Integrating Patient-Centred Research in the Canadian Cancer Trials Group
Abstract
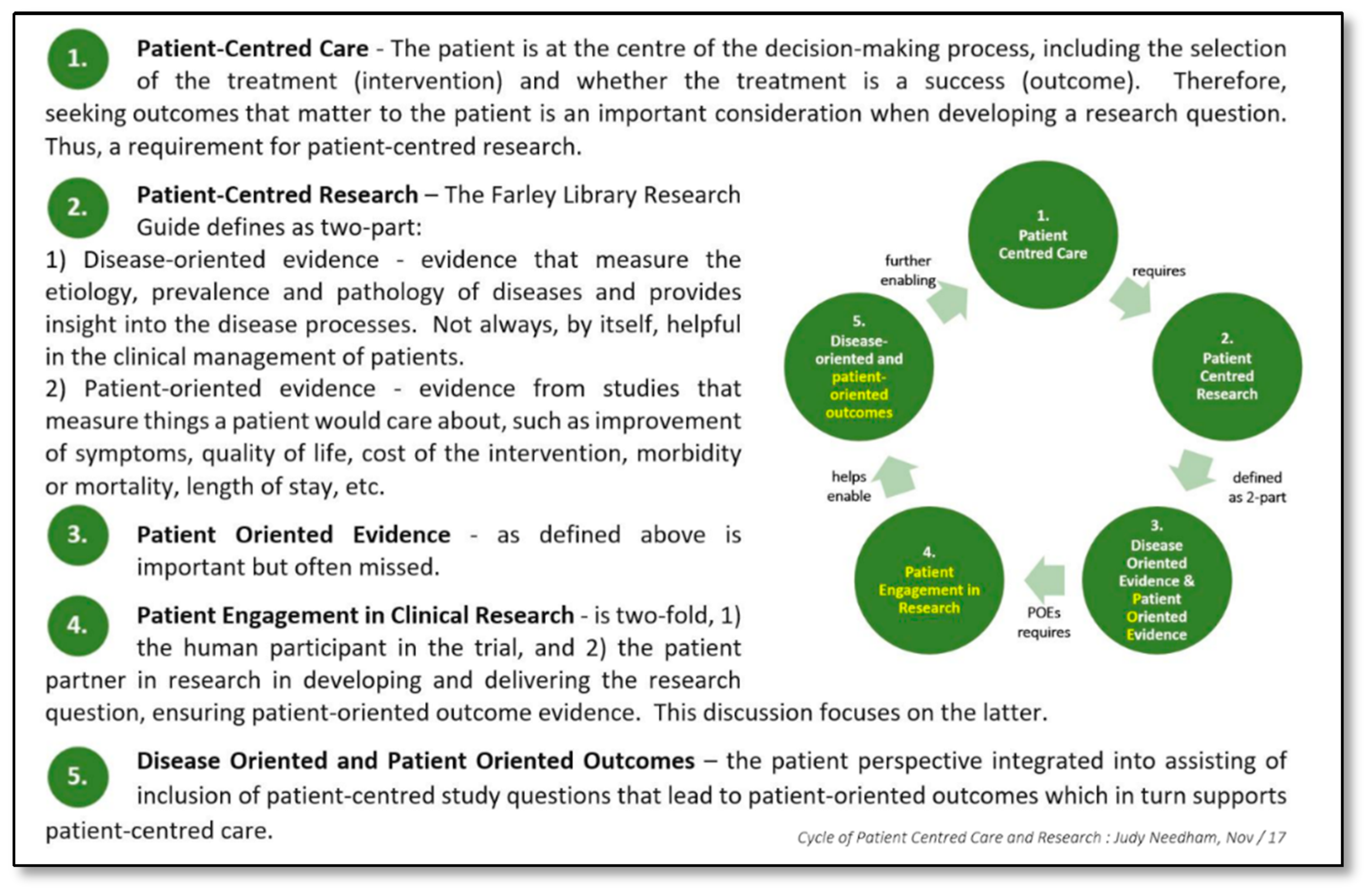
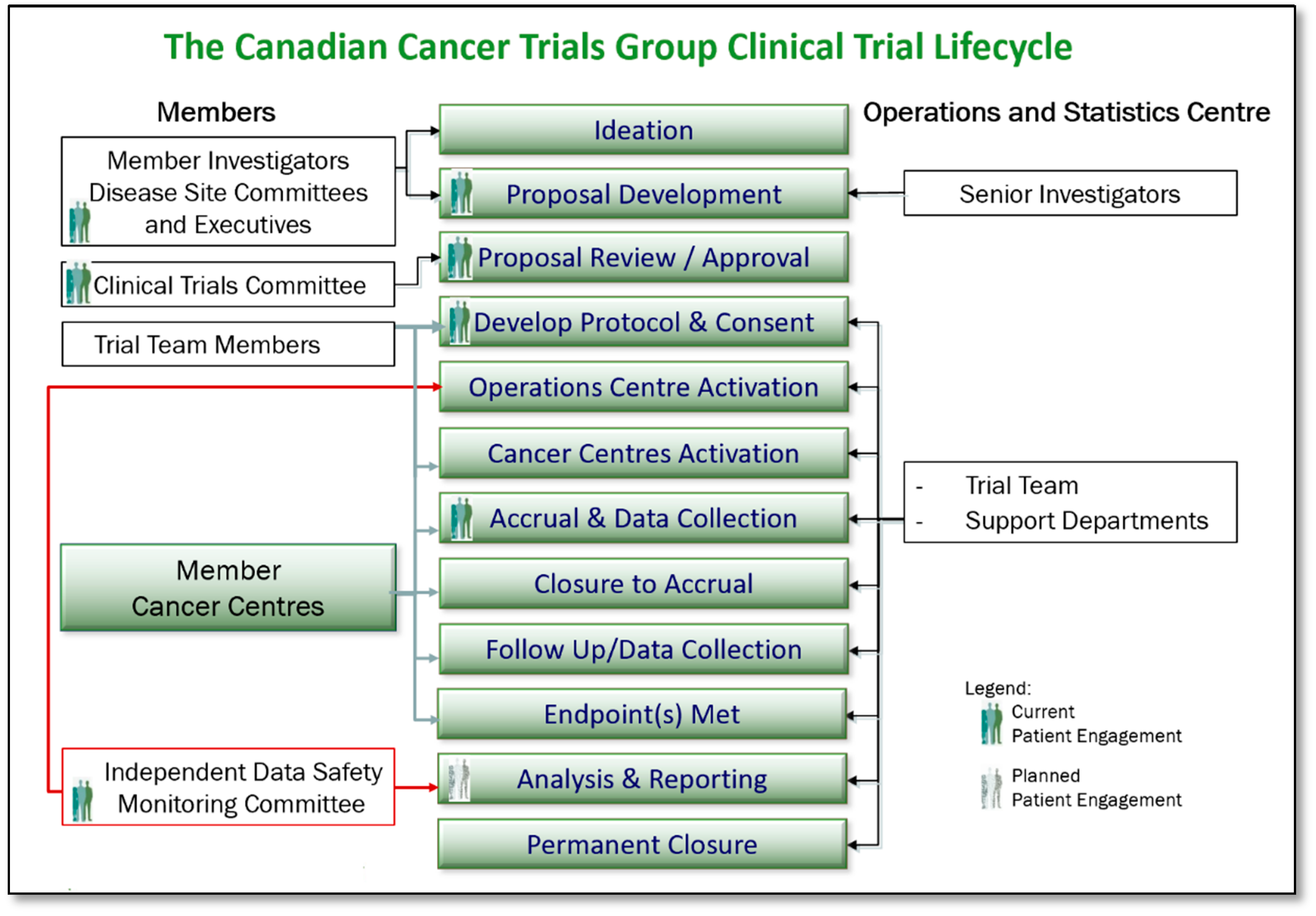
1. Introduction
2. Materials and Methods
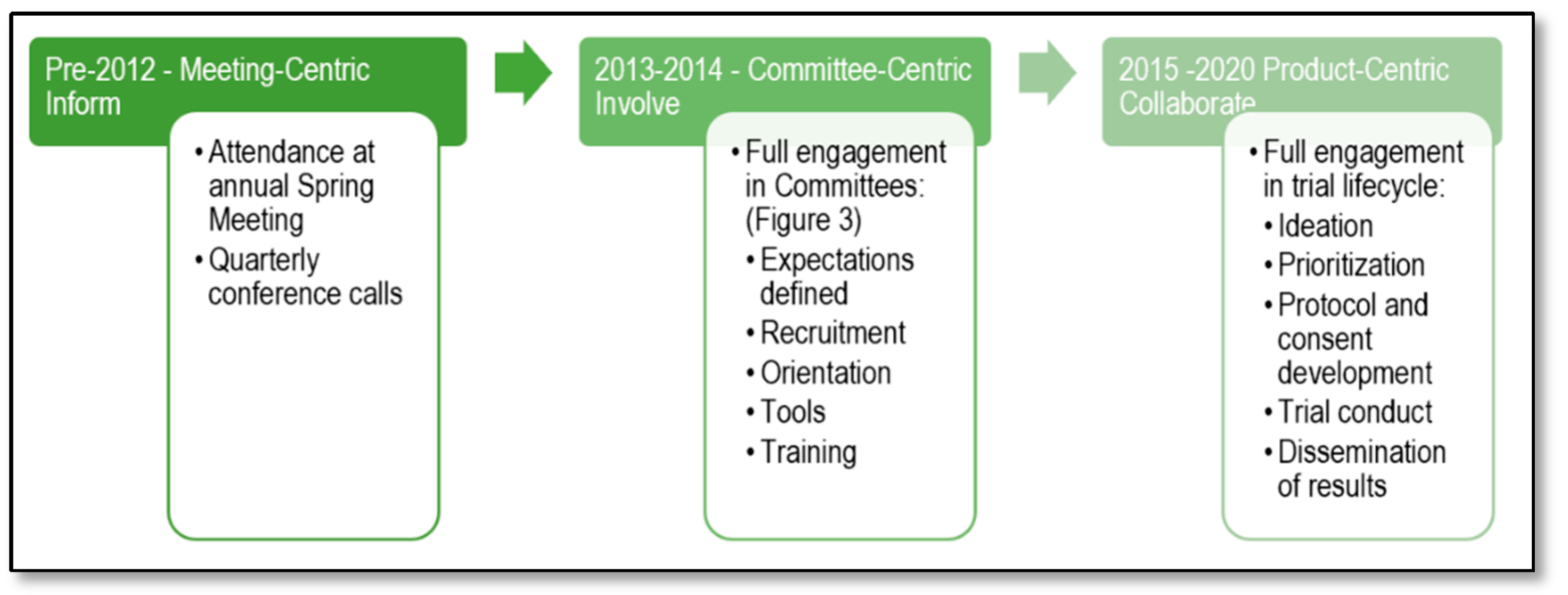
2.1. Phase I: Pre 2012 Meeting-Centric—Inform Level of Engagement
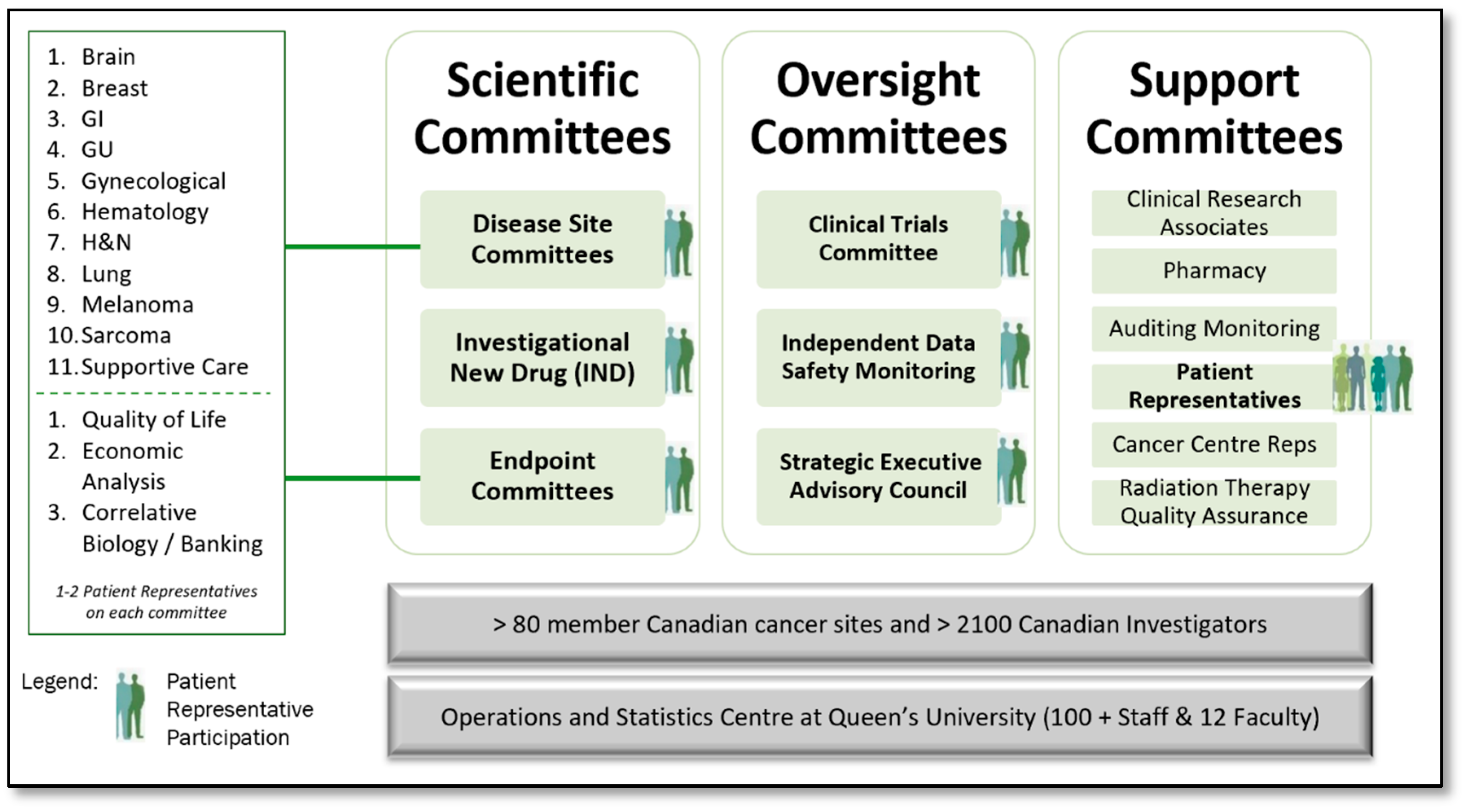
2.2. Phase II: Meeting-Centric to Committee-Centric—Involve Level of Engagement
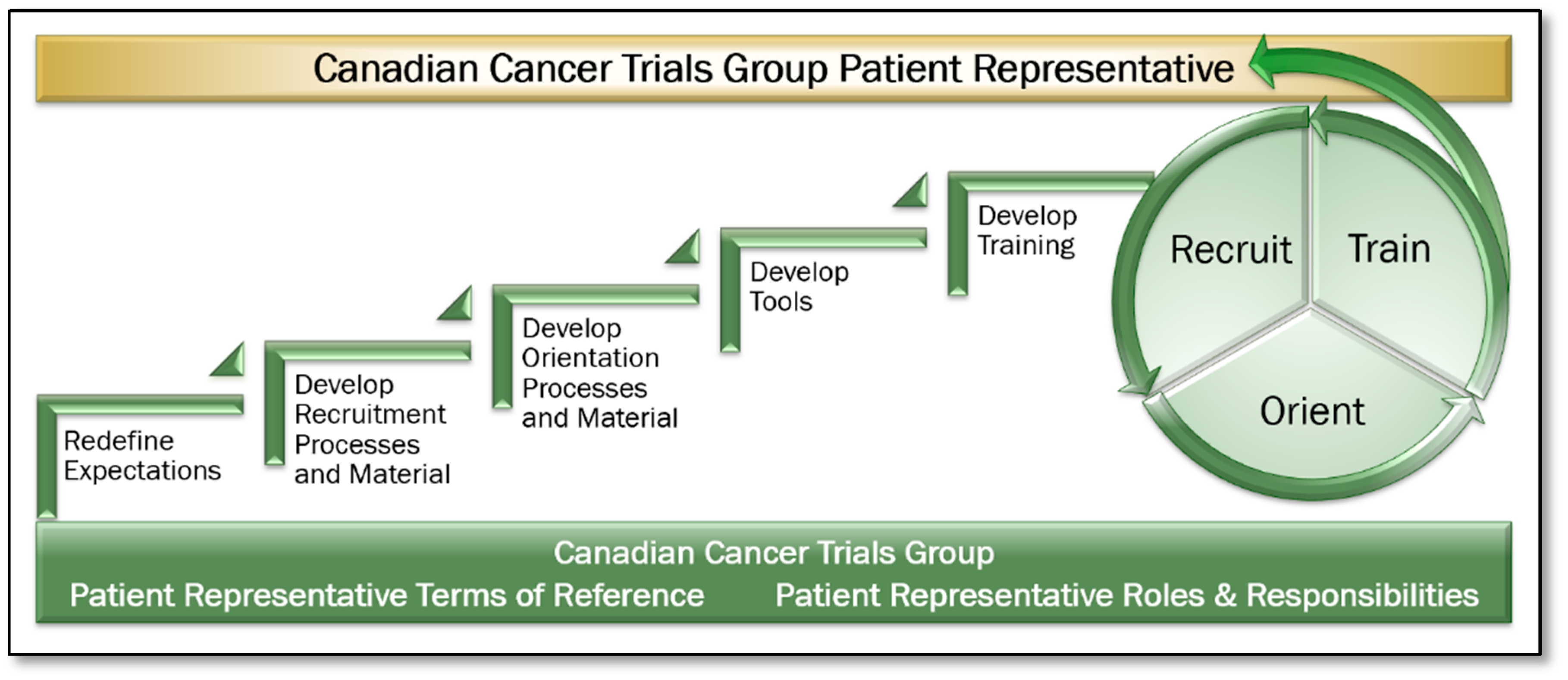
- Expectations: Patient Representative Terms of Reference and Roles and Responsibilities documents were created and approved. Expectations of Patient Representative