Cost-Effectiveness Analysis of 12-Versus 4-Weekly Administration of Bone-Targeted Agents in Patients with Bone Metastases from Breast and Castration-Resistant Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Costs
2.2. Outcomes
2.3. Assumptions
2.4. Analysis
3. Results
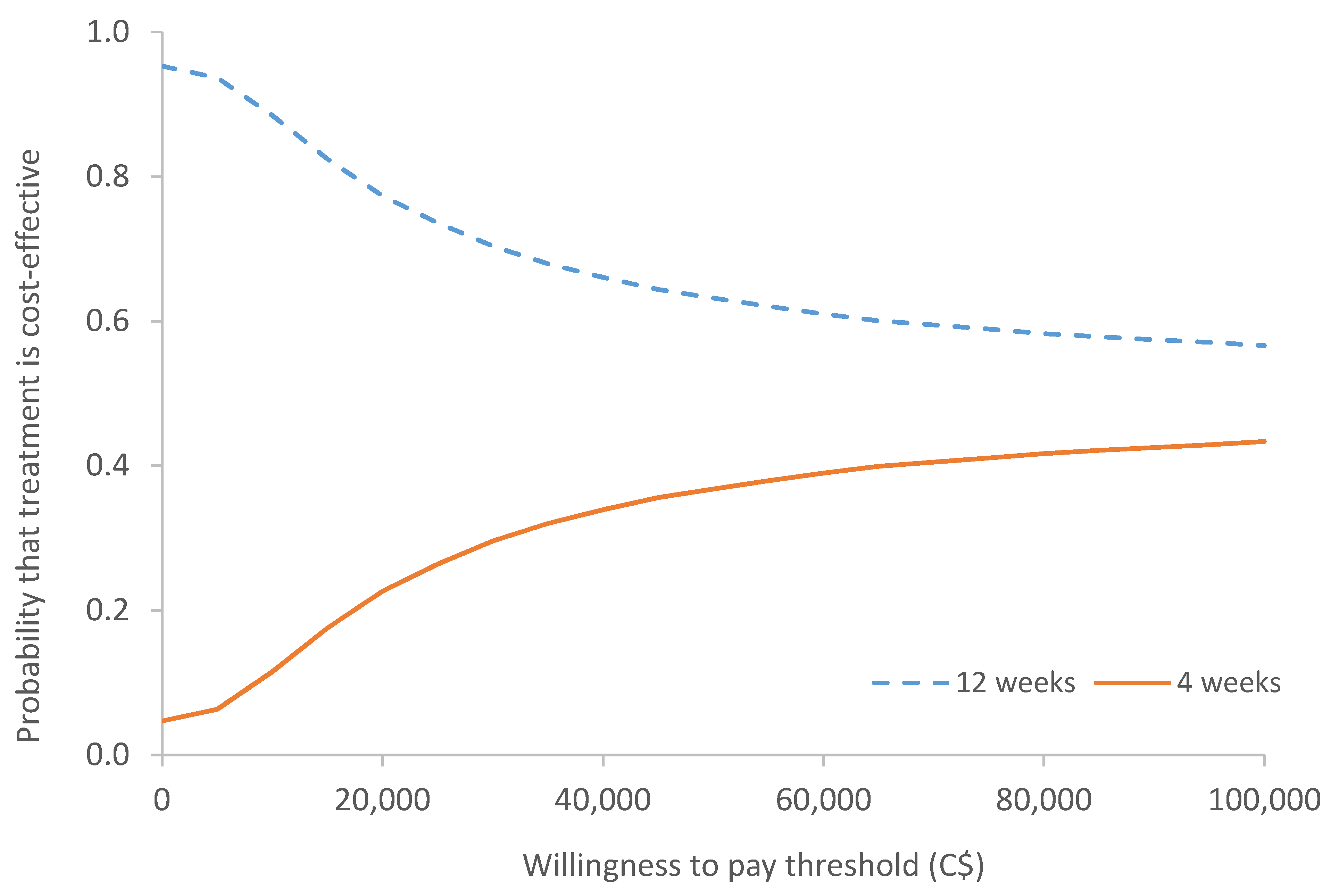
Sensitivity Analysis
4. Discussion
4.1. Strengths and Limitations
4.2. Interpretation Considering Other Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| 4-Weekly | 12-Weekly | |||||
|---|---|---|---|---|---|---|
| Cost of Drug (C$) [8] | Cost of Administration (C$) [6] | Total Cost (C$) | Cost of Drug (C$) [8] | Cost of Administration (C$) [6] | Total Cost (C$) | |
| Denosumab (Xgeva) | 7387.34 | 372.00 | 7759.34 | 2462.45 | 124.00 | 2586.45 |
| Pamidronate | 130.68 | 227.64 | 358.32 | 43.56 | 975.88 | 1019.44 |
| Zoledronate | 96.72 | 2370.00 | 266.72 | 32.24 | 790.00 | 822.24 |
Appendix B
| Variable | Value (C$) | Source |
|---|---|---|
| Radiotherapy to relieve bone pain | ||
| Average cost of external beam radiotherapy to axial skeleton (1AW27JA,1EA27JA,1SQ27JA) | 18,223 | [7] |
| Radiation oncologist—consultation (A345) | 152.40 | [9] |
| Radiation oncologist—radiation treatment planning—level 3 (X312) | 680.45 | [9] |
| Total cost of radiotherapy to relieve bone pain | 19,055.85 | |
| Pathological fracture | ||
| Average cost of pathological fracture, pelvic region and thigh (M8445) | 4890 | [7] |
| Orthopedic surgeon—consultation (A065) | 83.10 | [9] |
| Orthopedic surgeon—fractures—open reduction (F096)—“femoral nail fixation” = $493.80 | 492.38 | [9] |
| Orthopedic surgeon—fractures—open reduction—primary prosthesis, femur only (F101) “partial hip replacement for neck fracture” = $490.95 | [9] | |
| Radiation oncologist—radiation treatment planning—level 3 (X312) | 680.45 | [9] |
| Anesthesiologist (see Appendix C for cost breakdown) | 405.27 | [9] |
| Total cost to treat pathological fracture | 6145.93 | |
| Spinal cord compression | ||
| Average cost of cord compression (G952) | 6496 | [7] |
| Radiation oncologist—consultation (A345) | 152.40 | [9] |
| Radiation oncologist—radiation treatment planning—level 3 (X312) | 680.45 | [9] |
| Total cost to treat spinal cord compression | 7328.85 | |
