Identification and In-Depth Analysis of the Novel FGFR2-NDC80 Fusion in a Cholangiocarcinoma Patient: Implication for Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. Image Acquisition
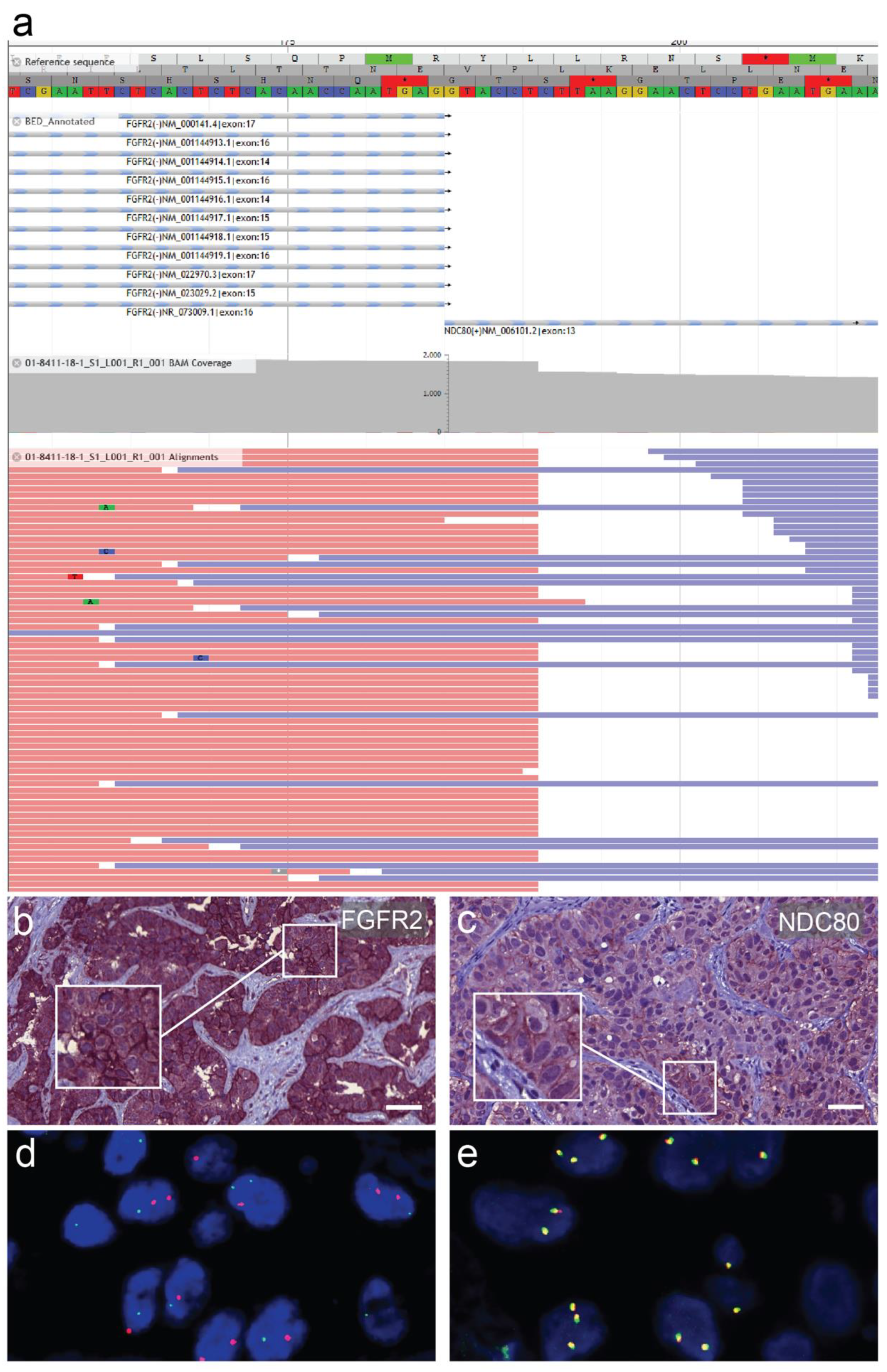
2.3. Fluorescence In Situ Hybridization (FISH)
2.4. Next-Generation DNA and RNA Sequencing
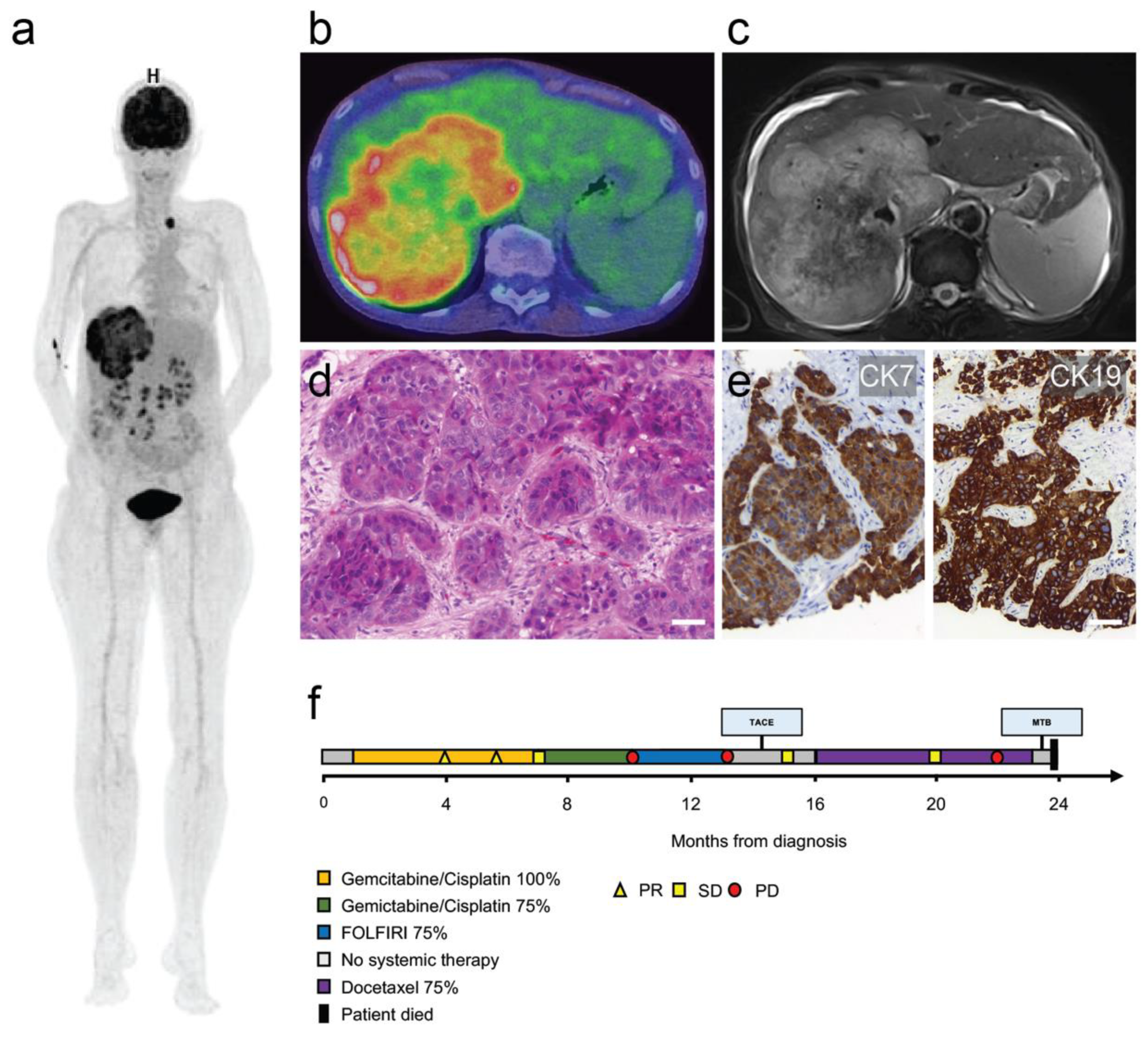
3. Case Report
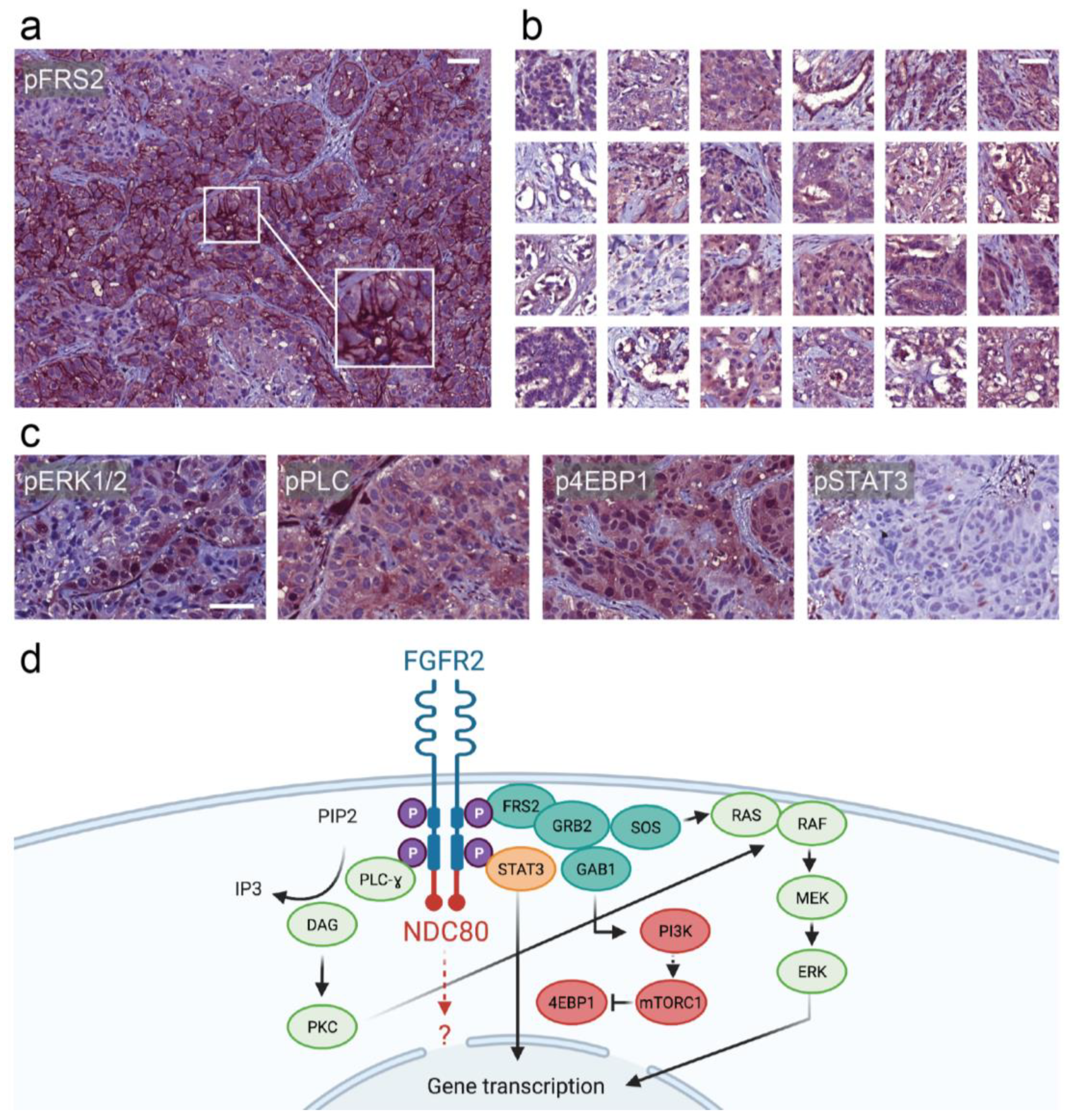
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Weigt, J.; Malfertheiner, P. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Machida, N.; Morizane, C.; Kasuga, A.; Takahashi, H.; Sudo, K.; Nishina, T.; Tobimatsu, K.; Ishido, K.; Furuse, J.; et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014, 105, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; André, F.; Soria, J.-C. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.H.; Toda, T. MAPping the Ndc80 loop in cancer: A possible link between Ndc80/Hec1 overproduction and cancer formation. BioEssays 2015, 37, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Gores, G.J. Emerging molecular therapeutic targets for cholangiocarcinoma. J. Hepatol. 