Prophylactic Surgery in the BRCA+ Patient: Do Women Develop Breast Cancer While Waiting?
Abstract
1. Introduction
2. Experimental Section
2.1. British Columbia (BC) Cancer Hereditary Cancer Program (HCP)
2.2. Patient Selection
2.3. Frequency of Surveillance and Surgical Techniques
2.4. Definition of Time Periods
2.5. Ethics
2.6. Statistical Analysis
2.7. Funding Support
3. Results
3.1. Patient Demographics
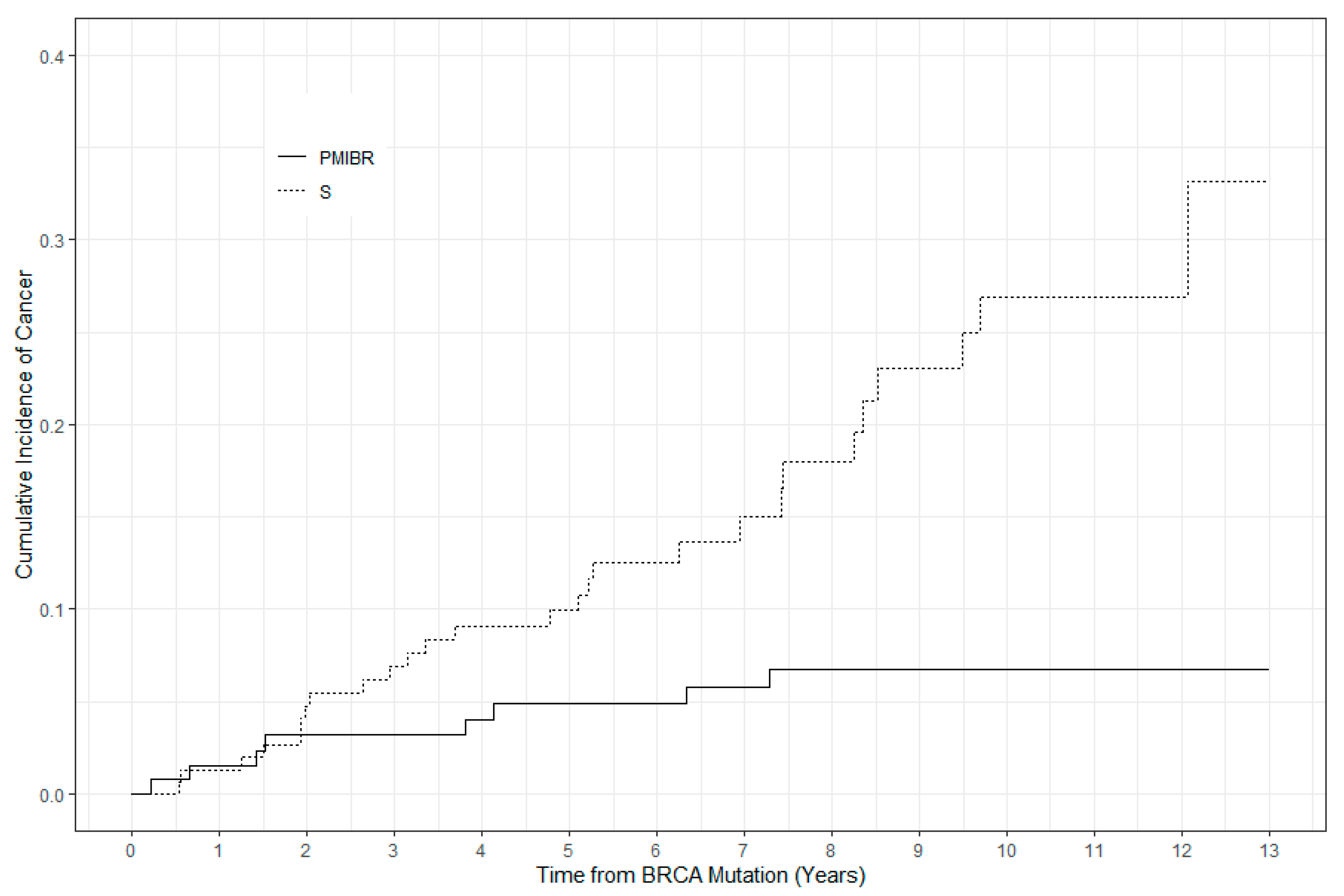
3.2. Incidence of Breast Cancer
3.3. Breast Cancer Characteristics
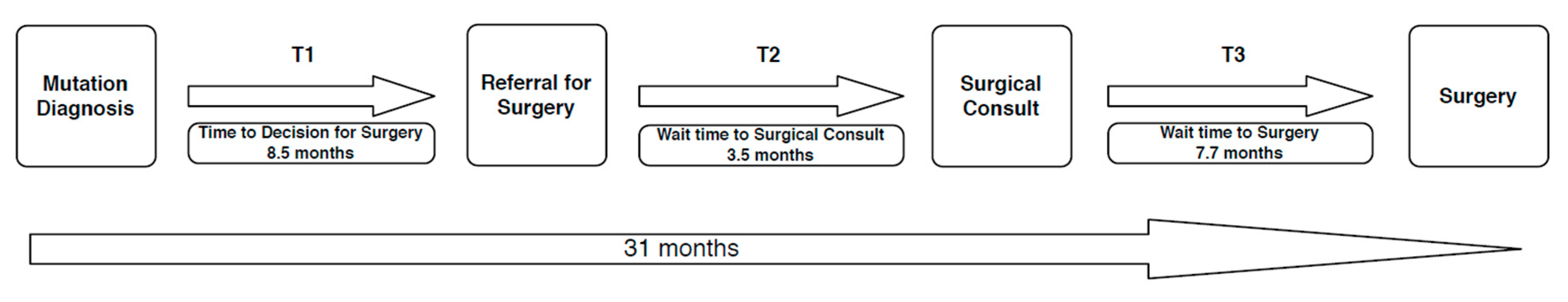
3.4. Time to Surgery
3.5. Reasons for Wait Time
3.6. Time to Development of Cancer
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015, 137, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 5 June 2017).
- King, M.C.; Marks, J.H.; Mandell, J.B. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.; Daly, M.; Garber, J.; Botkin, J.; Kahn, M.J.; Lynch, P.; McTiernan, A.; Offit, K.; Perlman, J.; Petersen, G.; et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA 1997, 277, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H. The management of women at high risk for the development of breast cancer: Risk estimation and preventative strategies. Cancer Treat. Rev. 2003, 29, 79–89. [Google Scholar] [CrossRef]
- Stoutjesdijk, M.J.; Boetes, C.; Jager, G.J.; Beex, L.; Bult, P.; Hendriks, J.C.L.; Laheij, R.J.F.; Massuger, L.; Van Die, L.E.; Barentsz, J.O.; et al. Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer. J. Natl. Cancer Inst. 2001, 93, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Meijers-Heijboer, E.J.; Verhoog, L.C.; Brekelmans, C.T.; Seynaeve, C.; Tilanus-Linthorst, M.M.; Wagner, A.; Dukel, L.; Devilee, P.; Van Den Ouweland, A.M.; Klijn, J.G.; et al. Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet 2000, 355, 2015–2020. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.; Menke-Pluijmers, M.B.; Jager, A.; Tilanus-Linthorst, M.M.A.; Koppert, L.B.; Obdeijn, I.M.A.; Van Deurzen, C.H.M.; Collée, J.M.; Seynaeve, C.; Hooning, M.J.; et al. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: A prospective analysis. Ann. Oncol. 2013, 24, 2029–2035. [Google Scholar] [CrossRef]
- Pijpe, A.; Andrieu, N.; Easton, D.F.; Kesminiene, A.; Cardis, E.; Noguès, C.; Gauthier-Villars, M.; Lasset, C.; Fricker, J.; Peock, S.; et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: Retrospective cohort study (GENE-RAD-RISK). BMJ 2012, 345, e5660. [Google Scholar] [CrossRef]
- Lodder, L.N.; Frets, P.G.; Trijsburg, R.W.; Meijers-Heijboer, E.J.; Klijn, j.M.; Seynaeve, C.; van Geel, A.N.; Tilanus, M.M.A.; Bartels, C.C.M.; Verhoog, L.C.; et al. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: Emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res. Treat. 2002, 73, 97–112. [Google Scholar] [CrossRef]
- Howard, A.F.; Balneaves, L.G.; Bottorff, J.L.; Rodney, P. Preserving the self: The process of decision making about hereditary breast cancer and ovarian cancer risk reduction. Qual Health Res. 2011, 21, 502–519. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). NICE Clinical Guideline 164 Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. June 2013. Available online: https://www.nice.org.uk/guidance/cg164 (accessed on 7 March 2017).
