Prognostic Impact of APOBEC3B Expression in Metastatic Urothelial Carcinoma and Its Association with Tumor-Infiltrating Cytotoxic T Cells
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
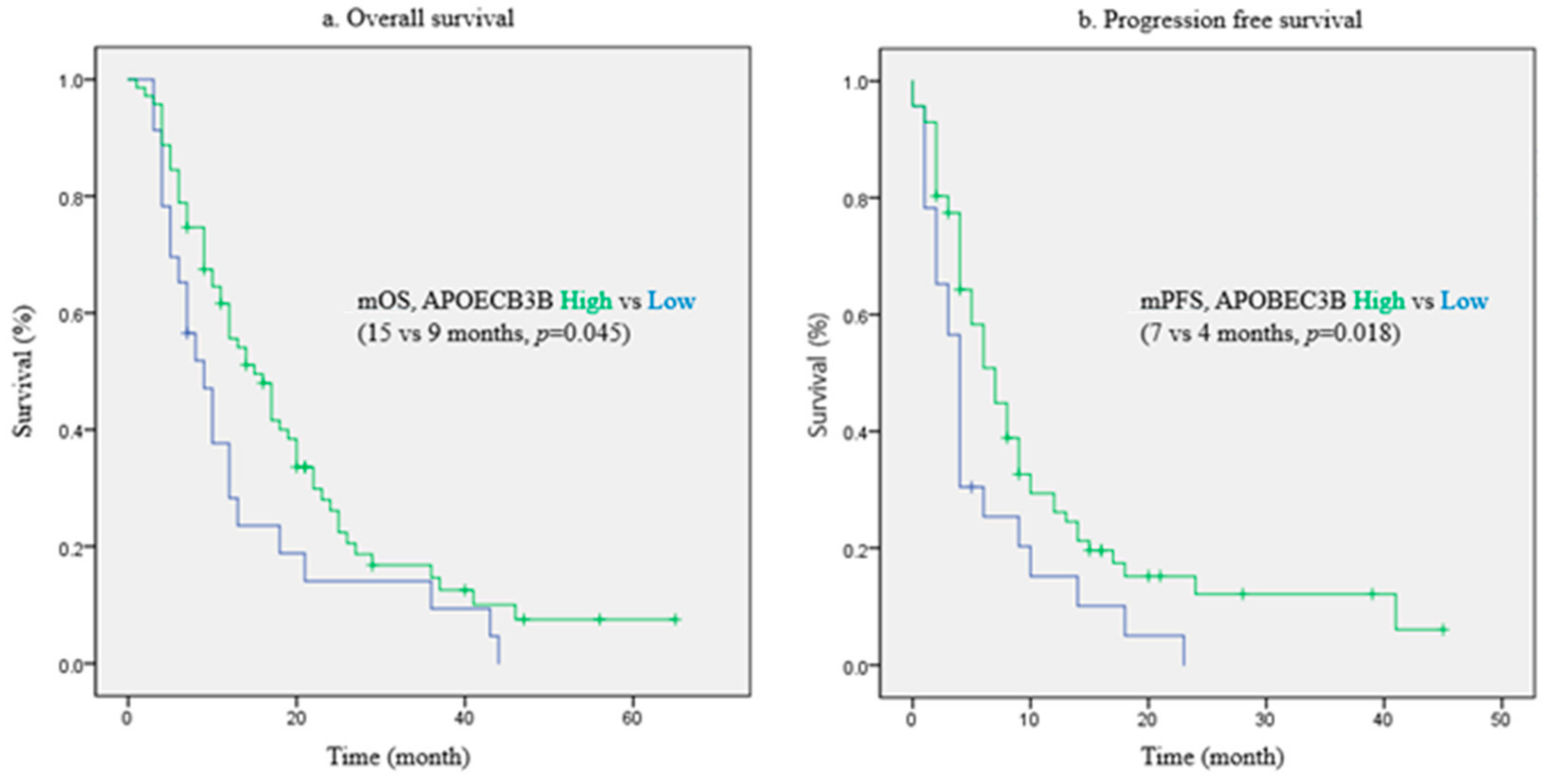
2.2. Prognostic Value of APOBEC3B for Patient Survival
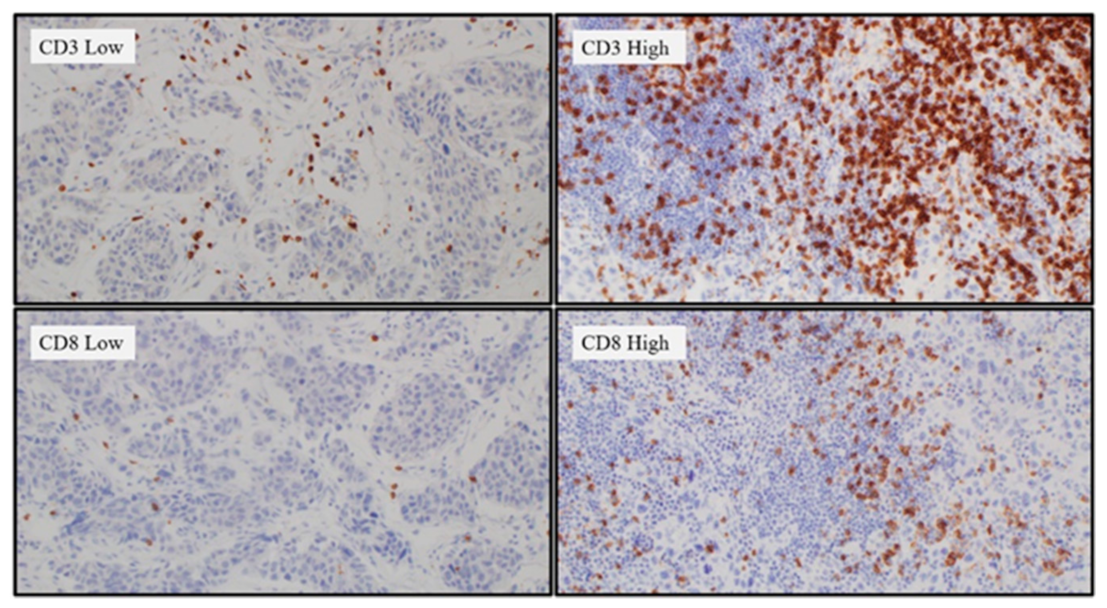
2.3. Tumor-Infiltrating Lymphocytes (TIL) Differences According to the APOBEC3B Expression Status
2.4. Evaluation of APOBEC3B Expression as a Predictive Marker for Cytotoxic Chemotherapy
3. Discussion
4. Materials and Methods
4.1. Study Population and Design
4.2. Immunohistochemistry (IHC)
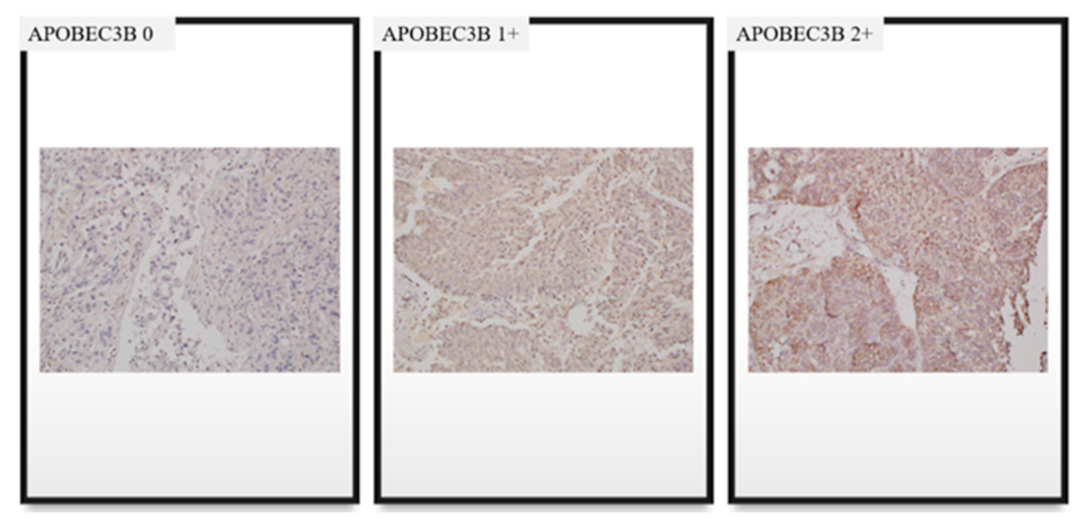
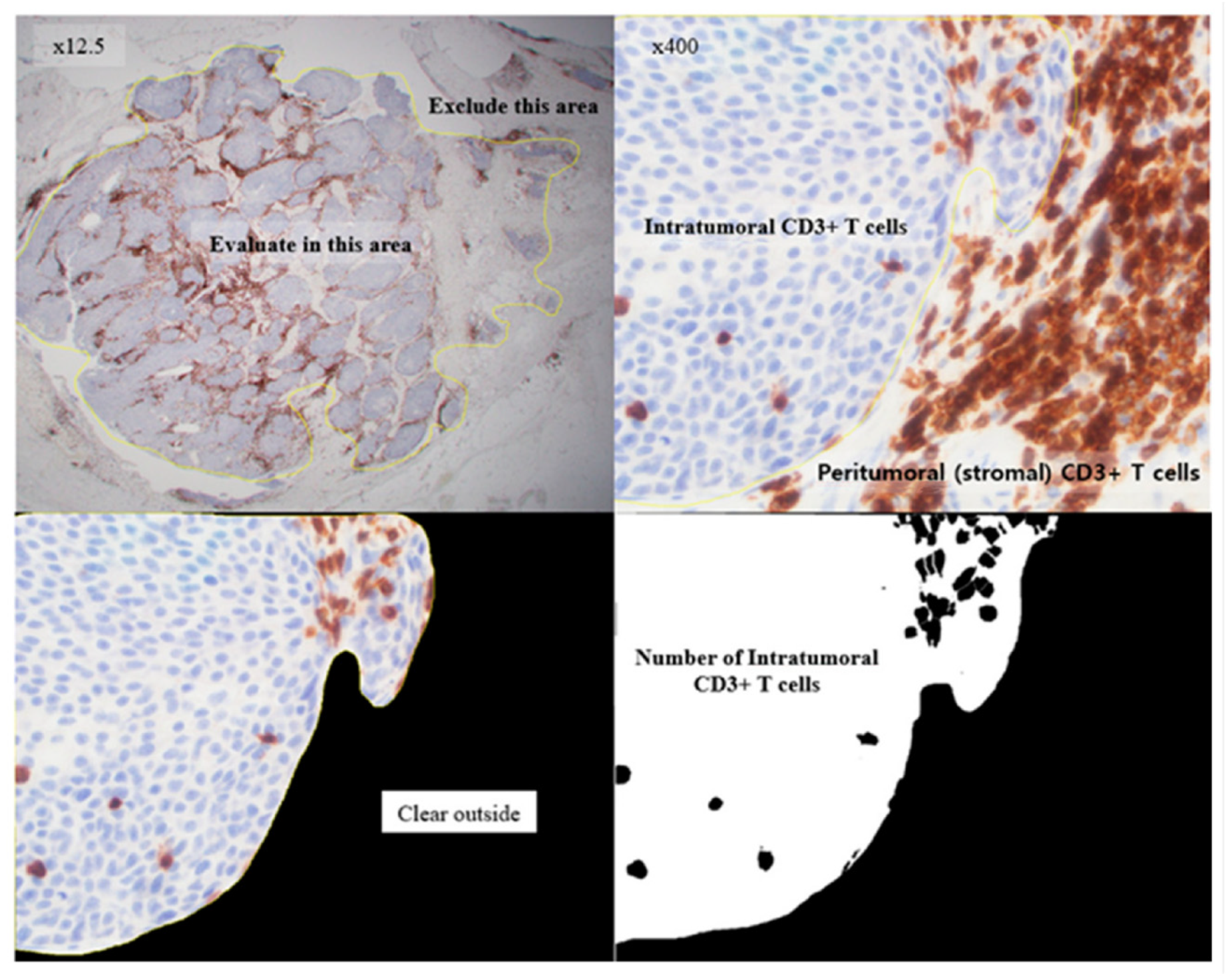
4.3. Interpretation of APOBEC3B Expression and TILs Based on IHC Staining
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reuter, V.E. The pathology of bladder cancer. Urology 2006, 67, 11–17; Discussion 17–18. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef]
- Bellmunt, J.; Powles, T.; Vogelzang, N.J. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: The future is now. Cancer Treat. Rev. 2017, 54, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, L.; Carteni, G.; Siena, S.; Bertetto, O.; Martoni, A.; Bono, A.; Amadori, D.; Onat, H.; Marini, L. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: Results of a randomized phase 2 trial. Eur. Urol. 2007, 52, 134–141. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.K.; Noon, A.P. Epidemiology, aetiology and screening of bladder cancer. Transl. Androl. Urol. 2019, 8, 5–11. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Lerner, S.P.; Kwiatkowski, D.J. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Faltas, B.M. Treatment resistance in urothelial carcinoma: An evolutionary perspective. Nat. Rev. Clin. Oncol. 2018, 15, 495–509. [Google Scholar] [CrossRef]