| Tumor-related orthopedic surgical intervention | ||
| Average cost of prophylactic surgery (Z408, Z409)—day surgery | 2375 | [7] |
| Orthopedic surgeon—consultation (A065) | 83.1 | [9] |
| Orthopedic tumor surgery—biopsy of suspected sarcoma, or resection of a complex bone or complex soft tissue | 600 | [9] |
| tumor(s), $100 per 15 min (R226A), assume 90min—“general oncology” | ||
| Orthopedic surgeon—bone—major tumor resection (R330)—“tumor excision” | 629.65 | [9] |
| Average cost of the two surgeries | 614.83 | [9] |
| Anesthesiologist (see Appendix C for cost breakdown) | 405.27 | |
| Total cost for tumour-related orthopedic surgical intervention | 3478.195 | |
| Hypercalcemia | ||
| Average cost of disorder of calcium metabolism (E835) | 7448 | [7] |
| Medical oncologist—consultation (A445) | 157 | [9] |
| Total cost to treat hypercalcemia | 7605 |
Appendix C
| Description | Base price [9] | Units | Total | Average |
|---|---|---|---|---|
| Pathological fracture | C$405.27 | |||
| F096 (75–90 min (use 90 min) + 30 min pre/post-op) | C$15.01 | |||
| To start case | 8 | C$120.08 | ||
| First 60 min (1 unit per 15 min) | 4 | C$60.04 | ||
| 60–90 min (2 units per 15 min) | 4 | C$60.04 | ||
| >90 min (4 units per 15 min) | 8 | C$120.08 | ||
| ASAII (E022C × 2 units) | 2 | C$30.02 | ||
| C$390.26 | ||||
| F101 (75–90 min (use 90 min) + 30 min pre/post op) | C$15.01 | |||
| To start case | 10 | C$150.10 | ||
| First 60 min (1 unit per 15 min) | 4 | C$60.04 | ||
| 60–90 min (2 units per 15 min) | 4 | C$60.04 | ||
| >90 min (4 units per 15 min) | 8 | C$120.08 | ||
| ASAII (E022C × 2 units) | 2 | C$30.02 | ||
| C$420.28 | ||||
| Tumor-related orthopedic surgical intervention | C$405.27 | |||
| R226A (45 min–24 h (use 90 min) + 30 min pre/post op) | C$15.01 | |||
| To start case | 15 | C$225.15 | ||
| First 60 min (1 unit per 15 min) | 4 | C$60.04 | ||
| 60–90 min (2 units per 15 min) | 4 | C$60.04 | ||
| > 90 min (4 units per 15 min) | 8 | C$120.08 | ||
| C$465.31 | ||||
| R330 | C$15.01 | |||
| To start case | 7 | C$105.07 | ||
| First 60 min (1 unit per 15 min) | 4 | C$60.04 | ||
| 60–90 min (2 units per 15 min) | 4 | C$60.04 | ||
| >90 min (4 units per 15 min) | 8 | C$120.08 | ||