participation within their committees were defined, including accessing electronic trial, committee, and meeting information, how to prepare for committee meetings and the time commitments required.
- Recruitment: A formal recruitment process including definition of desired role attributes, a position posting, an interview guide, and associated processes were developed. The volunteer opportunities were distributed to Clinical Trial Units, Investigators, the Canadian Cancer Society, and other organizations throughout Canada. Applicants were interviewed and selected through a panel interview process. The Patient Representative term is a once renewable three-year term.
- Orientation: An orientation process was developed to provide introduction to the organization, leadership, key contacts, and an introduction to a progressive training program.
- Tool Development: Tools including a Patient Representative Manual, Portfolio Trial Summary Matrix Report and Trial Tracking Forms were developed. The Portfolio Trial Summary Matrix provides a snapshot of all trials in a disease site at a given point in time, including where each is at in its lifecycle, including current accrual status. The Trial Tracking Form enables the Patient Representative to identify all key aspects of the trial, and secondly, to record and track patient feasibility, accrual related issues, and other notes on a cumulative basis, enabling discussion of accrual related issues and outcomes within their respective committees. This also enabled a continuum from meeting to meeting.
- Education and Training: Training materials including the Patient Representative Manual and in-person or virtual training module were developed. Initial Patient Representative training was conducted in person at the CCTG Annual Spring Meeting of Participants. Ensuring all interdisciplinary committee members are supportive of and engaged in the process to enhance Patient Representatives’ roles within their committees was paramount. All onboarding methods and procedures, training materials, and tools were shared with committees in person also at the CCTG Annual Spring Meeting of Participants. The same materials were shared with the investigative community and staff in the CCTG Central Operations and Statistics Centre, including Study Coordinators (project and data managers on CCTG clinical trials) who are well positioned to support the Patient Representatives in their roles. Refresher training and awareness of the CCTG patient engagement model is provided based on member attrition and turnover.