- Meijers-Heijboer, H.; Van Geel, B.; Van Putten, W.L.J.; Henzen-Logmans, S.C.; Seynaeve, C.; Menke-Pluymers, M.B.; Bartels, C.C.; Verhoog, L.C.; Van Den Ouweland, A.M.; Niermeijer, M.F.; et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N. Engl. J. Med. 2001, 345, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Rebbick, T.R.; Friebel, T.M.; Lynch, H.T.; Neuhausen, S.L.; Veer, L.v.; Garber, J.E.; Evans, G.R.; Narod, S.A.; Isaacs, C.; Matloff, E.; et al. Bilateral Prophylactic Mastectomy Reduces Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers: The PROSE Study Group. J. Clin. Oncol. 2004, 22, 1055–1062. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Skytte, A.B.; Gerdes, A.M.; Andersen, M.K.; Sunde, L.; Brøndum-Nielsen, K.; Waldstrøm, M.; Kølvraa, S.; Crüger, D. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin. Genet. 2010, 77, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Ingham, S.L.; Sperrin, M.; Baildam, A.; Ross, G.L.; Clayton, R.; Lalloo, F.; Buchan, I.; Howell, A.; Evans, D.G.R. Risk-reducing surgery increases survival in BRCA1/2 mutation carriers unaffected at time of family referral. Breast Cancer Res. Treat. 2013, 142, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.; Verhoef, S.; Wesseling, J.; Rookus, M.A.; Oldenburg, H.S.A.; Peeters, M.V.; Rutgers, E.J.T. Prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers: Very low risk for subsequent breast cancer. Ann. Surg. 2010, 251, 488–492. [Google Scholar] [CrossRef]
- Ludwig, K.K.; Neuner, J.; Butler, A.; Geurts, J.L.; Kong, A.L. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, A systematic review. Am. J. Surg. 2016, 212, 660–669. [Google Scholar] [CrossRef]
- Li, X.; You, R.; Wang, X.; Liu, C.; Xu, Z.; Zhou, J.; Yu, B.; Xu, T.; Cai, H.; Zou, Q.; et al. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-analysis and Systematic Review. Clin. Cancer Res. 2016, 22, 3971–3981. [Google Scholar] [CrossRef]
- Yao, K.; Liederback, E.; Tang, R.; Lei, L.; Czechura, T.; Sisco, M.; Howard, M.; Hulick, P.J.; Weissman, S.; Winchester, D.J.; et al. Nipple-sparing mastectomy in BRCA1 and BRCA2 mutation carriers: An interim analysis and review of the literature. Ann. Surg. Oncol. 2015, 370, 370–376. [Google Scholar] [CrossRef]
- Beattie, M.S.; Crawford, B.; Lin, F.; Ziegler, E.V.J. Uptake, time course, and predictors of risk-reducing surgeries in BRCA carriers. Genet. Test. Mol. Biomark. 2009, 13, 51–56. [Google Scholar] [CrossRef]