2017, 67, 632–644. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM classification of malignant tumours—Towards common understanding and reasonable expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef]
- Chen, H.; Ma, J.; Li, W.; Eliseenkova, A.V.; Xu, C.; Neubert, T.A.; Miller, W.T.; Mohammadi, M. A Molecular Brake in the Kinase Hinge Region Regulates the Activity of Receptor Tyrosine Kinases. Mol. Cell 2007, 27, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Wimbish, R.T.; de Luca, J.G. Hec1/Ndc80 Tail Domain Function at the Kinetochore-Microtubule Interface. Front. Cell Dev. Biol. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2013, 59, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Chan, J.M.; Zoppoli, P.; Niola, F.; Sullivan, R.; Castano, A.; Liu, E.M.; Reichel, J.; Porrati, P.; Pellegatta, S.; et al. Transforming Fusions of FGFR and TACC Genes in Human Glioblastoma. Science 2012, 337, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Borad, M.J.; Champion, M.D.; Egan, J.B.; Liang, W.S.; Fonseca, R.; Bryce, A.H.; McCullough, A.E.; Barrett, M.T.; Hunt, K.; Patel, M.D.; et al. Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma. PLoS Genet. 2014, 10, e1004135. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Clone | Source | Dilution | Antigen Retrieval |
|---|---|---|---|---|
| FGFR2 | SP273 | Abcam | 1:1000 | citrate |
| NDC80/HEC | polyclonal | Abcam | 1:1000 | citrate |
| pERK1/2 | D13.14.4E | Cell Signaling | 1:400 | citrate |
| p4E-BP1 | 236B4 | Cell Signaling | 1:50 | citrate |
| pFRS2 | polyclonal | Abcam | 1:400 | citrate |
| pSTAT3 | D3A7 | Cell Signaling | 1:200 | Tris-EDTA |
| pPLCγ | D25A9 | Cell Signaling | 1:100 | citrate |
| CK7 | OV-TL12/30 | Dako | 1:400 | - |
| CK19 | KS19.1 | Progen | 1:400 | - |
| HepPar1 | OCH1E5 | Dako | 1:500 | - |
| Arginase1 | 380R-15 | Cell Mark | 1:50 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheiter, A.; Keil, F.; Lüke, F.; Grosse, J.; Verloh, N.; Opitz, S.; Schlosser, S.; Kandulski, A.; Pukrop, T.; Dietmaier, W.; et al. Identification and In-Depth Analysis of the Novel FGFR2-NDC80 Fusion in a Cholangiocarcinoma Patient: Implication for Therapy. Curr. Oncol. 2021, 28, 1161-1169. https://doi.org/10.3390/curroncol28020112
