Abstract
Background: Myxofibrosarcoma is a type of soft-tissue sarcoma that is associated with high rates of local recurrence and distant metastases. The first-line treatment for metastatic soft-tissue sarcoma has conventionally been doxorubicin-based. Recent evidence suggests that myxofibrosarcoma may be molecularly similar to undifferentiated pleomorphic sarcoma (UPS), which is particularly sensitive to gemcitabine-based therapy. The goal of this study was to evaluate the activity of gemcitabine-containing regimens for the treatment of metastatic myxofibrosarcoma refractory to doxorubicin. Material and Methods: We retrospectively evaluated seven consecutive cases of metastatic myxofibrosarcoma at our institution treated with gemcitabine-based therapy in the second-line setting, after progression on doxorubicin. Baseline clinical and baseline characteristics were collected. Primary endpoints were objective response rate (ORR), progression-free survival (PFS) and overall survival (OS). Results: After progression on first-line doxorubicin, a partial, or complete radiological response was observed in four of seven patients who received gemcitabine-based chemotherapy. With a median follow-up of 14 months, median progression-free and overall survival were 8.5 months and 11.4 months, respectively. Conclusions: Gemcitabine-based chemotherapy was associated with encouraging response rates in this cohort, similar to those seen in UPS. Both entities could be studied together for novel gemcitabine-based regimens.
1. Introduction
Soft-tissue sarcomas are a relatively rare tumor group and first-line treatment for metastatic disease has conventionally been doxorubicin-based [1]. Although there have been notable advances in systemic therapies in the past years, prognosis still remains poor with advanced disease, with studies reporting a median overall survival (OS) between 14 and 17 months [2].
Given the rarity of soft-tissue sarcoma, subgroups have usually been studied altogether for potential therapies. However, recent evidence has shown that systemic therapy response differs depending on histology. Notably, undifferentiated pleomorphic sarcomas (UPS) and leiomyosarcomas are sensitive to regimens containing gemcitabine [3].
Myxofibrosarcoma (MFS) is another subtype of soft-tissue sarcoma which usually arises in the extremities and is associated with high rates of local recurrence and distant metastases [4]. Tumor grade, percentage of myxoid component, and positive margins after resection are predictors of recurrence. Median progression-free survival (PFS) of advanced myxofibrosarcoma treated with anthracycline-based therapy is four months, and response rates to second-line chemotherapy are reported to be as low as 10% [5]. Because of sparse data, there is currently no consensus on second-line treatment in the metastatic setting. Recent studies suggest that MFS may be molecularly similar to UPS [6], which is particularly sensitive to gemcitabine-based therapy [6]. However, the activity of gemcitabine-based therapy in MFS remains unknown. Therefore, the goal of this study was to evaluate the role of gemcitabine-containing regimens for the treatment of metastatic myxofibrosarcoma refractory to doxorubicin.
2. Material and Methods
Between 2010 and 2019, a total of 17 cases of metastatic MFS refractory to doxorubicin were identified and retrospectively evaluated at the McGill University Health Centre (MUHC). Inclusion criteria were a diagnosis of metastatic MFS on second- line treatment with gemcitabine-based therapy after progression on doxorubicin; availability of radiological and clinical data for assessment of response. Ten patients were excluded due to lack of follow-up, other agent used as second-line therapy or no further treatment offered after doxorubicin. A total of 7 patients were included in our cohort (Figure S1 for Consort diagram). Clinical data were retrieved retrospectively from the MUHC sarcoma database and included age, sex, performance status, location and size of initial tumor, stage, status of margin after resection, histological grade, depth of tumor, area of metastasis, treatment agent, time of recurrence, death, last follow-up, and response rate at last follow-up. Primary outcomes were objective response rate (ORR), PFS, and OS. PFS was defined as the time of first administration of gemcitabine-based therapy until objective radiological disease progression according to the RECIST criteria or death, and overall survival was defined as the time until last follow-up or death. The study was approved by the MUHC REB Ethics Committee (Ethics number 2021-7060).
3. Results
The baseline characteristics of all seven patients are summarized in Table 1. Median age was 66 years, and 43% of patients were male. Six patients had a performance status of less than one. All patients had a diagnosis of myxofibrosarcoma originating in the lower extremities with previous primary surgical resection. As per the American Joint Committee on Cancer R classification criteria, five of seven had margins which were involved after primary resection (R1) and four of them had deep margins involved. More than half of the patients had high-grade disease on histology.

Table 1.
Baseline characteristics.
