The Relationship between Fatigue and Actigraphy-Derived Sleep and Rest–Activity Patterns in Cancer Survivors
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Study Design
2.3. Actigraphy
2.4. Sleep Diary
2.5. Cancer-Related Fatigue
2.6. Insomnia Severity
2.7. Data Analysis
2.8. Statistical Methods
3. Results
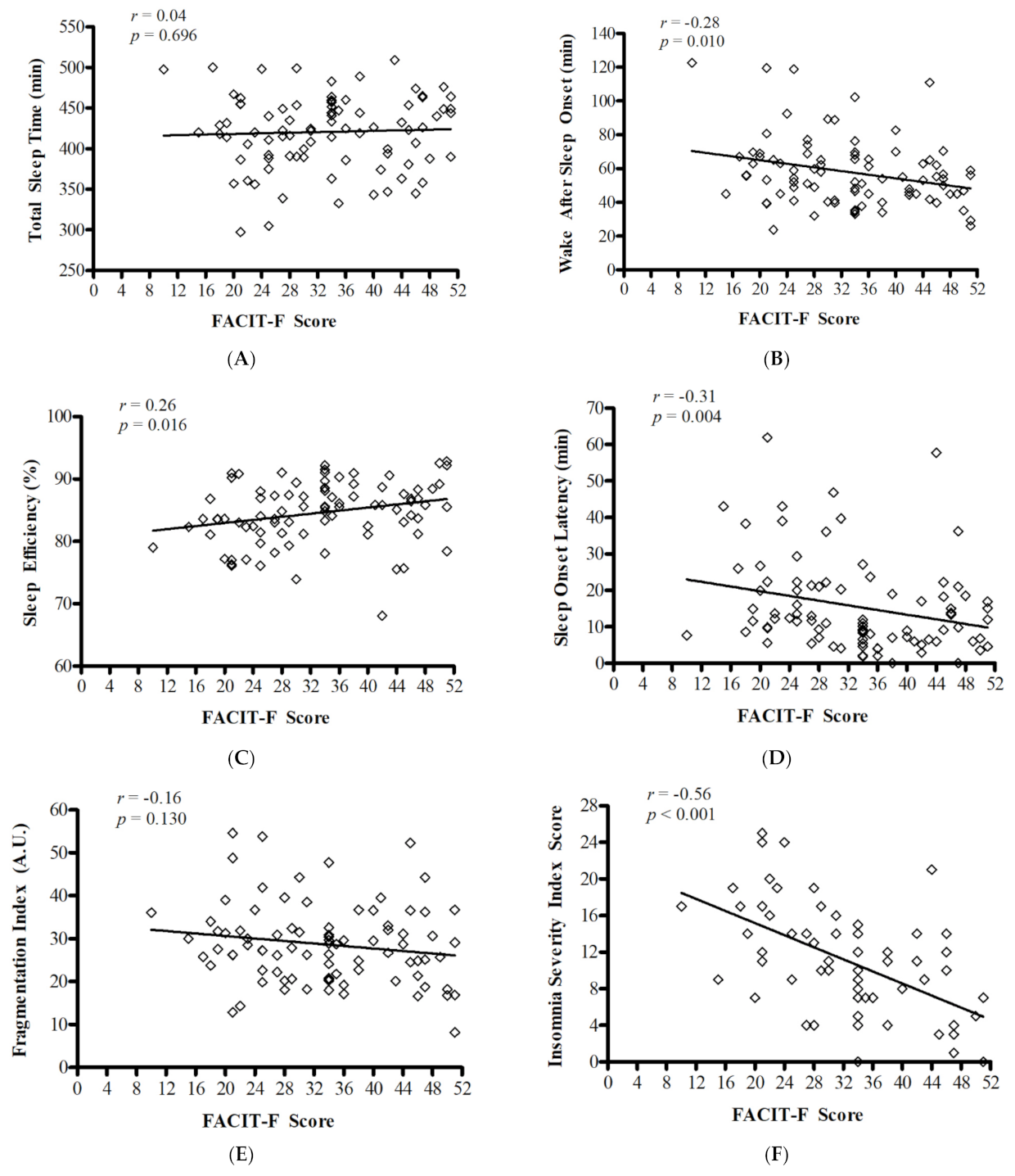
3.1. Objective Measures of Sleep
3.2. Rest–Activity Cycles
3.3. Perception of Sleep Disruption
4. Discussion
4.1. The Relationship between Fatigue and Actigraphy-Derived Sleep and Comparison to Reference Data for Sleep Duration and Quality
4.2. The Relationship between Fatigue and Rest–Activity Cycles in Cancer Survivors
4.3. The Mismatch between Subjective and Objective Measures of Sleep
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prue, G.; Rankin, J.; Allen, J.; Gracey, J.; Cramp, F. Cancer-related fatigue: A critical appraisal. Eur. J. Cancer 2006, 42, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef]
- Jones, J.M.; Olson, K.; Catton, P.; Catton, C.N.; Fleshner, N.E.; Krzyzanowska, M.K.; McCready, D.R.; Wong, R.K.S.; Jiang, H.; Howell, D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J. Cancer Surviv. 2016, 10, 51–61. [Google Scholar] [CrossRef] [PubMed]
- O’Higgins, C.M.; Brady, B.; O’Connor, B.; Walsh, D.; Reilly, R.B. The pathophysiology of cancer-related fatigue: Current controversies. Support. Care Cancer 2018, 26, 3353–3364. [Google Scholar] [CrossRef]
- Ryan, J.L.; Carroll, J.K.; Ryan, E.P.; Mustian, K.M.; Fiscella, K.; Morrow, G.R. Mechanisms of cancer-related fatigue. Oncologist 2007, 12, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, J.A.; Kaufman, M.E.; Matteson-Rusby, S.E.; Palesh, O.G.; Ryan, J.L.; Kohli, S.; Perlis, M.L.; Morrow, G.R. Cancer-Related Fatigue and Sleep Disorders. Oncology 2007, 12, 35–42. [Google Scholar] [CrossRef]
- Howell, D.; Keshavarz, H.; Broadfield, L.; Hack, T.; Hamel, M.; Harth, T.J. On behalf of the Cancer Journey Advisory Group of the Canadian Partnership Against Cancer. A Pan Canadian Practice Guideline for Screening, Assessment, and Management of Cancer-Related Fatigue in Adults Version 2-2015; Canadian Partnership Against Cancer (Cancer Journey Advisory Group) and the Canadian Association of Psychosocial Oncology: Toronto, ON, Canada, 2015. [Google Scholar] [CrossRef]
- Minton, O.; Stone, P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann. Oncol. 2009, 20, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yellen, S.B.; Cella, D.F.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74. [Google Scholar] [CrossRef]
- Van Belle, S.; Paridaens, R.; Evers, G. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: Proposal for use as a screening tool. Support Care Cancer Off. J. Multinatl. Assoc. Support Care Cancer 2005, 13, 246–254. [Google Scholar] [CrossRef]