2.3. Phase III: Committee-Centric to Product-Centric—Collaborate Level of Engagement
- Ideation and Proposal Development: Patient Representatives’ roles on CCTG Disease Site Committee Executives facilitate the ability to contribute to trial design, to inclusion of patient-centred end points, and to decisions regarding which proposals are sufficiently meritorious to be presented to the Clinical Trials Committee (CTC) for approval. A Patient Representative New Proposal Review form has been developed as a tool to facilitate the review and input.
- Trial Proposal Review and Approval: As full members of the CTC, Patient Representatives review and score proposals that come forward for consideration. Those proposals that are approved by the CTC move on to become fully developed and activated trials conducted by the CCTG. Thus, Patient Representatives on the CTC are directly impactful on those trials that are made available to patients. Tools that support the Patient Representative in their role on the CTC are the same as afforded to all members of the committee, including CTC review, and scoring guidelines and a CTC New Proposal Review Form.
- Protocol and Consent Development: As protocols and consents for CCTG trials are developed and finalized, Disease Site Committee Patient Representatives review, provide input, and make suggestions to help ensure the trial design is acceptable to patients. A Patient Representative Protocol Review Form and Consent Review Form have been developed to assist Patient Representatives.
- Trial Activation and Launch: Patient Representatives have contributed to the development of a plain language template and now contribute to the development of plain language patient-facing materials (Research Ethics Board approved) for all CCTG trials. These are distributed to regional cancer centres to assist in raising awareness of clinical trials at Canadian centres as applicable, with the goals of assisting patient understanding and thereby influencing accrual.
- Trial Accrual and Progression Monitoring: As members of the Disease Site Committee Executives, CCTG Disease Site Committee Patient Representatives are able to monitor trial progress within their portfolios and contribute to discussions around those trials that are having challenges. Tools including the Portfolio Trial Matrix Report, the Trial Tracking Form, Monthly Accrual Reports, and the online Accrual Tracking Utility assist Patient Representatives in this role. Additionally, as equal members of the Data Safety Monitoring Committee, Patient Representatives are aware of and contribute on each trial’s status, including trial progress and safety.
- Trial Closure, Analysis, and Reporting: A planned next step in the evolution of the CCTG patient engagement model includes development of processes for informing knowledge translation associated with this research, and methods of supporting widespread dissemination of CCTG study findings across appropriate patient communities in Canada.
3. Results
3.1. CCTG Experiencing Trial Activity and Accrual at its Highest in Six Years: Qualitative Evidence of Patient Representatives Having Contributed to this Success
- 100% of CCTG trials are reviewed at key touchpoints as identified earlier, including new proposal, funding review, protocol, and consent development, launch and execution for inclusion of patient-centred end points, potential patient barriers to accrual and patient safety.
- The inclusion of patient-centred end points to augment the scientific question, and proactive review and identification of patient barriers in CCTG trials has contributed to increased trial enrollment. In addition to informing practice, patient-centred end points inform patients by providing outcomes that are important to them and their quality of life, thereby motivating enrollment and retention. This is related to a broader result of also making a difference to Canadian patients.
- An appeal influencing industry to reverse a decision to terminate drug supply for CCTG trial PA6 enabled trial continuation versus trial closure (Needham, J. Letter to Meryem Maoui, June 2015. Maoui, M. Letter to Judy Needham, July 2015).
- An appeal influencing United Kingdom participation in CO21 influencing broader global accrual (Needham, J. Letter to Vicky Coyle, Centre for Cancer Research and Cell Biology, Queen’s University Belfast, August 2013).
- Influencing new trials in the pipeline–MYX.1 A Phase II Study of High Dose Weekly Carfilzomib plus Cyclophosphamide and Dexamethasone in the Treatment of Relapsed Multiple Myeloma; coordinated through Mr. Aldo Del Col, CCTG Patient Representative on the Hematology DSC and then Chief Scientific Advisory of Myeloma Canada (1917).
3.2. CCTG has Realized a 50% Increase in Research Funding Realized in the Past Four Years: Patient Representative Committee Contributions to These Results
- The Patient Representative Committee scored high in the core programmatic grant application from the Canadian Cancer Society Research (Presentation; 2016 CCSRI Grant Review), thereby influencing the overall CCTG score. Committee structure and plans were well received with no changes requested.
