Canadian Consensus for Biomarker Testing and Treatment of TRK Fusion Cancer in Adults †
Abstract
1. Introduction
1.1. Targeted Therapy for TRK Fusion Cancer
- ALKA-372-001 (adult Phase I basket trial) (NCT02097810)
- STARTRK-1 (adult Phase I basket trial) (NCT02097810)
- STARTRK-2 (adult Phase I basket trial) (NCT02568267)
- STARTRK-NG (adolescent/pediatric {≤20 years of age} Phase I/II basket trial) (NCT02650401)
1.2. Development of Resistance to TRK Inhibitors
1.3. Regulatory and Funding Status of TRK Inhibitors in Canada, as of 2020
1.4. NTRK Gene Fusions Testing
2. Method to Achieve Consensus on TRK Fusion Cancer Algorithms
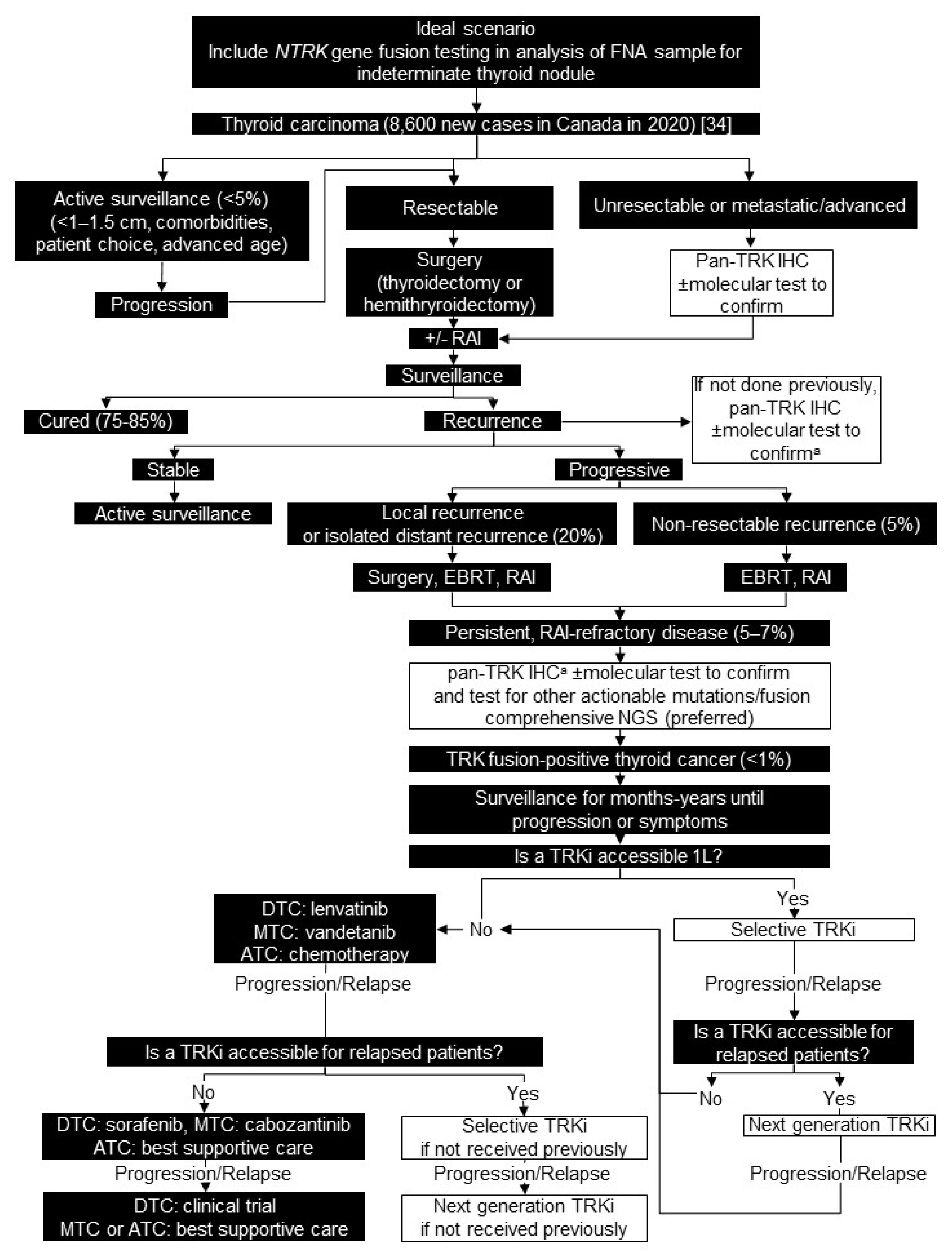
3. Thyroid Carcinoma
3.1. Background