One of seven patients received gemcitabine with docetaxel as a second-line treatment, five patients received gemcitabine and dacarbazine and one received gemcitabine as a single agent. Four of seven patients showed either a partial or a complete radiological response (Figure 1A.) One patient (case 3) with oligometastatic lung involvement experienced partial response (PR) and underwent consolidative stereotactic body radiotherapy to the remaining lung nodule, resulting in a prolonged disease-free status. Another one (case 7) with complete resolution of lung nodules for 10 months had an aggressive disease recurrence and died one month later. With a median follow-up of 14 months, median progression-free and overall survival were 8.5 months and 11.4 months, respectively (Figure 1B.)
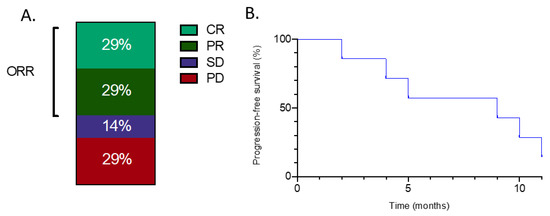
Figure 1.
(A) Objective response rates with gemcitabine-containing regimens for patients with metastatic myxofibrosarcoma treated in the second-line setting. ORR: Objective response rate. PD; progressive disease. CR; complete response SD; Stable disease PR; Partial response. (B) Progression-free survival with gemcitabine-containing regimens for patients with metastatic myxofibrosarcoma treated in the second-line setting.
The American Joint Committee on Cancer R classification R0: wide negative margin. R1: microscopically positive margin. R2: macroscopically positive margin.
4. Discussion and Conclusions
MFS and UPS, both formerly known as malignant fibrous histiocytoma, can be difficult to distinguish. Some authors have proposed that a threshold of at least 10% of myxoid component be an attribute of MFS [7]. It is thought that the hypocellular myxoid component predisposes patients to local recurrence. With recurrence, MFS can become more cellular and resemble UPS morphologically, with no difference in overall survival between recurrent MFS with myxoid area of <10% and primary UPS [7]. Indeed, The Cancer Genome Atlas (TCGA) sarcoma genetically analyzed different subtypes of sarcomas and concluded that, on a molecular basis, UPS and MFS are a spectrum of the same pathology [8]. Although the biology of MFS and UPS is still poorly understood [9], next-generation sequencing in a study of 94 pathology samples of patients diagnosed with either disease revealed frequent loss of Rb and p53, two tumor suppressor genes, from chromosomal deletions or loss-of-function mutations, in both entities [10]. MFS and UPS presenting with these mutations rely on Skp2, an oncogene that promotes cell turnover, to proliferate, and thus Li et al. suggest targeting Skp2 in order to suppress the cancer with novel therapies. Gemcitabine, an antimetabolite that incorporates into DNA and blocks replication, has shown its activity in tumor cells presenting with loss of function for p53 in different tumor subgroups [11].
A phase II randomized trial comparing gemcitabine monotherapy to gemcitabine with docetaxel in metastatic soft-tissue sarcomas refractory to initial treatment showed superiority in terms of overall survival and progression-free survival with the combination [3]. The response to combination therapy was particularly notable in the leiomyosarcoma and UPS subgroups, and therefore this is generally the preferred second-line regimen in recurrent UPS. Similarly, a phase II randomized study comparing gemcitabine with dacarbazine versus dacarbazine alone in patients with recurrent soft-tissue sarcoma revealed superior median overall survival of 16.8 months compared to 8.2 months in the combined regimen [12]. Gemcitabine has thus a role in previously treated soft-tissue sarcomas, especially UPS and leiomyosarcoma, but to our knowledge, there are no current data evaluating the response of myxofibrosarcoma specifically to gemcitabine. As such, our study showed that gemcitabine in second-line setting in metastatic myxofibrosarcoma was associated with encouraging response rates, similar to those observed in UPS, further hinting at the similarities between these two sarcoma types. The exact contribution of docetaxel or dacarbazine to the activity of gemcitabine-based combinations as second-line treatment is unclear, and it is not known whether one is superior to the other in that setting. In our institution, we have noted greater convenience and better tolerability with the gemcitabine/dacarbazine regimen, hence our frequent use of it.
Regarding newer treatments, immunotherapy with pembrolizumab is active in UPS, as shown in a prospective multicenter study [13]. Although there are no comparable data for MFS, some case reports are also suggestive of the potential benefit of immune checkpoint inhibitors in this disease [14,15]. Furthermore, the Melanoma-associated antigen 3 (MAGE-A3) is expressed in both UPS and MFS [16] and is, therefore, a potential immunotherapy target for both entities.
On the basis of the above-mentioned relationship between UPS and MFS and the promising response of MFS to gemcitabine, we agree that both entities could be studied together for novel gemcitabine-based regimens, as already done by some investigators [17].