- Evidence of patient engagement has become a criteria for many granting organizations. CCTG grant applications (CIHR, PCORI, etc.) now clearly articulate all patient representative touchpoints and engagement including specific associated activities throughout the lifecycle of the specific project and the trial team resulting in higher scores versus criticism and loss of points for lack of meaningful patient engagement.
3.3. Evidence of a Productive Well-Working Model and Elimination of Duplication of Effort Regarding Best Practices in Patient Engagement
- Internally
- -
- Patient Representatives are highly sought out for secondary responsibilities in addition to their primary duties outlined in this publication, not limited to, but including, activities such as cross-committee projects and working groups, grant applications, trial teams, and molecular tumour board membership.
- Externally
- -
- CCTG’s patient engagement model has been sought out by and shared with the Canadian Cancer Clinical Trials Network, Myeloma Canada, British Columbia Cancer, the Ontario Institute of Cancer Research, the Canadian Initiative for Patient Advocacy Groups in Clinical Trials, and National Cancer Institute (NCI) National Clinical Trials Network (NCTN) partner groups.
- -
- CCTG Patient Representatives have been requested to and support international committees including the NCI NCTN Accrual Core Team, Correlative Science Tumour Biology Committee, Patient Advocacy Chairs’ Committee, Social Media Workshop, Bethesda, MD, and NCI NCTN Plain Language Committee.
- -
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parry, M.; Bjørnnes, A.; Toupin-April, K.; Najam, A.; Wells, D.; Sivakumar, A.; Richards, D.P.; Ceroni, T.; Park, M.; Ellis, A.K.; et al. Patient Engagement Partnerships in Clinical Trials: Development of Patient Partner and Investigator Decision Aids. Patient 2020, 13, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Heckert, A.; Forsythe, L.; Carman, K.; Frank, L.; Hemphill, R.; Elstad, E.; Esmail, L.; Lesch, J.K. Researchers, patients, and other stakeholders′ perspectives on challenges to and strategies for engagement. Res. Involv. Engagem. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Skovlund, P.; Nielsen, B.; Thaysen, H.; Schmidt, H.; Finset, A.; Hansen, K.; Lomborg, K. The impact of patient involvement in research: A case study of the planning, conduct and dissemination of a clinical, controlled trial. Res. Involv. Engagem. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- nihr.ac.uk. I Want to Help with Research. Available online: https://www.nihr.ac.uk/patients-carers-and-the-public/i-want-to-help-with-research/ (accessed on 19 September 2020).
- Deverka, P.A.; Bangs, R.; Kreizenbeck, K.; Delaney, D.M.; Hershman, D.L.; Blanke, C.D.; Ramsey, S.D. A New Framework for Patient Engagement in Cancer Clinical Trials Cooperative Group Studies. JNCI J. Natl. Cancer Inst. 2018, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, L.; Heckert, A.; Margolis, M.; Schrandt, S.; Frank, L. Methods and impact of engagement in research, from theory to practice and back again: Early findings from the Patient-Centered Outcomes Research Institute. Qual. Life Res. 2018, 27, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Forsythe, L.; Ellis, L.; Schrandt, S.; Sheridan, S.; Gerson, J.; Konopka, K.; Daugherty, S. Conceptual and practical foundations of patient engagement in research at the patient-centered outcomes research institute. Qual. Life Res. 2015, 24, 1033–1041. [Google Scholar] [CrossRef]
- Clinical Trials Transformation Initiative. CTTI Recommendations: Patient Groups and Clinical Trials. 2015. Available online: https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/7-revised_pgct-recommendations-2019_final.pdf (accessed on 19 September 2020).
- Patrick-Lake, B. Patient engagement in clinical trials: The Clinical Trials Transformation Initiative’s leadership from theory to practical implementation. Clin. Trials. 2018, 15, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Needham, J. (Canadian Cancer Trials Group, Kingston, ON, Canada). Advancing the Role of the Lay Representative in NCIC CTG. Personal Communication, 2013. [Google Scholar]
- Iap2 International Association for Public Participation. Available online: https://cdn.ymaws.com/www.iap2.org/resource/resmgr/pillars/Spectrum_8.5x11_Print.pdf (accessed on 12 December 2020).