- Twomey, R.; Martin, T.; Temesi, J.; Culos-Reed, S.N.; Millet, G.Y. Tailored exercise interventions to reduce fatigue in cancer survivors: Study protocol of a randomized controlled trial. BMC Cancer 2018, 18, 757. [Google Scholar] [CrossRef]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, J.C.; Targum, S.D.; Harrison, J.E. Cognitive Impairment Associated with Cancer. Innov. Clin. Neurosci. 2018, 15, 36–44. [Google Scholar]
- Otte, J.L.; Carpenter, J.S.; Manchanda, S.; Rand, K.L.; Skaar, T.C.; Weaver, M.T.; Chernyak, Y.; Zhong, X.; Igega, C.; Landis, C. Systematic review of sleep disorders in cancer patients: Can the prevalence of sleep disorders be ascertained? Cancer Med. 2014, 4, 183–200. [Google Scholar] [CrossRef]
- Harvey, A.G.; Tang, N.K.Y. (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychol. Bull. 2012, 138, 77–101. [Google Scholar] [CrossRef] [PubMed]
- Reinsel, R.A.; Starr, T.D.; O’Sullivan, B.; Passik, S.D.; Kavey, N.B. Polysomnographic Study of Sleep in Survivors of Breast Cancer. J. Clin. Sleep Med. 2015, 11, 1361–1370. [Google Scholar] [CrossRef]
- Banthia, R.; Malcarne, V.L.; Ko, C.M.; Varni, J.W.; Sadler, G.R. Fatigued breast cancer survivors: The role of sleep quality, depressed mood, stage and age. Psychol. Health 2009, 24, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rissling, M.; Neikrug, A.; Fiorentino, L.; Natarajan, L.; Faierman, M.; Sadler, G.R.; Dimsdale, J.E.; Mills, P.J.; Parker, B.A.; et al. Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: A controlled study. Fatigue Biomed. Health Behav. 2013, 1, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Medysky, M.E.; Temesi, J.; Culos-Reed, S.N.; Millet, G.Y. Exercise, sleep and cancer-related fatigue: Are they related? Neurophysiol. Clin. Neurophysiol. 2017, 47, 111–122. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- van Someren, E.J. Improving actigraphic sleep estimates in insomnia and dementia: How many nights? J. Sleep Res. 2007, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, T.I.; Lee-Chiong, T.; Alessi, C.; Friedman, L.; Aurora, R.N.; Boehlecke, B.; Brown, T.; Chesson, A.L.; Kapur, V.; Maganti, R.; et al. Practice Parameters for the Clinical Evaluation and Treatment of Circadian Rhythm Sleep Disorders. Sleep 2007, 30, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef]
- Andersen, C.; Rørth, M.; Ejlertsen, B.; Stage, M.; Møller, T.; Midtgaard, J.; Quist, M.; Bloomquist, K.; Adamsen, L. The effects of a six-week supervised multimodal exercise intervention during chemotherapy on cancer-related fatigue. Eur. J. Oncol. Nurs. 2013, 17, 331–339. [Google Scholar] [CrossRef]