Supplementary Materials
The following are available online at https://www.mdpi.com/1718-7729/28/1/78/s1, Figure S1: Consort diagram for study inclusion.
Author Contributions
Conceptualization, T.A.; Methodology, A.E., S.K., T.A.; Drafting of manuscript, A.E., S.K., T.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Frezza, A.M.; Stacchiotti, S.; Gronchi, A. Systemic treatment in advanced soft tissue sarcoma: What is standard, what is new. BMC Med. 2017, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Maki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002 [corrected]. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.L.; Wang, W.L.; Lazar, A.J.; Torres, K.E. Myxofibrosarcoma. Surg. Oncol. Clin. N. Am. 2016, 25, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Colia, V.; Fiore, M.; Provenzano, S.; Fumagalli, E.; Bertulli, R.; Morosi, C.; Tos, A.P.D.; Barisella, M.; Gronchi, A.; Casali, P.G.; et al. Activity of anthracycline- and ifosfamide-based chemotherapy in a series of patients affected by advanced myxofibrosarcoma. Clin. Sarcoma Res. 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhou, S.; Hu, X.; Zhang, H.; Huang, S.; Wang, Y. Systematic screening identifies a 2-gene signature as a high-potential prognostic marker of undifferentiated pleomorphic sarcoma/myxofibrosarcoma. J. Cell Mol. Med. 2020, 24, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Yamada, Y.; Ishihara, S.; Kohashi, K.; Toda, Y.; Ito, Y.; Yamamoto, H.; Furue, M.; Nakashima, Y.; Oda, Y. Comparative Study of Myxofibrosarcoma with Undifferentiated Pleomorphic Sarcoma: Histopathologic and Clinicopathologic Review. Am. J. Surg. Pathol. 2020, 44, 87–97. [Google Scholar] [CrossRef]
- Abeshouse, A.; Adebamowo, C.; Adebamowo, S.N.; Akbani, R.; Akeredolu, T.; Ally, A.; Anderson, M.L.; Anur, P.; Appelbaum, E.L.; Armenia, J.; et al. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e28. [Google Scholar] [CrossRef]
- Sambri, A.; De Paolis, M.; Spinnato, P.; Donati, D.M.; Bianchi, G. The Biology of Myxofibrosarcoma: State of the Art and Future Perspectives. Oncol. Res. Treat. 2020, 43, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Okada, T.; Kim, Y.M.; Agaram, N.P.; Sanchez-Vega, F.; Shen, Y.; Tsubokawa, N.; Rios, J.; Martin, A.S.; Dickson, M.A.; et al. Rb and p53-Deficient Myxofibrosarcoma and Undifferentiated Pleomorphic Sarcoma Require Skp2 for Survival. Cancer Res. 2020, 80, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Rabb, M.; Madureira, P.A.; Clements, D.; Gujar, S.A.; Waisman, D.M.; Giacomantonio, C.A.; Lee, P.W.K. Gemcitabine-mediated tumour regression and p53-dependent gene expression: Implications for colon and pancreatic cancer therapy. Cell Death Dis. 2013, 4, e791. [Google Scholar] [CrossRef] [PubMed]
- García-Del-Muro, X.; López-Pousa, A.; Maurel, J.; Martín, J.; Martínez-Trufero, J.; Casado, A.; Gómez-España, A.; Fra, J.; Cruz, J.; Poveda, A.; et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J. Clin. Oncol. 2011, 29, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Song, H.-N.; Kang, M.G.; Park, J.R.; Hwang, J.-Y.; Kang, J.H.; Lee, W.S.; Lee, G.-W. Pembrolizumab for Refractory Metastatic Myxofibrosarcoma: A Case Report. Cancer Res. Treat. 2018, 50, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Pan, D.; Zhou, R. Combination nivolumab and bevacizumab for metastatic myxofibrosarcoma: A case report and review of the literature. Mol. Clin. Oncol. 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.P.; Wang, W.L.; Livingston, J.A.; Ravi, V.; Tsai, J.W.; Ali, A.; Ingram, D.R.; Lowery, C.D.; Roland, C.L.; Somaiah, N.; et al. MAGE-A3 is a Clinically Relevant Target in Undifferentiated Pleomorphic Sarcoma/Myxofibrosarcoma. Cancers 2019, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Chawla, S.P.; Attia, S.; Schöffski, P.; Gelderblom, H.; Chmielowski, B.; Le Cesne, A.; Van Tine, B.A.; Trent, J.C.; Patel, S.; et al. A phase 1 and randomized controlled phase 2 trial of the safety and efficacy of the combination of gemcitabine and docetaxel with ontuxizumab (MORAb-004) in metastatic soft-tissue sarcomas. Cancer 2019, 125, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).