Trajectory of End-of-Life Pain and Other Physical Symptoms among Cancer Patients Receiving Home Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Data Sources
2.3. Outcomes
2.4. Covariates
2.5. Analysis
3. Results
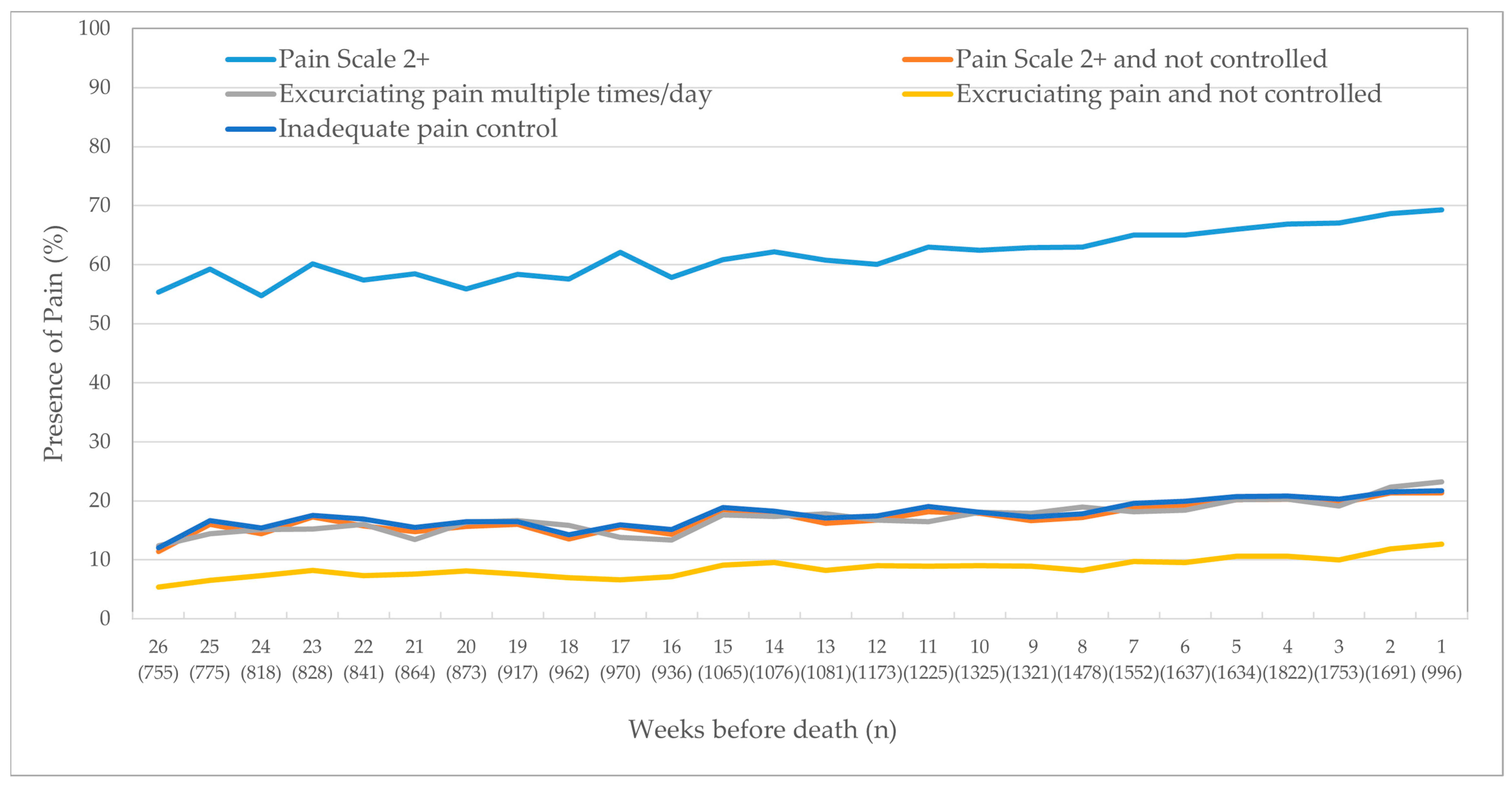
3.1. Pain
3.2. Other Non-Pain-Related Physical Outcomes
3.3. Regression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenz, K.; Lynn, J.; Dy, S.; Hughes, R.; Mularski, R.; Shugarman, L.R.; Wilkinson, A.M. Cancer care quality measures: Symptoms and end-of-life care. Évid. Rep. Assess. 2006, 2006, 1–77. [Google Scholar]
- Lorenz, K.A.; Lynn, J.; Dy, S.M.; Shugarman, L.R.; Wilkinson, A.; Mularski, R.A.; Morton, S.C.; Hughes, R.G.; Hilton, L.K.; Maglione, M.; et al. Evidence for Improving Palliative Care at the End of Life: A Systematic Review. Ann. Intern. Med. 2008, 148, 147–159. [Google Scholar] [CrossRef]
- Carr, D.; Goudas, L.; Lawrence, D.; Pirl, W.; Lau, J.; Devine, D.; Kupelnick, B.; Miller, K. Management of Cancer Symptoms: Pain, Depression, and Fatigue: Evidence Report /Technology Assessment, Number. PsycEXTRA Dataset 2002, 2002, 1–5. [Google Scholar] [CrossRef][Green Version]
- Barbera, L.; Seow, H.; Howell, D.; Sutradhar, R.; Earle, C.; Liu, Y.; Stitt, A.; Husain, A.; Sussman, J.; Dudgeon, D. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer 2010, 116, 5767–5776. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.E.; Snyder, C.F.; Abernethy, A.P.; Basch, E.; Potosky, A.L.; Roberts, A.C.; Loeffler, D.R.; Reeve, B.B. Review of Electronic Patient-Reported Outcomes Systems Used in Cancer Clinical Care. J. Oncol. Pr. 2014, 10, e215–e222. [Google Scholar] [CrossRef]
- Seow, H.; Barbera, L.; Sutradhar, R.; Howell, D.; Dudgeon, D.; Atzema, C.; Liu, Y.; Husain, A.; Sussman, J.; Earle, C. Trajectory of Performance Status and Symptom Scores for Patients with Cancer During the Last Six Months of Life. J. Clin. Oncol. 2011, 29, 1151–1158. [Google Scholar] [CrossRef]
- Barbera, L.; Seow, H.; Sutradhar, R.; Chu, A.; Burge, F.; Fassbender, K.; McGrail, K.; Lawson, B.; Liu, Y.; Pataky, R.; et al. Quality of End-of-Life Cancer Care in Canada: A Retrospective Four-Province Study Using Administrative Health Care Data. Curr. Oncol. 2015, 22, 341–355. [Google Scholar] [CrossRef]