- Savard, M.-H.; Savard, J.; Simard, S.; Ivers, H. Empirical validation of the Insomnia Severity Index in cancer patients. Psycho-Oncol. 2004, 14, 429–441. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Lockley, S.W.; Skene, D.J.; Arendt, J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J. Sleep Res. 1999, 8, 175–183. [Google Scholar] [CrossRef]
- Bonmati-Carrion, M.A.; Middleton, B.; Revell, V.L.; Skene, D.J.; Rol, M.A.; Madrid, J.A.; Rol, A. Validation of an innovative method, based on tilt sensing, for the assessment of activity and body position. Chrono Int. 2015, 32, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.; Brissos, S.; Figueira, M.L.; Paiva, T. Schizophrenia patients with predominantly positive symptoms have more disturbed sleep–wake cycles measured by actigraphy. Psychiatry Res. 2011, 189, 62–66. [Google Scholar] [CrossRef]
- Martin, T.; Moussay, S.; Bulla, I.; Bulla, J.; Toupet, M.; Etard, O.; Denise, P.; Davenne, D.; Coquerel, A.; Quarck, G. Exploration of Circadian Rhythms in Patients with Bilateral Vestibular Loss. PLoS ONE 2016, 11, e0155067. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Manhaw, NJ, USA, 1988. [Google Scholar]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Dahlui, M.; Majid, H.A.; Nahar, A.M.; Taib, N.A.M.; Su, T.T. MyBCC study group Factors associated with return to work of breast cancer survivors: A systematic review. BMC Public Health 2014, 14, S8. [Google Scholar] [CrossRef]
- Berger, A.M. Update on the State of the Science: Sleep-Wake Disturbances in Adult Patients With Cancer. Oncol. Nurs. Forum 2009, 36, E165–E177. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Liu, L.; Rissling, M.; Natarajan, L.; Neikrug, A.B.; Palmer, B.W.; Mills, P.J.; Parker, B.A.; Sadler, G.R.; Maglione, J. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: A 1-year longitudinal study. Support. Care Cancer 2014, 22, 2535–2545. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.; Innominato, P.F.; Boreggiani, M.; Tonetti, L.; Filardi, M.; Parganiha, A.; Fabbri, M.; Martoni, M.; Levi, F. The difference between in bed and out of bed activity as a behavioral marker of cancer patients: A comparative actigraphic study. Chrono Int. 2015, 32, 925–933. [Google Scholar] [CrossRef]
- Palesh, O.; Aldridge-Gerry, A.; Zeitzer, J.; Koopman, C.; Neri, E.; Giese-Davis, J.; Jo, B.; Kraemer, H.; Nouriani, B.; Spiegel, D. Actigraphy-Measured Sleep Disruption as a Predictor of Survival among Women with Advanced Breast Cancer. Sleep 2014, 37, 837–842. [Google Scholar] [CrossRef]
- Costa, A.R.; Fontes, F.; Pereira, S.; Gonçalves, M.; Azevedo, A.; Lunet, N. Impact of breast cancer treatments on sleep disturbances—A systematic review. Breast 2014, 23, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.; Pjrek, E.; Praschak-Rieder, N.; Willeit, M.; Pezawas, L.; Konstantinidis, A.; Stastny, J.; Kasper, S. Actigraphy in Patients with Seasonal Affective Disorder and Healthy Control Subjects Treated with Light Therapy. Biol. Psychiatry 2005, 58, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Mormont, M.C.; Waterhouse, J.; Bleuzen, P.; Giacchetti, S.; Jami, A.; Bogdan, A.; Lellouch, J.; Misset, J.L.; Touitou, Y.; Lévi, F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 2000, 6, 3038–3045. [Google Scholar]