| $345.23 |
Appendix D
| SSE | Number of Events [Proportion of Total Events] | Weighted Cost (C$) of Each SSE | ||
|---|---|---|---|---|
| 4-Weekly | 12-Weekly | 4-Weekly | 12-Weekly | |
| Radiotherapy to bone | 35 [0.78] | 38 [0.86] | 14,821.22 | 16,457.33 |
| Hypercalcemia | 4 [0.089] | 1 [0.023] | 676.00 | 172.84 |
| Pathological fracture | 4 (0.089) | 2 [0.045] | 546.30 | 279.36 |
| Spinal cord compression | 2 [0.044] | 1 [0.023] | 325.73 | 166.56 |
| Surgery to bone | 0 [0] | 2 [0.045] | 0.00 | 158.10 |
| Total | 45 | 44 | 16,369.25 | 17,234.19 |
References
- Clemons, M.; Gelmon, K.; Pritchard, K.; Paterson, A. Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: The state of the art. Curr. Oncol. 2012, 19, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Van Poznak, C.; Somerfield, M.R.; Barlow, W.E.; Biermann, J.S.; Bosserman, L.D.; Clemons, M.J.; Dhesy-Thind, S.K.; Dillmon, M.S.; Eisen, A.; Frank, E.S.; et al. Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology–Cancer Care Ontario Focused Guideline Update. J. Clin. Oncol. 2017, 35, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, R.; Costa, L.; Scagliotti, G.; Sleeboom, H.; Goldwasser, F.; Hirsh, V.; Spencer, A.; Radcliffe, H.-S.; Niepel, D.; Henry, D. Symptomatic skeletal events (SSEs) versus skeletal-related events (SREs) in patients with advanced cancer and bone metastases treated with denosumab or zoledronic acid. Ann. Oncol. 2016, 27, vi507. [Google Scholar] [CrossRef]
- Clemons, M.; Ong, M.; Stober, C.; Ernst, S.; Booth, C.; Canil, C.; Mates, M.; Robinson, A.; Blanchette, P.; Joy, A.A.; et al. A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer 2021, 142, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L.; Moriarty, J.P.; Dusetzina, S.; Himelstein, A.L.; Foster, J.C.; Grubbs, S.S.; Novotny, P.J.; Borah, B.J. Cost-Effectiveness Analysis of Monthly Zoledronic Acid, Zoledronic Acid Every 3 Months, and Monthly Denosumab in Women with Breast Cancer and Skeletal Metastases: CALGB 70604 (Alliance). J. Clin. Oncol. 2017, 35, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- CADTH Common Drug Reviews. In Denosumab (Xgeva); Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2016.
