriatric Assessment on Treatment Decision in Geriatric Oncology. BMC Cancer 2020, 20, 384. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.H.; Hsu, T. Comprehensive Geriatric Assessment in the Older Adult with Cancer: A Review. Eur. Urol. Focus 2017, 3, 330–339. [Google Scholar] [CrossRef]
- Feng, M.A.; McMillan, D.T.; Crowell, K.; Muss, H.; Nielsen, M.E.; Smith, A.B. Geriatric Assessment in Surgical Oncolo-gy: A Systematic Review. J. Surg. Res. 2015, 193, 265–272. [Google Scholar] [CrossRef]
- Yokom, D.W.; Alibhai, S.M.; Sattar, S.; Krzyzanowska, M.K.; Puts, M.T. Geriatric oncology screening tools for CGA-based interventions: Results from a phase II study of geriatric assessment and management for older adults with cancer. J. Geriatr. Oncol. 2018, 9, 683–686. [Google Scholar] [CrossRef]
- Mitry, E.; Fields, A.L.; Bleiberg, H.; Labianca, R.; Portier, G.; Tu, D.; Nitti, D.; Torri, V.; Elias, D.; O’Callaghan, C.; et al. Adjuvant Chemotherapy after Po-tentially Curative Resection of Metastases from Colorectal Cancer: A Pooled Analysis of Two Randomized Trials. J. Clin. Oncol. 2008, 26, 4906–4911. [Google Scholar] [CrossRef] [PubMed]
- Benedix, F.; Kube, R.; Meyer, F.; Schmidt, U.; Gastinger, I.; Lippert, H. Comparison of 17,641 Patients With Right- and Left-Sided Colon Cancer: Differences in Epidemiology, Perioperative Course, Histology, and Survival. Dis. Colon Rectum 2010, 53, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Suttie, S.A.; Shaikh, I.; Mullen, R.; Amin, A.I.; Daniel, T.; Yalamarthi, S. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Color. Dis. 2010, 13, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. Folfiri Plus Cetuximab Versus Folfiri Plus Bevacizumab as First-Line Treatment for Patients with Metastatic Colorectal Cancer (Fire-3): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Boeckx, N.; Koukakis, R.; de Beeck, K.O.; Rolfo, C.; van Camp, G.; Siena, S.; Tabernero, J.; Douillard, J.Y.; André, T.; Peeters, M. Primary Tumor Sidedness Has an Impact on Prognosis and Treatment Outcome in Metastatic Colorectal Cancer: Results from Two Randomized First-Line Panitumumab Studies. Ann. Oncol. 2017, 28, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Pfau, P.R.; O’Connor, E.S.; King, J.; LoConte, N.; Kennedy, G.; Smith, M.A. Mortality by Stage for Right- Versus Left-Sided Colon Cancer: Analysis of Surveillance, Epidemiology, and End Results–Medicare Data. J. Clin. Oncol. 2011, 29, 4401–4409. [Google Scholar] [CrossRef]
- Engstrand, J.; Strömberg, C.; Nilsson, H.; Freedman, J.; Jonas, E. Synchronous and Metachronous Liver Metastases in Pa-tients with Colorectal Cancer-Towards a Clinically Relevant Definition. World J. Surg. Oncol. 2019, 17, 228. [Google Scholar] [CrossRef]
- Suthananthan, A.E.; Bhandari, M.; Platell, C. Influence of primary site on metastatic distribution and survival in stage IV colorectal cancer. ANZ J. Surg. 2017, 88, 445–449. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Y.; Peng, J.; Sui, Q.; Lin, J.; Qiu, M.; Pan, Z. Does the Preoper-ative Prognostic Nutritional Index Predict Survival in Patients with Liver Metastases from Colorectal Cancer Who Under-went Curative Resection? J. Cancer 2018, 9, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Romero, P.; Palucka, A.K.; Marincola, F.M. Tumour immunity: Effector response to tumour and role of the microenvironment. Lancet 2008, 371, 771–783. [Google Scholar] [CrossRef]
- Mallappa, S.; Sinha, A.; Gupta, S.; Chadwick, S.J. Preoperative Neutrophil to Lymphocyte Ratio >5 Is a Prognostic Factor for Recurrent Colorectal Cancer. Colorectal. Dis. 2013, 15, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Cook, E.; Goulder, F.; Justin, T.; Keeling, N. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, N.; Felekouras, E.; Karavokyros, I.; Alexandrou, A.; Pikoulis, E.; Griniatsos, J. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer 2018, 18, 1202. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).