- Roberts, S.A.; Lawrence, M.S.; Klimczak, L.J.; Grimm, S.A.; Fargo, D.; Stojanov, P.; Kiezun, A.; Kryukov, G.V.; Carter, S.L.; Saksena, G.; et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat. Genet. 2013, 45, 970–976. [Google Scholar] [CrossRef]
- Glaser, A.P.; Fantini, D.; Wang, Y.; Yu, Y.; Rimar, K.J.; Podojil, J.R.; Miller, S.D.; Meeks, J.J. APOBEC-mediated mutagenesis in urothelial carcinoma is associated with improved survival, mutations in DNA damage response genes, and immune response. Oncotarget 2018, 9, 4537–4548. [Google Scholar] [CrossRef]
- Baras, A.S.; Drake, C.; Liu, J.J.; Gandhi, N.; Kates, M.; Hoque, M.O.; Meeker, A.; Hahn, N.; Taube, J.M.; Schoenberg, M.P.; et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology 2016, 5, e1134412. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed]
- Mullane, S.A.; Werner, L.; Rosenberg, J.; Signoretti, S.; Callea, M.; Choueiri, T.K.; Freeman, G.J.; Bellmunt, J. Correlation of Apobec Mrna Expression with overall Survival and pd-l1 Expression in Urothelial Carcinoma. Sci. Rep. 2016, 6, 27702. [Google Scholar] [CrossRef] [PubMed]
- Kanu, N.; Cerone, M.A.; Goh, G.; Zalmas, L.P.; Bartkova, J.; Dietzen, M.; McGranahan, N.; Rogers, R.; Law, E.K.; Gromova, I.; et al. DNA replication stress mediates APOBEC3 family mutagenesis in breast cancer. Genome. Biol. 2016, 17, 185. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, Q.; Park, D.; Deng, X. Targeting DNA Replication Stress for Cancer Therapy. Genes 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Buisson, R.; Lawrence, M.S.; Benes, C.H.; Zou, L. APOBEC3A and APOBEC3B Activities Render Cancer Cells Susceptible to ATR Inhibition. Cancer Res. 2017, 77, 4567–4578. [Google Scholar] [CrossRef]