- Ontario Case Costing Initiative (OCCI). 2019. Available online: https://data.ontario.ca/dataset/ontario-case-costing-initiative-occi (accessed on 5 November 2019).
- Drugs Reimbursed by the Provincial Drug Reimbursement Programs (PDRP); Cancer Care Ontario: Toronto, ON, Canada, 2019.
- Schedule of Benefits. Physician Services Under the Health Insurance Act. 1 March 2016. Available online: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20160401.pdf (accessed on 5 November 2019).
- Dranitsaris, G.; Castel, L.D.; Baladi, J.F.; A Schulman, K. Zoledronic acid versus pamidronate as palliative therapy in cancer patients: A Canadian time and motion analysis. J. Oncol. Pharm. Pract. 2001, 7, 27–33. [Google Scholar] [CrossRef]
- McTaggart-Cowan, H.; King, M.T.; Norman, R.; Costa, D.S.J.; Pickard, A.S.; Regier, D.A.; Viney, R.; Peacock, S.J. The EORTC QLU-C10D: The Canadian Valuation Study and Algorithm to Derive Cancer-Specific Utilities from the EORTC QLQ-C30. MDM Policy Pract. 2019, 4, 2381468319842532. [Google Scholar] [CrossRef] [PubMed]
- Crott, R.; Briggs, A. Mapping the QLQ-C30 quality of life cancer questionnaire to EQ-5D patient preferences. Eur. J. Health Econ. 2010, 11, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Rowen, D.; Brazier, J.; Young, T.; Gaugris, S.; Craig, B.M.; King, M.T.; Velikova, G. Deriving a preference-based measure for cancer using the EORTC QLQ-C30. Value Health 2011, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.; et al. The european organization for research and treatment of cancer qlq-c30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.; Tu, M.M.; Ibrahim, M.F.K.; Basulaiman, B.; McGee, S.F.; Srikanthan, A.; Fernandes, R.; VanderMeer, L.; Stober, C.; Sienkiewicz, M.; et al. Long-term impact of bone-modifying agents for the treatment of bone