- Armstrong, J.J.; Stolee, P.; Hirdes, J.P.; Poss, J.W. Examining three frailty conceptualizations in their ability to predict negative outcomes for home-care clients. Age Ageing 2010, 39, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.N.; Fries, B.E.; Steel, K.; Ikegami, N.; Bernabei, R.; Carpenter, G.I.; Gilgen, R.; Hirdes, J.P.; Topinková, E. Comprehensive Clinical Assessment in Community Setting: Applicability of the MDS-HC. J. Am. Geriatr. Soc. 1997, 45, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Hawes, C.; Fries, B.E.; James, M.L.; Guihan, M. Prospects and pitfalls: Use of the RAI-HC assessment by the Department of Veterans Affairs for home care clients. Gerontologist 2007, 47, 378–387. [Google Scholar] [CrossRef]
- Barbera, L.; Seow, H.; Husain, A.; Howell, D.; Atzema, C.; Sutradhar, R.; Earle, C.; Sussman, J.; Liu, Y.; Dudgeon, D. Opioid Prescription After Pain Assessment: A Population-Based Cohort of Elderly Patients with Cancer. J. Clin. Oncol. 2012, 30, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- de Arriba, J.J.; Nerin, C.; Ortiz, M.C.; del Soto Sesma, M.; Beltran, S.; Vila, B. Symptom scores and delirium in advanced cancer. J. Clin. Oncol. 2011, 29, 2833–2834. [Google Scholar] [CrossRef]
- Cook, R.J.; Berg, K.; Lee, K.-A.; Poss, J.W.; Hirdes, J.P.; Stolee, P. Rehabilitation in Home Care Is Associated With Functional Improvement and Preferred Discharge. Arch. Phys. Med. Rehabil. 2013, 94, 1038–1047. [Google Scholar] [CrossRef]
- Hirdes, J.P.; Ljunggren, G.; Morris, J.N.; Frijters, D.H.M.; Soveri, H.F.; Gray, L.; Björkgren, M.; Gilgen, R. Reliability of the interRAI suite of assessment instruments: A 12-country study of an integrated health information system. BMC Heal. Serv. Res. 2008, 8, 277. [Google Scholar] [CrossRef]
- Kim, H.; Jung, Y.; Sung, M.; Lee, J.; Yoon, J.; Yoon, J. Reliability of the interRAI Long Term Care Facilities (LTCF) and interRAI Home Care (HC). Geriatr. Gerontol. Int. 2015, 15, 220–228. [Google Scholar] [CrossRef]
- Morris, J.N.; Bernabei, R.; Ikegami, N.; Gilgen, R.; Frijters, D.; Hirdes, J.P.; Fries, B.E.; Steel, K.; Carpenter, I.; Du Pasquier, J.; et al. RAI-Home Care (RAI-HC) Assessment Manual for Version 2; interRAI Corporation: Washington, DC, USA, 1999. [Google Scholar]