Scheiter A, Keil F, Lüke F, Grosse J, Verloh N, Opitz S, Schlosser S, Kandulski A, Pukrop T, Dietmaier W, et al. Identification and In-Depth Analysis of the Novel FGFR2-NDC80 Fusion in a Cholangiocarcinoma Patient: Implication for Therapy. Current Oncology. 2021; 28(2):1161-1169. https://doi.org/10.3390/curroncol28020112
Chicago/Turabian StyleScheiter, Alexander, Felix Keil, Florian Lüke, Jirka Grosse, Niklas Verloh, Sabine Opitz, Sophie Schlosser, Arne Kandulski, Tobias Pukrop, Wolfgang Dietmaier, and et al. 2021. "Identification and In-Depth Analysis of the Novel FGFR2-NDC80 Fusion in a Cholangiocarcinoma Patient: Implication for Therapy" Current Oncology 28, no. 2: 1161-1169. https://doi.org/10.3390/curroncol28020112
APA StyleScheiter, A., Keil, F., Lüke, F., Grosse, J., Verloh, N., Opitz, S., Schlosser, S., Kandulski, A., Pukrop, T., Dietmaier, W., Evert, M., Calvisi, D. F., & Utpatel, K. (2021). Identification and In-Depth Analysis of the Novel FGFR2-NDC80 Fusion in a Cholangiocarcinoma Patient: Implication for Therapy. Current Oncology, 28(2), 1161-1169. https://doi.org/10.3390/curroncol28020112