- Flippo-Morton, T.; Walsh, K.; Chambers, K.; Amacker-North, L.; White, B.; Sarantou, T.; Boselli, D.M.; White, R.L., Jr. Surgical decision making in the BRCA-positive population: Institutional experience and comparison with recent literature. Breast 2016, 22, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lerman, C.; Narod, S.; Schulman, K.; Chanita Hughes, M.S.; Andres Gomez-Caminero, M.P.H.; George Bonney, P.; Karen Gold, P.; Bruce Trock, P.; David Main, M.S. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision-making and outcomes. JAMA 1996, 275, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.A.; Liede, A.; Hoodfar, E.; Scott, A.; Foulkes, W.D.; Narod, S.A. An evaluation of needs of female BRCA1 and BRCA2 carriers undergoing genetic counseling. J. Med. Genet. 2000, 37, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.A.; Dennis, C.L.; Poll, A.; Armel, S.; Demsky, R.; Carlsson, L.; Nanda, S.; Kiss, A.; Narod, S.A. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: A multisite, randomized, controlled trial. Gen. Med. 2017, 19, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Valdimarsdottir, H.B.; DeMarco, T.A.; Peshkin, B.N.; Lawrence, W.; Rispoli, J.; Brown, K.; Isaacs, C.; O’Neill, S.; Shelby, R.; et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: Impact on measures of decision making and satisfaction. Health Psychol. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Siciliani, L.; Hurst, J. Explaining Waiting Times Variations for Elective Surgery Across OECD Countries; OECD Health Working Papers, No. 7; OECD Publishing: Paris, France, 2003. [Google Scholar] [CrossRef]
| Variable | Surveillance n = 201 | Prophylactic Mastectomy/ Immediate Reconstruction n = 132 |
|---|---|---|
| Age at BRCA diagnosis | ||
| Mean (SD)—yr | 43 (14.1) | 40 (9.6) |
| Median—yr | 43 | 38 |
| 10th, 90th Percentile | 28, 66 | 29, 54 |
| Length of follow-up after BRCA diagnosis | ||
| Mean (SD)—yr | 6.8 (4.2) | 3.4 (2.7) |
| Median—yr | 5.9 | 2.7 |
| 10th, 90th Percentile | 1.6, 12.7 | 0.6, 7.2 |
| BMI | ||
| Mean (SD)—kg/m2 | 26 (6.7) | 26 (5.6) |
| Median—kg/m2 | 24 | 24 |
| 10th, 90th Percentile | 20, 35 | 20, 33 |
| Ovarian hormone exposure | ||
| Mean (SD)—yr | 33 (13.8) | 29 (9.3) |
| Median—yr | 33 | 27.5 |
| 10th, 90th Percentile | 16, 52 | 18, 41 |
| Mutation—no. (%) | ||
| BRCA1 | 87 (43.3) | 78 (59.1) |
| BRCA2 | 112 (55.7) | 53 (40.2) |
| BRCA1&2 | 1 (0.5) | 1 (0.8) |
| Ethnicity—no. (%) | ||
| Caucasian | 142 (70.6) | 107 (81.1) |
| Asian | 6 (3) | 2 (1.5) |
| South Asian | 14 (7) | 5 (3.8) |
| Hispanic | 0 (0) | 0 (0) |
| White Hispanic | 0 (0) | 0 (0) |
| Aboriginal | 4 (2) | 5 (3.8) |
| Multiple | 2 (1) | 1 (0.8) |
| Unknown | 33 (16.4) | 12 (9) |
| All Tumors (n = 28) | BRCA1 (n = 13) | BRCA2 (n = 15) | |||||
|---|---|---|---|---|---|---|---|
| Median (10th, 90th Percentile) | Median (10th, 90th Percentile) | Median (10th, 90th Percentile) | |||||
| Age at Diagnosis | 47 (32, 61) | 47 (30, 51) | 46 (33, 62) | ||||
| Time From BRCA Diagnosis to Cancer Diagnosis (Months) | 59.3 (14.9, 116.4) | 63.2 (6.7, 144.9) | 44.4 (23.1, 102.4) | ||||
| n | (%) | n | (%) | n | (%) | ||