metastases: A systematic review. Support. Care Cancer 2021, 29, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Lamy, O.; Gonzalez-Rodriguez, E.; Stoll, D.; Hans, D.; Aubry-Rozier, B. Severe Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: 9 Clinical Cases Report. J. Clin. Endocrinol. Metab. 2017, 102, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; von Moos, R.A.F.; Hayoz, S.; Hawle, H.; Cathomas, R.; Rothermundt, C.A.; Anchisi, S.; Mueller, A.; Wehrhahn, T.; Burmeister, H.; et al. Incidence of hypocalcemia in patients with castration-resistant prostate cancer treated with denosumab: Data from a non-inferiority phase III trial assessing prevention of symptomatic skeletal events (SSE) with denosumab administered every four weeks (q4w) versus every 12 weeks (q12w)—SAKK 96/12 (REDUSE). J. Clin. Oncol. 2019, 37 (Suppl. 7), 139. [Google Scholar]
- AlZahrani, M.; Clemons, M.; Vandermeer, L.; Sienkiewicz, M.; Awan, A.A.; Hutton, B.; Pond, G.R.; Ng, T.L. Real-world practice patterns and attitudes towards de-escalation of bone-modifying agents in patients with bone metastases from breast and prostate cancer: A physician survey. J. Bone Oncol. 2021, 26, 100339. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Namjoshi, M.; Wu, E.Q.; Parikh, K.; Diener, M.; Yu, A.P.; Guo, A.; Culver, K.W. Economic Evaluation of Denosumab Compared with Zoledronic Acid in Hormone-Refractory Prostate Cancer Patients with Bone Metastases. J. Manag. Care Pharm. 2011, 17, 621–643. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Diener, M.; Sorg, R.; Wu, E.Q.; Namjoshi, M. Cost-effectiveness of denosumab compared with zoledronic acid in patients with breast cancer and bone metastases. Clin. Breast Cancer 2012, 12, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, S.J.; Carter, J.A.; Kaura, S.; Botteman, M.F. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin. Ther. 2012, 34, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.; Rader, M.; Henry, D.; Danese, M.; Halperin, M.; Cong, Z.; Qian, Y.; Dansey, R.; Chung, K. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J. Med. Econ. 2012, 15, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, S.J.; Carter, J.A.; Kaura, S.; Botteman, M.F. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A cost-effectiveness analysis. J. Med. Econ. 2013, 16, 19–29. [Google Scholar] [CrossRef] [PubMed]
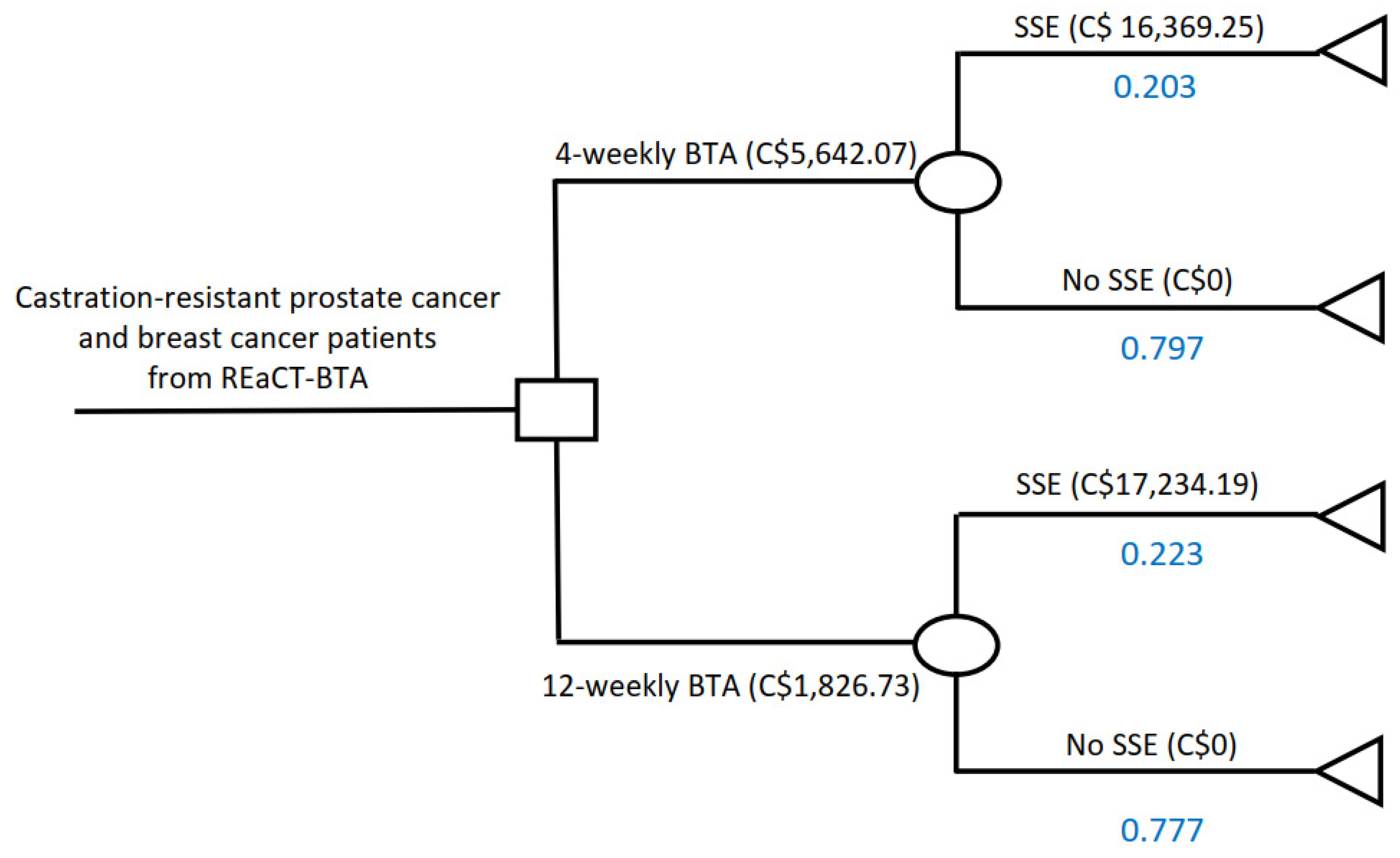
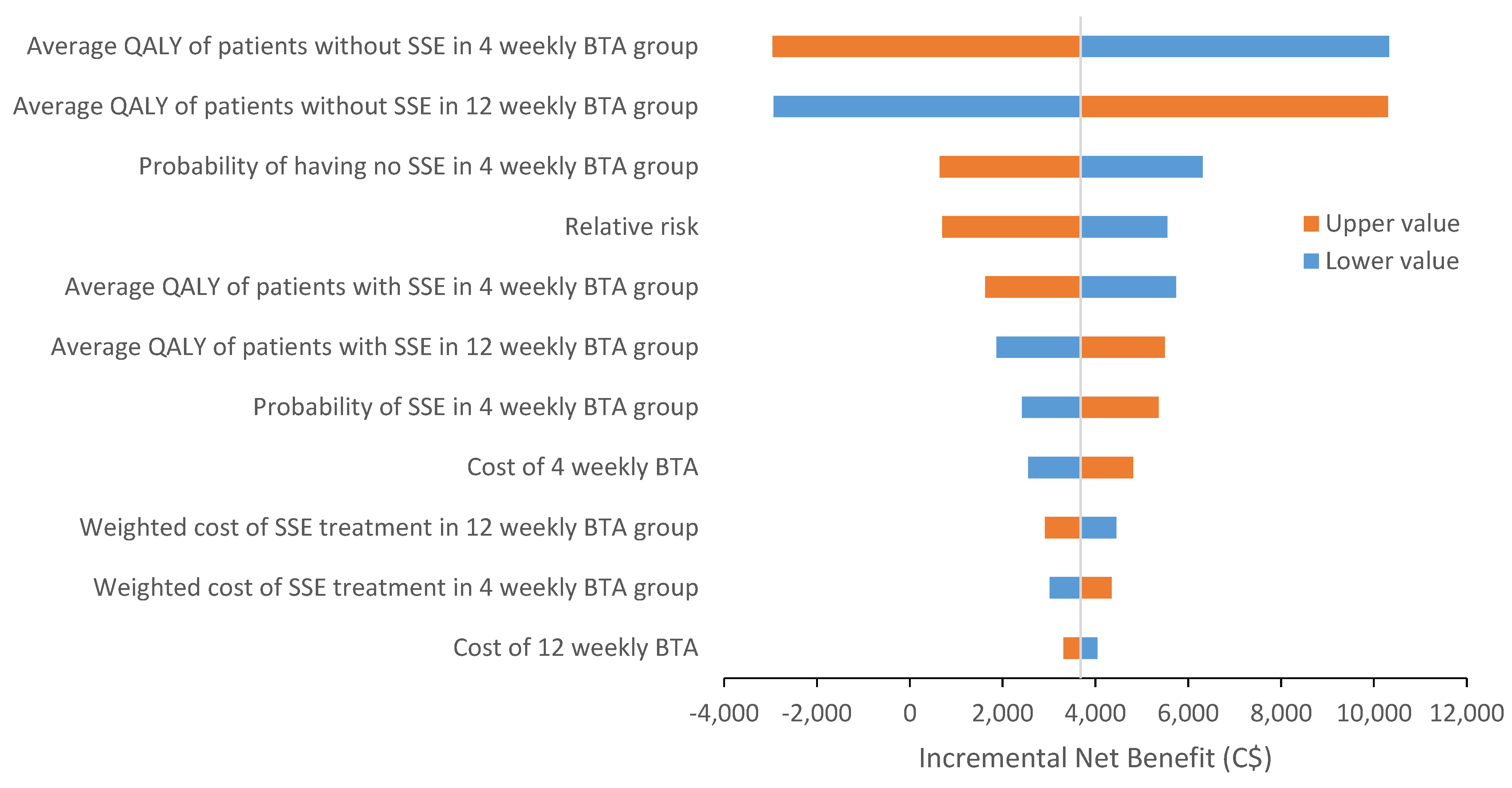
| Baseline | Lower limit | Upper limit | Reference | |
|---|---|---|---|---|
| Probabilities | ||||
| Probability of SSE with 4-weekly BTA | 0.203 | 0.1338 | 0.2954 | [4] |
| Probability of no SSE with 4-weekly BTA | 0.797 | 0.6525 | 0.9639 | [4] |
| Probability of SSE with 12-weekly BTA | 0.223 | [4] | ||
| Probability of no SSE with 12-weekly BTA | 0.777 | [4] | ||
| De-escalation effect (relative risk) | ||||
| Reduction of BTA treatment from 4-weekly to 12-weekly | 1.099 | 0.6902 | 1.7496 | [4] |
| Costs per year | ||||
| Weighted cost of 4-weekly BTA | C$5642.07 | C$4513.66 | C$6770.48 | Appendix A |
| Weighted cost of 12-weekly BTA | C$1826.73 | C$1461.38 | C$2192.08 | Appendix A |
| Weighted cost of SSE in 4-weekly BTA group | C$16,369.25 | C$13,095.40 | C$19,643.10 | Appendix B |
| Weighted cost of SSE in 12-weekly BTA group | C$17,234.19 | C$13,787.35 | C$20,681.03 | Appendix B |
| Costs | QALY | |
|---|---|---|
| 4-weekly BTA | C$8965.03 | 0.605 |
| 12-weekly BTA | C$5671.28 | 0.612 |
| Incremental | −C$3293.75 | 0.008 |
| ICER (∆ cost/∆ QALY) | 12-weekly dominates 4-weekly | |
| Incremental net benefit (INB) * | C$3681.37 | |