- Fries, B.E.; Simon, S.E.; Morris, J.N.; Flodstrom, C.; Bookstein, F.L. Pain in U.S. nursing homes: Validating a pain scale for the minimum data set. Gerontologist 2001, 41, 173–179. [Google Scholar] [CrossRef]
- Hirdes, J.P.; Frijters, D.H.; Teare, G.F. The MDS-CHESS Scale: A New Measure to Predict Mortality in Institutionalized Older People. J. Am. Geriatr. Soc. 2003, 51, 96–100. [Google Scholar] [CrossRef]
- Burrows, A.B.; Morris, J.N.; Simon, S.; Hirdes, J.P.; Phillips, C. Development of a minimum data set-based depression rating scale for use in nursing homes. Age Ageing 2000, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.N.; Fries, B.E.; Mehr, D.R.; Hawes, C.; Phillips, C.; Mor, V.; Lipsitz, L.A. MDS Cognitive Performance Scale(C). J. Gerontol. 1994, 49, M174–M182. [Google Scholar] [CrossRef]
- Morris, J.N.; Fries, B.E.; Morris, S.A. Scaling ADLs Within the MDS. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1999, 54, M546–M553. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.N.; Berg, K.; Fries, B.; Steel, K.; Howard, E.P. Scaling functional status within the interRAI suite of assessment instruments. BMC Geriatr. 2013, 13, 128. [Google Scholar] [CrossRef]
- Freeman, S.; Hirdes, J.P.; Stolee, P.; Garcia, J.; Smith, T.F. Correlates and Predictors of Changes in Dyspnea Symptoms Over Time Among Community-Dwelling Palliative Home Care Clients. J. Pain Symptom Manag. 2015, 50, 793–805. [Google Scholar] [CrossRef] [PubMed]
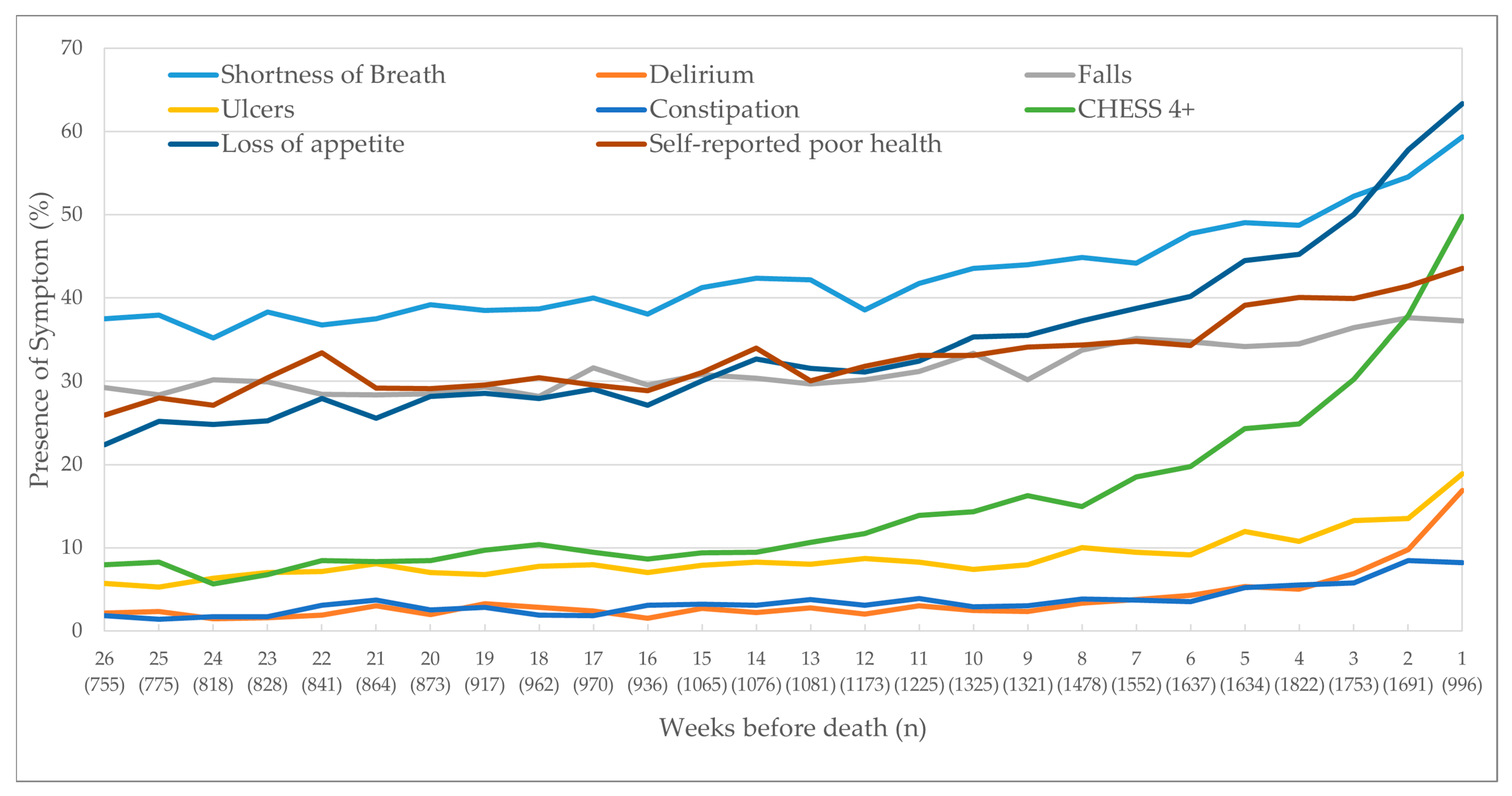

| Non-Pain Physical Items | Description of Questions in the RAI-HC |
|---|---|
| Shortness of breath | Client experiences shortness of breath at any point in the last three days |
| Delirium | Client experiences a sudden change in mental function within the last seven days |
| Falls | Client has experienced one or more falls within the last 90 days |
| Ulcers | Presence of any ulcer (pressure or stasis) at the time of the assessment |
| Loss of appetite | Client experiences a loss of appetite on at least two of the last three days |
| Constipation | Client has not had a bowel movement within the last three days |
| High health instability using the CHESS Scale score of 4 or more | Client shows high or very high health instability. Client receives a score of 1 for having the presence of a symptom variable, up to a maximum of 2 (e.g., dyspnea, weight loss, dehydration, fluid loss), then another 1 point for having the presence of each of these three variables: change in decision making, change in ADL status, and evidence of end-stage disease, i.e., prognosis of less than six months (scale scored from 0 (no health instability) to 5 (very high health instability)) [18] |