| DCIS | 4 | 14 | 1 | 7 | 3 | 20 | |
| IDC | 24 | 86 | 12 | 93 | 12 | 80 | |
| ER status | Negative | 9 | 32 | 8 | 62 | 1 | 7 |
| Positive | 18 | 64 | 4 | 31 | 14 | 93 | |
| Unknown | 1 | 4 | 1 | 7 | 0 | 0 | |
| PR status | Negative | 13 | 46 | 9 | 69 | 4 | 27 |
| Positive | 11 | 39 | 3 | 23 | 8 | 53 | |
| Unknown | 4 | 15 | 1 | 8 | 3 | 20 | |
| Her2 status | Negative | 18 | 64 | 8 | 62 | 10 | 67 |
| Positive | 5 | 18 | 4 | 31 | 1 | 7 | |
| Unknown | 5 | 18 | 1 | 8 | 4 | 27 | |
| Triple negative | Yes | 7 | 25 | 6 | 46 | 1 | 7 |
| Tumor size (mm) | <20 | 24 | 89 | 11 | 85 | 13 | 87 |
| >21 | 3 | 10 | 2 | 15 | 1 | 6.5 | |
| Unknown | 1 | 0 | 0 | 1 | 6.5 | ||
| Node positive | Yes | 5 | 18 | 2 | 15 | 3 | 20 |
| Unknown | 2 | 7 | 0 | 0 | 2 | 13 | |
| Compliant | Yes | 23 | 79 | 10 | 77 | 13 | 87 |
| All Tumors (n = 8) | BRCA1 (n = 4) | BRCA2 (n = 4) | ||
|---|---|---|---|---|
| Median (10th, 90th percentile) | Median (10th, 90th percentile) | Median (10th, 90th percentile) | ||
| Age at Diagnosis | 41 (34, 63) | 39 (34, 48) | 51 (34, 63) | |
| Time from BRCA Diagnosis to Cancer Diagnosis (months) | 32.0 (2.7, 87.5) | 47.2 (16.9, 87.5) | 26.9 (2.7, 49.6) | |
| n | n | n | ||
| DCIS | 2 | 2 | 0 | |
| IDC | 6 | 2 | 4 | |
| ER status | Negative | 4 | 2 | 2 |
| Positive | 4 | 2 | 2 | |
| PR status | Negative | 6 | 4 | 2 |
| Positive | 2 | 0 | 2 | |
| Her2 status | Negative | 4 | 2 | 2 |
| Positive | 3 | 1 | 2 | |
| Unknown | 1 | 1 | 0 | |
| Triple negative | Yes | 2 | 2 | 0 |
| Tumor size (mm) | <20 | 6 | 3 | 3 |
| >21 | 2 | 1 | 1 | |
| Node positive | Yes | 2 | 0 | 2 |
| Unknown | 3 | 2 | 1 | |
| Compliant | Yes | 7 | 4 | 3 |
| T1 (BRCA Mutation Diagnosis to Decision for Surgery) | Frequency (n) | Percent (%) |
|---|---|---|
| HCP visit and referral for PM/IBR </= 3 months from BRCA mutation disclosure | 41 | 39.1 |
| Initially declined surgical consult then changed mind and proceeded for unknown reason | 24 | 22.9 |
| Initially declined surgical consult then changed mind due to family influence (i.e., new cancer) | 9 | 8.6 |
| Delayed in order to proceed with BSO first | 7 | 6.7 |
| Initially declined surgical consult then changed mind after completing child-bearing or breast feeding | 5 | 4.8 |
| Delayed due to patient distance from tertiary care center | 5 | 4.8 |
| Initially declined surgical consult then changed mind after imaging abnormality | 4 | 3.8 |
| Initially declined surgical consult then changed mind due to new information (discussion with new physician, media influence) | 2 | 1.9 |
| Interested in PM/IBR but deemed unable to have reconstruction | 3 | 2.9 |
| Delay for unknown reason | 3 | 2.9 |
| Delay for patient health issues | 2 | 1.9 |
| T2 (HCP Referral to Consultation with Surgeon) | Frequency (n) | Percent (%) |
| HCP referral to consultation with surgeon </= 6 months | 64 | 70.3 |