- Davis, M.P.; Khoshknabi, D.; Walsh, D.; Lagman, R.; Platt, A. Insomnia in Patients With Advanced Cancer. Am. J. Hosp. Palliat. Med. 2014, 31, 365–373. [Google Scholar] [CrossRef]
- Fleming, L.; Randell, K.; Stewart, E.; Espie, C.A.; Morrison, D.S.; Lawless, C.; Paul, J. Insomnia in breast cancer: A prospective observational study. Sleep 2018, 42. [Google Scholar] [CrossRef]
- Dirksen, S.R.; Belyea, M.J.; Epstein, D.R. Fatigue-Based Subgroups of Breast Cancer Survivors With Insomnia. Cancer Nurs. 2009, 32, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Ritterband, L.M.; Bailey, E.T.; Thorndike, F.P.; Lord, H.R.; Farrell-Carnahan, L.; Baum, L.D. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psycho-Oncol. 2012, 21, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Carter, P.; Page, M.; Dean, G.; Berger, A. Sleep-Wake Disturbance: A Systematic Review of Evidence-Based Interventions for Management in Patients With Cancer. Clin. J. Oncol. Nurs. 2018, 22, 37–52. [Google Scholar] [CrossRef]
- Jungquist, C.R.; Pender, J.J.; Klingman, K.J.; Mund, J. Validation of Capturing Sleep Diary Data via a Wrist-Worn Device. Sleep Disord. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
| All | Fatigued | Non-Fatigued | ||
|---|---|---|---|---|
| N | 87 | 51 | 36 | |
| Age (years) | 55.8 ± 10.2 | 54.2 ± 8.9 | 58.2 ± 11.6 | |
| Months Since Treatment | 33.4 ± 28.7 | 40.1 ± 28.9 | 29.9 ± 27.1 | |
| Sex | Female | 53 (61) | 32 (63) | 21 (58) |
| Male | 34 (39) | 19 (37) | 15 (42) | |
| Ethnicity (self-identified) a | White | 76 (89) | 43 (88) | 33 (98) |
| Middle Eastern | 1 (1) | 0 (0) | 1 (3) | |
| Asian | 5 (6) | 4 (8) | 1 (3) | |
| Black | 1 (1) | 1 (2) | 0 (0) | |
| First Nations | 2 (2) | 1 (2) | 1 (3) | |
| Marital Status a | Married/Common Law | 64 (75) | 34 (69) | 30 (83) |
| Divorced | 12 (14) | 6 (12) | 6 (17) | |
| Single | 7 (8) | 7 (14) | 0 (0) | |
| Widowed | 2 (2) | 2 (4) | 0 (0) | |
| Education a | University | 42 (49) | 25 (51) | 17 (48) |
| College | 26 (31) | 16 (33) | 10 (28) | |
| Secondary School | 14 (17) | 5 (10) | 9 (25) | |
| Other | 3 (4) | 3 (6) | 0 (0) | |
| Employment Status | Part-Time | 14 (17) | 6 (12) | 8 (22) |
| Full-Time | 34 (40) | 23 (47) | 11 (31) | |
| Retired | 20 (24) | 6 (12) | 14 (39) | |
| Unemployed | 7 (8) | 5 (10) | 2 (6) | |
| Disability/Leave | 10 (12) | 9 (18) | 1 (3) | |
| Cancer Type | Breast | 38 (44) | 22 (43) | 16 (44) |
| Prostate | 15 (17) | 4 (8) | 11 (31) | |