- Jiang, T.; Shi, J.; Dong, Z.; Hou, L.; Zhao, C.; Li, X.; Mao, B.; Zhu, W.; Guo, X.; Zhang, H.; et al. Genomic landscape and its correlations with tumor mutational burden, PD-L1 expression, and immune cells infiltration in Chinese lung squamous cell carcinoma. J. Hematol. Oncol. 2019, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Faraj, S.F.; Munari, E.; Guner, G.; Taube, J.; Anders, R.; Hicks, J.; Meeker, A.; Schoenberg, M.; Bivalacqua, T.; Drake, C.; et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 2015, 85, e701–e706. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Muller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Leonard, B.; Starrett, G.J.; Maurer, M.J.; Oberg, A.L.; Van Bockstal, M.; Van Dorpe, J.; De Wever, O.; Helleman, J.; Sieuwerts, A.M.; Berns, E.M.; et al. APOBEC3G Expression Correlates with T-Cell Infiltration and Improved Clinical Outcomes in High-grade Serous Ovarian Carcinoma. Clin. Cancer Res. 2016, 22, 4746–4755. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chang, Y.; An, H.; Zhu, Y.; Yang, Y.; Xu, J. High APOBEC3B expression is a predictor of recurrence in patients with low-risk clear cell renal cell carcinoma. Urol. Oncol. 2015, 33, 340.e1–340.e8. [Google Scholar] [CrossRef]
- Huang, H.S.; Su, H.Y.; Li, P.H.; Chiang, P.H.; Huang, C.H.; Chen, C.H.; Hsieh, M.C. Prognostic impact of tumor infiltrating lymphocytes on patients with metastatic urothelial carcinoma receiving platinum based chemotherapy. Sci. Rep. 2018, 8, 7485. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Suzuki, T.; Suzuki, S.; Moriya, T.; Kaneko, C.; Takizawa, T.; Sunamori, M.; Handa, M.; Kondo, T.; Sasano, H. Sex steroid hormone receptors in human thymoma. J. Clin. Endocrinol. Metab. 2003, 88, 2309–2317. [Google Scholar] [CrossRef]