- Canadian Institute of Health Research Strategy for Patient-Oriented Research. Available online: https://cihr-irsc.gc.ca/e/48413.html (accessed on 12 December 2020).
- cihr-irsc.gc.ca. Patient Engagement. 2019. Available online: http://www.cihr-irsc.gc.ca/e/45851.html (accessed on 20 May 2020).
- AMA Journal of Ethics. Available online: https://journalofethics.ama-assn.org/article/they-are-people-first-then-patients/2017-05 (accessed on 12 December 2020).
- Ennis, L.; Wykes, T. Impact of patient involvement in mental health research: Longitudinal study. Br. J. Psychiatry 2013, 203, 381–386. [Google Scholar] [CrossRef]
- Needham, J.; Nomikos, D.; Stanton, H.; on behalf of the CCTG Lay Representatives Committee. Integrating Patient/Public Involvement in the Canadian Cancer Trials Group (CCTG). In Proceedings of the International Society for Clinical Trials Conference, Montreal, QU, Canada, 15–18 May 2016. [Google Scholar]
- Needham, J.; Nomikos, D.; Stanton, H.; on behalf of the CCTG Lay Representatives Committee. Integrating Patient/Public Involvement in the Canadian Cancer Trials Group (CCTG). In Proceedings of the Canadian Cancer Research Association (CCRA) Conference, Vancouver, BC, Canada, 5–7 November 2017. [Google Scholar]
- Needham, J. Effective Examples of Collaborations in Clinical Trials. In Proceedings of the Patient Group Pathway Model to Accessing Cancer Clinical Trials Follow Up and World Evidence Conference, Montreal, QU, Canada, 6–7 November 2018. [Google Scholar]
- Snyder, C.; Smith, K.; Holzner, B.; Rivera, Y.M.; Bantug, E.; Brundage, M. Making a picture worth a thousand numbers: Recommendations for graphically displaying patient-reported outcomes data. Qual. Life Res. 2019, 28, 345–356. [Google Scholar] [CrossRef]
- Ghent, H.; Petrie, J.; on behalf of the members of the Lay Representatives Committee, NCIC Clinical Trials Group. Whose Tissue is it Anyway? Available online: https://www.cmaj.ca/content/185/2/135/tab-e-letters#whose-tissue-is-it-anyway (accessed on 10 June 2013).
- Piche, L.; Needham, J.; Hamm, C.; Nixon, N.; Presseau, J. Stakeholder and Patient Engagement in Clinical Trials and Patient-Oriented Research. In Proceedings of the Canadian Cancer Research Conference, Ottawa, ON, Canada, 3–5 November 2019. [Google Scholar]
- Domecq, J.P.; Prutsky, G.; Elraiyah, T.; Wang, Z.; Nabhan, M.; Shippee, N.; Brito, J.P.; Boehmer, K.; Hasan, R.; Firwana, B. Patient engagement in research: A systematic review. BMC Health Serv. Res. 2014, 14, 89. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Needham, J.; Taylor, J.; Nomikos, D. Integrating Patient-Centred Research in the Canadian Cancer Trials Group. Curr. Oncol. 2021, 28, 630-639. https://doi.org/10.3390/curroncol28010062
Needham J, Taylor J, Nomikos D. Integrating Patient-Centred Research in the Canadian Cancer Trials Group. Current Oncology. 2021; 28(1):630-639. https://doi.org/10.3390/curroncol28010062
Chicago/Turabian StyleNeedham, J., J. Taylor, and D. Nomikos. 2021. "Integrating Patient-Centred Research in the Canadian Cancer Trials Group" Current Oncology 28, no. 1: 630-639. https://doi.org/10.3390/curroncol28010062
APA StyleNeedham, J., Taylor, J., & Nomikos, D. (2021). Integrating Patient-Centred Research in the Canadian Cancer Trials Group. Current Oncology, 28(1), 630-639. https://doi.org/10.3390/curroncol28010062