| Self-reported poor heath | Client feels they have poor health |
| Pain Items | |
| Pain Scale score of 2 or more | The Pain Scale is scored from 0 to 3. A score of 0 means a patient experiences no pain; 1 means less than daily pain; 2 means daily pain that is not severe; 3 means daily pain that is severe [17] |
| Pain Scale 2 or more and not controlled | Pain Scale score of 2 or more, and the pain is not adequately controlled by medications |
| Excruciating pain multiple times per day | Client experiences pain that is excruciating or horrible multiple times per day |
| Excruciating pain and not controlled | Excruciating pain multiple times per day, and the pain is not adequately controlled by medications |
| Inadequate pain control by medications | From a client’s point of view, medications do not adequately control pain |
| Cohort Characteristics | Overall | Died in Hospital | Died at Home | Absolute Standardized Difference | |
|---|---|---|---|---|---|
| % (N) | |||||
| Total | 100.0 (27,295) | 58.2 (15,888) | 41.8 (11,407) | ||
| Age | Under 65 | 22.1 (6021) | 22.4 (3562) | 21.6 (2459) | 0.02 |
| 65–74 | 22.0 (6009) | 22.0 (3496) | 22.0 (2513) | 0.00 | |
| 75–84 | 32.9 (8988) | 33.6 (5340) | 32.0 (3648) | 0.03 | |
| 85+ | 23.0 (6275) | 22.0 (3488) | 24.4 (2787) | 0.06 | |
| Most recent assessment’s date before death | 0–4 weeks | 22.8 (6219) | 22.1 (3504) | 23.8 (2714) | 0.04 |
| 5–12 weeks | 39.3 (10,717) | 39.4 (6251) | 39.1 (4465) | 0.01 | |
| 13–26 weeks | 38.0 (10,359) | 38.6 (6131) | 37.1 (4228) | 0.03 | |
| Sex | Female | 46.8 (12,777) | 46.7 (7411) | 47.0 (5365) | 0.01 |
| Pain Scale | Moderate to Severe Pain (score of 2–4) | 62.8 (17,154) | 61.4 (9751) | 64.9 (7403) | 0.07 |
| Depression Rating Scale | Signs/symptoms of depression (score of 3–14) | 22.3 (6076) | 21.6 (3434) | 23.2 (2642) | 0.04 |
| Cognitive Performance Scale | Moderate–severe impairment (score of 2–6) | 31.8 (8683) | 29.5 (4681) | 35.1 (4002) | 0.12 |
| ADL* Self-Performance Hierarchy Scale | Moderate–severe impairment (score of 3–6) | 20.3 (5543) | 15.9 (2530) | 26.4 (3013) | 0.26 |