| Change in requested surgeon | 8 | 8.8 |
| Long wait time for consultation | 7 | 7.7 |
| Unknown | 7 | 7.7 |
| Patient cancelled or postponed | 4 | 4.4 |
| Delay for patient health issues | 1 | 1.1 |
| T3 (Surgical Consultation to Surgery) | Frequency (n) | Percent (%) |
| Surgery </= 1 year from surgical consult | 58 | 61.7 |
| Patient undecided after consult or patient timing preference | 12 | 12.8 |
| Patient requested second opinion | 8 | 8.5 |
| Long surgical wait list | 7 | 7.5 |
| Patient health (BMI, smoking, nursing) | 5 | 5.3 |
| Unknown | 2 | 2.1 |
| Patient moved before surgery completed | 1 | 1.1 |
| Patient elected to have BSO completed first | 1 | 1.1 |
| Patient | Interval from BRCA Diagnosis to Cancer Diagnosis (Months) | Age at Cancer Diagnosis | Tumor Size (mm) | No. Positive Nodes/ Nodes Assessed | Histologic Type | Grade | ER/PR Status | Her2 Status | Findings CBE | Findings MRI | Findings MMG | BRCA | Compliance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | 2 | 63 | <1 | N/A | IDC | 2 | -/- | + | N | N/A | N/A | 2 | Y |
| 2 * | 7 | 35 | 20 | 0/2 | IDC | 2 | +/+ | + | N/A | N/A | N | 2 | Y |
| 3 | 16 | 39 | 5 | 0/4 | DCIS | 3 | +/- | + | N | Atypical | N | 1 | Y |
| 4 | 18 | 43 | 70 | N/A | DCIS | 2 | +/- | N/A | N | Suspicious | Biopsy recommended | 1 | Y |
| 5 | 45 | 65 | 29 | 5/17 | IDC | 2 | -/- | - | N | N/A | N/A | 2 | Y |
| 6 | 49 | 44 | 10 | 7/12 | IDC | 2 | +/+ | - | Mass detected | N/A | Indeterminate | 2 | N |
| 7 * | 76 | 55 | 2 | N/A | IDC | 3 | -/- | - | N | N | N | 1 | Y |
| 8 | 85 | 41 | 9 | 0/5 | IDC | 3 | -/- | - | Mass detected | Malignant mass | Malignant mass | 1 | Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macadam, S.A.; Slater, K.; Cheifetz, R.E.; Jansen, L.; Chia, S.; Brasher, P.M.A.; Bovill, E.S. Prophylactic Surgery in the BRCA+ Patient: Do Women Develop Breast Cancer While Waiting? Curr. Oncol. 2021, 28, 702-715. https://doi.org/10.3390/curroncol28010069
Macadam SA, Slater K, Cheifetz RE, Jansen L, Chia S, Brasher PMA, Bovill ES. Prophylactic Surgery in the BRCA+ Patient: Do Women Develop Breast Cancer While Waiting? Current Oncology. 2021; 28(1):702-715. https://doi.org/10.3390/curroncol28010069
Chicago/Turabian StyleMacadam, Sheina A., Karen Slater, Rona E. Cheifetz, Leigh Jansen, Stephen Chia, Penelope M. A. Brasher, and Esta S. Bovill. 2021. "Prophylactic Surgery in the BRCA+ Patient: Do Women Develop Breast Cancer While Waiting?" Current Oncology 28, no. 1: 702-715. https://doi.org/10.3390/curroncol28010069
APA StyleMacadam, S. A., Slater, K., Cheifetz, R. E., Jansen, L., Chia, S., Brasher, P. M. A., & Bovill, E. S. (2021). Prophylactic Surgery in the BRCA+ Patient: Do Women Develop Breast Cancer While Waiting? Current Oncology, 28(1), 702-715. https://doi.org/10.3390/curroncol28010069