| Head & Neck | 8 (9) | 6 (12) | 2 (6) | |
| Colorectal | 7 (8) | 5 (10) | 2 (6) | |
| Hematologic | 1 (1) | 1 (2) | 0 (0) | |
| Other | 18 (21) | 13 (26) | 5 (14) | |
| FACIT-F Score | 33 ± 10 | 26 ± 6 | 43 ± 6 |
| Fatigued (n = 51) | Non-Fatigued (n = 36) | |||||
|---|---|---|---|---|---|---|
| Outcome | Mean | SD | Mean | SD | P (q) | Effect Size |
| Sleep (Actigraphy) | ||||||
| Total Sleep Time (min) | 422.6 | 44.9 | 417.9 | 46.3 | 0.632 (0.100) | 0.10 |
| Sleep Efficiency (%) | 84.1 | 4.7 | 85.3 | 5.3 | 0.285 (0.071) | 0.23 |
| Sleep Onset Latency (min) np | 17.3 | 13.1 | 12.9 | 11.9 | 0.053 a (0.064) | 0.35 |
| Wake After Sleep Onset (min) | 61.2 | 22.4 | 52.9 | 15.7 | 0.046 a (0.057) | 0.43 |
| Fragmentation Index | 29.5 | 9.4 | 27.5 | 8.9 | 0.305 (0.086) | 0.23 |
| Perceptions of Sleep | ||||||
| Insomnia Severity Index | 12.7 | 5.6 | 8.0 | 5.6 | 0.020 a (0.043) | 0.84 |
| Rest–activity Cycle | ||||||
| Relative amplitude np | 0.88 | 0.07 | 0.91 | 0.05 | 0.017 a (0.029) | 0.58 |
| Bed times | 23 h 21 | 0 h 57 | 22 h 55 | 00 h 48 | 0.033 a (0.05) | 0.48 |
| Wake up times | 7 h 40 | 01 h 07 | 6 h 59 | 01 h 11 | 0.009 a (0.021) | 0.58 |
| Peak Time (hh:mm) | 14 h 40 | 01 h 14 | 14 h 00 | 00 h 53 | 0.007 a (0.007) | 0.62 |
| Mean Sleep Actigraphy (MW8 counts) np | 15.6 | 6.9 | 12.8 | 6.5 | 0.018 a (0.036) | 0.42 |
| Mean Wake Actigraphy (MW8 counts) | 101.3 | 33.6 | 109.6 | 37.0 | 0.289 (0.079) | 0.23 |
| Index of Activity during Wake (%) | 68.9 | 9.3 | 70.8 | 10.0 | 0.364 (0.093) | 0.20 |
| Index of Activity during Sleep (%) | 14.3 | 3.8 | 12.8 | 3.7 | 0.008 a (0.014) | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, T.; Twomey, R.; Medysky, M.E.; Temesi, J.; Culos-Reed, S.N.; Millet, G.Y. The Relationship between Fatigue and Actigraphy-Derived Sleep and Rest–Activity Patterns in Cancer Survivors. Curr. Oncol. 2021, 28, 1170-1182. https://doi.org/10.3390/curroncol28020113
Martin T, Twomey R, Medysky ME, Temesi J, Culos-Reed SN, Millet GY. The Relationship between Fatigue and Actigraphy-Derived Sleep and Rest–Activity Patterns in Cancer Survivors. Current Oncology. 2021; 28(2):1170-1182. https://doi.org/10.3390/curroncol28020113
Chicago/Turabian StyleMartin, Tristan, Rosie Twomey, Mary E. Medysky, John Temesi, S. Nicole Culos-Reed, and Guillaume Y. Millet. 2021. "The Relationship between Fatigue and Actigraphy-Derived Sleep and Rest–Activity Patterns in Cancer Survivors" Current Oncology 28, no. 2: 1170-1182. https://doi.org/10.3390/curroncol28020113
APA StyleMartin, T., Twomey, R., Medysky, M. E., Temesi, J., Culos-Reed, S. N., & Millet, G. Y. (2021). The Relationship between Fatigue and Actigraphy-Derived Sleep and Rest–Activity Patterns in Cancer Survivors. Current Oncology, 28(2), 1170-1182. https://doi.org/10.3390/curroncol28020113