| Total (n = 94) | APOBEC3B Low * (n = 23) | APOBEC3B High ** (n = 71) | p-Value | ||
|---|---|---|---|---|---|
| Age | median (range) | 68 (36–93) | 68 (48–88) | 70 (36–93) | |
| <65 | 33 | 6 | 27 | 0.297 | |
| ≥65 | 61 | 17 | 44 | ||
| Sex | Female | 22 | 5 | 17 | 0.828 |
| Male | 72 | 18 | 54 | ||
| ECOG-PS *** | 0 | 16 | 1 | 15 | |
| 1 | 23 | 5 | 18 | ||
| 2 | 10 | 2 | 8 | ||
| unknown | 45 | 15 | 30 | ||
| Primary Site | Renal pelvis | 22 | 8 | 14 | 0.314 |
| Ureter | 22 | 4 | 18 | ||
| Bladder | 50 | 11 | 39 | ||
| Disease presentation | Recurrent | 57 | 13 | 44 | 0.642 |
| Metastatic | 37 | 10 | 27 | ||
| Metastatic site | Lymphnode only | 36 | 10 | 26 | 0.556 |
| Liver, Lung, bone, others | 58 | 13 | 45 | ||
| Regimen | Gem/Cis | 29 | 4 | 25 | 0.108 |
| Gem/Carbo | 65 | 19 | 46 | ||
| previous chemotherapy | No | 83 | 21 | 62 | 1.000 |
| Yes | 11 | 2 | 9 | ||
| Subsequent chemotherapy | No | 43 | 9 | 34 | 0.464 |
| Yes | 51 | 14 | 37 | ||
| Surgery | No | 41 | 13 | 28 | 0.151 |
| Yes | 53 | 10 | 43 | ||
| H-score of APOBEC3B | median (range) | 110 (0–280) | 70 (0–90) | 120 (95–280) | |
| OS | PFS | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Response | CR or PR | 1.000 | 1.000 | ||||
| SD or PD | 3.918 | 1.955–7.849 | < 0.001 | 4.478 | 2.206–9.089 | < 0.001 | |
| APOBEC3B | Low | 1.000 | 1.000 | ||||
| High | 0.292 | 0.118–0.723 | 0.008 | 0.335 | 0.139–0.806 | 0.015 | |
| Intra-Tumoral and Stromal TIL Number | ||||
|---|---|---|---|---|
| CD8/CD3 ratio | Total | APOEC3B low | APOBEC3B high | p value |
| Low | 52 | 17 | 35 | 0.039 |
| High | 42 | 6 | 36 | |
| Intra-Tumoral and Stromal TIL Area | ||||
| CD8/CD3 ratio | Total | APOBEC3B low | APOBEC3B high | p value |