| * The INB is based upon an assumption that the willingness to pay for one QALY is C$50,000 | ||
| If INB > 0 = intervention is cost effective | ||
| If INB < 0 = not cost effective | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, M.M.; Clemons, M.; Stober, C.; Jeong, A.; Vandermeer, L.; Mates, M.; Blanchette, P.; Joy, A.A.; Aseyev, O.; Pond, G.; et al. Cost-Effectiveness Analysis of 12-Versus 4-Weekly Administration of Bone-Targeted Agents in Patients with Bone Metastases from Breast and Castration-Resistant Prostate Cancer. Curr. Oncol. 2021, 28, 1847-1856. https://doi.org/10.3390/curroncol28030171
Tu MM, Clemons M, Stober C, Jeong A, Vandermeer L, Mates M, Blanchette P, Joy AA, Aseyev O, Pond G, et al. Cost-Effectiveness Analysis of 12-Versus 4-Weekly Administration of Bone-Targeted Agents in Patients with Bone Metastases from Breast and Castration-Resistant Prostate Cancer. Current Oncology. 2021; 28(3):1847-1856. https://doi.org/10.3390/curroncol28030171
Chicago/Turabian StyleTu, Megan M., Mark Clemons, Carol Stober, Ahwon Jeong, Lisa Vandermeer, Mihaela Mates, Phillip Blanchette, Anil Abraham Joy, Olexiy Aseyev, Gregory Pond, and et al. 2021. "Cost-Effectiveness Analysis of 12-Versus 4-Weekly Administration of Bone-Targeted Agents in Patients with Bone Metastases from Breast and Castration-Resistant Prostate Cancer" Current Oncology 28, no. 3: 1847-1856. https://doi.org/10.3390/curroncol28030171
APA StyleTu, M. M., Clemons, M., Stober, C., Jeong, A., Vandermeer, L., Mates, M., Blanchette, P., Joy, A. A., Aseyev, O., Pond, G., Fergusson, D., Ng, T. L., & Thavorn, K. (2021). Cost-Effectiveness Analysis of 12-Versus 4-Weekly Administration of Bone-Targeted Agents in Patients with Bone Metastases from Breast and Castration-Resistant Prostate Cancer. Current Oncology, 28(3), 1847-1856. https://doi.org/10.3390/curroncol28030171