| IADL § Involvement Scale | Moderate–severe impairment (score of 14–48) | 48.9 (13,361) | 44.1 (7006) | 55.7 (6355) | 0.23 |
| Social decline | Decline in social activities as compared to 90 days ago | 62.0 (19,622) | 59.0 (9374) | 66.2 (7548) | 0.15 |
| Caregiver items | Primary caregiver lives with client | 67.9 (18,532) | 67.1 (10,659) | 69.0 (7873) | 0.04 |
| Caregiver expresses feelings of anger, distress or depression | 18.8 (5143) | 18.1 (2877) | 19.9 (2266) | 0.05 | |
| Marital status | Married, common-law | 58.2 (15,878) | 57.8 (9178) | 58.7 (6700) | 0.02 |
| Widowed, separated, divorced | 37.1 (10,116) | 36.9 (5858) | 37.3 (4258) | 0.01 | |
| Never married | 4.8 (1301) | 5.4 (852) | 3.9 (449) | 0.07 | |
| Education completed µ | Grade 11 or less | 38.7 (7233) | 40.6 (4481) | 36.0 (2752) | 0.09 |
| High school | 24.6 (4587) | 23.9 (2640) | 25.5 (1947) | 0.04 | |
| College, university, trade | 36.8 (6867) | 35.5 (3915) | 38.6 (2952) | 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seow, H.; Guthrie, D.M.; Stevens, T.; Barbera, L.C.; Burge, F.; McGrail, K.; Chan, K.K.W.; Peacock, S.J.; Sutradhar, R. Trajectory of End-of-Life Pain and Other Physical Symptoms among Cancer Patients Receiving Home Care. Curr. Oncol. 2021, 28, 1641-1651. https://doi.org/10.3390/curroncol28030153
Seow H, Guthrie DM, Stevens T, Barbera LC, Burge F, McGrail K, Chan KKW, Peacock SJ, Sutradhar R. Trajectory of End-of-Life Pain and Other Physical Symptoms among Cancer Patients Receiving Home Care. Current Oncology. 2021; 28(3):1641-1651. https://doi.org/10.3390/curroncol28030153
Chicago/Turabian StyleSeow, Hsien, Dawn M. Guthrie, Tara Stevens, Lisa C. Barbera, Fred Burge, Kimberlyn McGrail, Kelvin K. W. Chan, Stuart J. Peacock, and Rinku Sutradhar. 2021. "Trajectory of End-of-Life Pain and Other Physical Symptoms among Cancer Patients Receiving Home Care" Current Oncology 28, no. 3: 1641-1651. https://doi.org/10.3390/curroncol28030153
APA StyleSeow, H., Guthrie, D. M., Stevens, T., Barbera, L. C., Burge, F., McGrail, K., Chan, K. K. W., Peacock, S. J., & Sutradhar, R. (2021). Trajectory of End-of-Life Pain and Other Physical Symptoms among Cancer Patients Receiving Home Care. Current Oncology, 28(3), 1641-1651. https://doi.org/10.3390/curroncol28030153