| Low | 52 | 17 | 35 | 0.039 |
| High | 42 | 6 | 36 | |
| Total | APOBEC3B * Low | APOBEC3B ** High | p-Value | |
|---|---|---|---|---|
| CR or PR | 59 | 12 | 24 | 0.227 |
| SD or PD | 35 | 11 | 47 | |
| CR, PR or SD | 73 | 15 | 58 | 0.099 |
| PD | 21 | 8 | 13 | |
| Total | APOBEC3B low | APOBEC3B high | ||
| CR | 18 | 2 | 16 | |
| PR | 41 | 10 | 31 | |
| SD | 14 | 3 | 11 | |
| PD | 21 | 8 | 13 | |
| Total | 94 | 23 | 71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, O.; Lee, M.A.; Lee, J.Y.; Hong, S.-H.; Ha, U.-S.; Yim, K.; Kim, I.-H. Prognostic Impact of APOBEC3B Expression in Metastatic Urothelial Carcinoma and Its Association with Tumor-Infiltrating Cytotoxic T Cells. Curr. Oncol. 2021, 28, 1652-1662. https://doi.org/10.3390/curroncol28030154
Kim H, Kim O, Lee MA, Lee JY, Hong S-H, Ha U-S, Yim K, Kim I-H. Prognostic Impact of APOBEC3B Expression in Metastatic Urothelial Carcinoma and Its Association with Tumor-Infiltrating Cytotoxic T Cells. Current Oncology. 2021; 28(3):1652-1662. https://doi.org/10.3390/curroncol28030154
Chicago/Turabian StyleKim, Hyunho, Okran Kim, Myung Ah Lee, Ji Youl Lee, Sung-Hoo Hong, U-Syn Ha, Kwangil Yim, and In-Ho Kim. 2021. "Prognostic Impact of APOBEC3B Expression in Metastatic Urothelial Carcinoma and Its Association with Tumor-Infiltrating Cytotoxic T Cells" Current Oncology 28, no. 3: 1652-1662. https://doi.org/10.3390/curroncol28030154
APA StyleKim, H., Kim, O., Lee, M. A., Lee, J. Y., Hong, S.-H., Ha, U.-S., Yim, K., & Kim, I.-H. (2021). Prognostic Impact of APOBEC3B Expression in Metastatic Urothelial Carcinoma and Its Association with Tumor-Infiltrating Cytotoxic T Cells. Current Oncology, 28(3), 1652-1662. https://doi.org/10.3390/curroncol28030154