3.2. Testing Consensus
3.3. Treatment Consensus
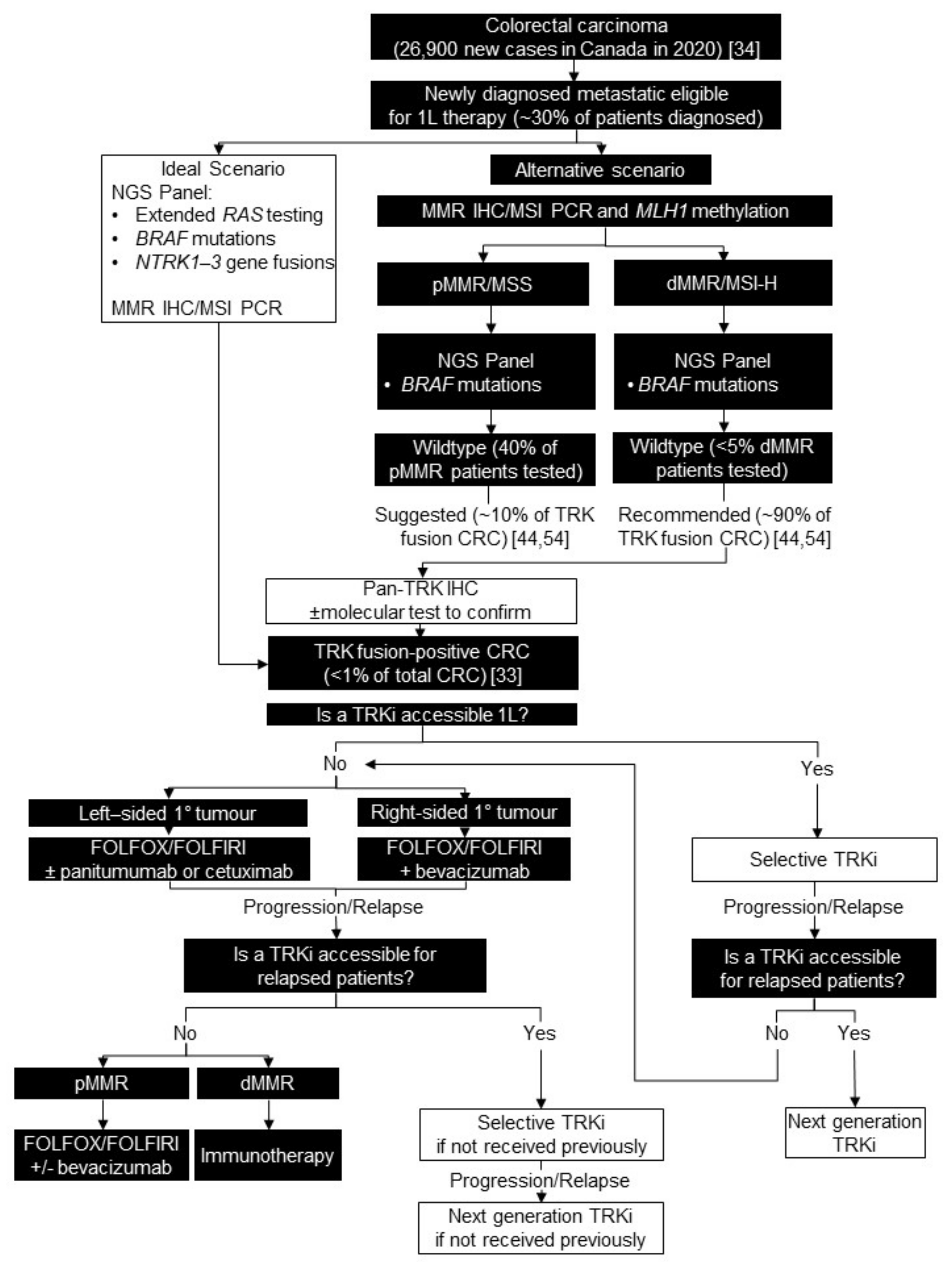
4. Colorectal Carcinoma
4.1. Background
4.2. Testing Consensus
4.3. Treatment Consensus
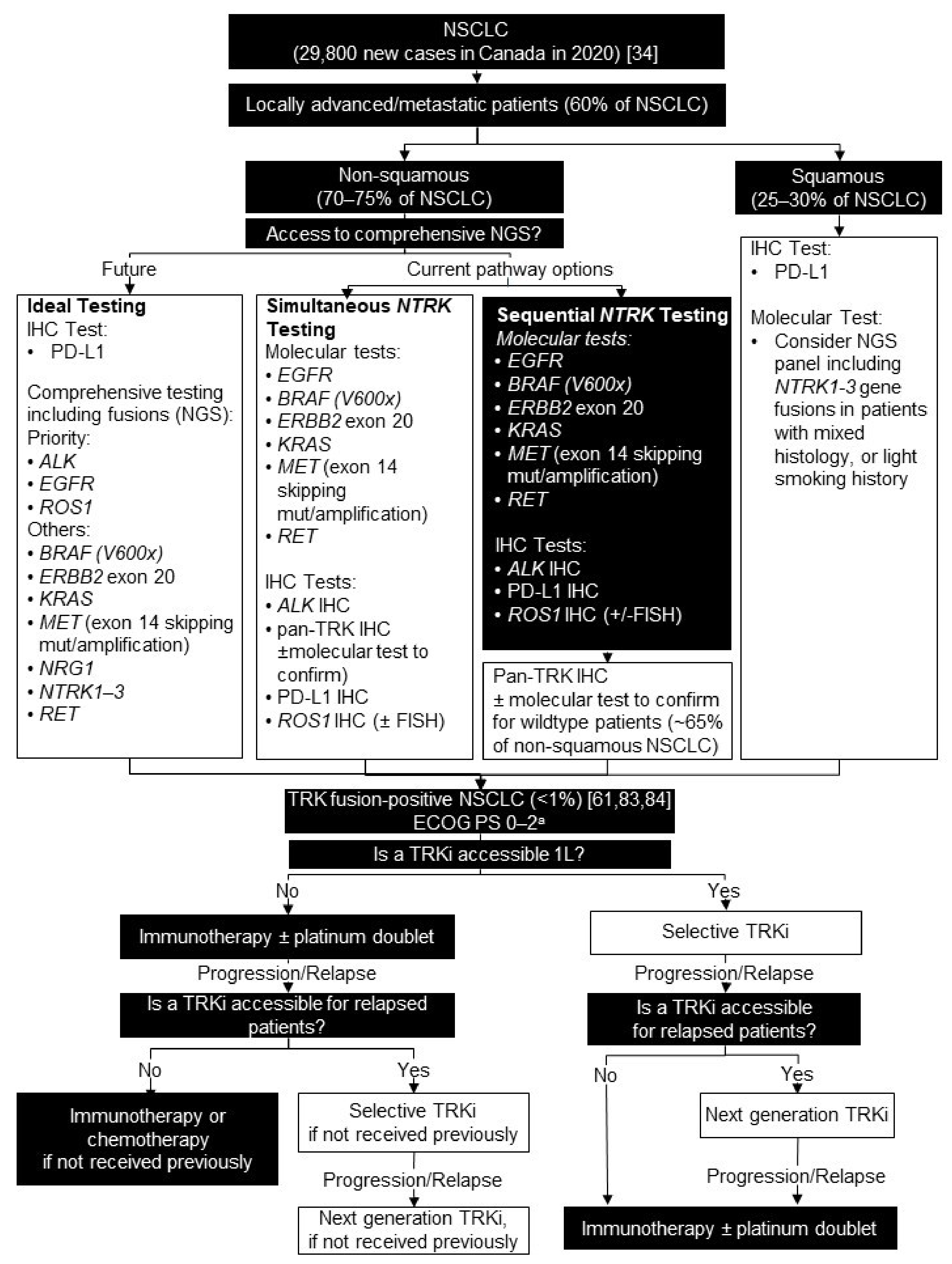
5. Non-Small Cell Lung Cancer
5.1. Background
5.2. Testing Consensus
5.3. Treatment Consensus
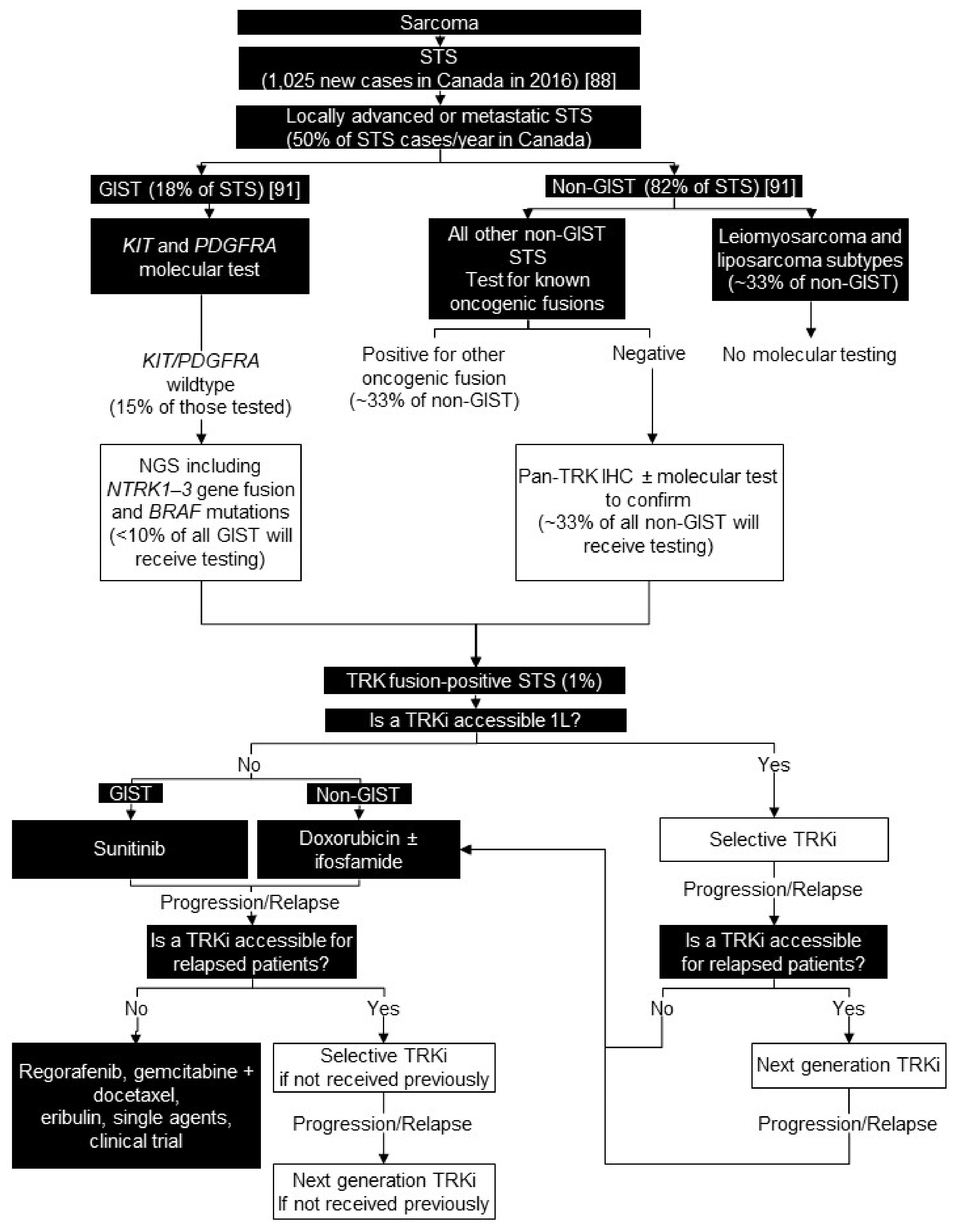
6. Soft Tissue Sarcoma
6.1. Background
6.2. Testing Consensus
6.3. Treatment Consensus
7. Salivary Gland Tumours
7.1. Background
7.2. Testing Consensus
7.3. Treatment Consensus
8. Tumour-Agnostic
8.1. Background
8.2. Testing Consensus
8.3. Treatment Consensus
9. Access to NTRK Gene Fusion Testing in Canada
10. Regulatory Landscape of TRK-Targeted Therapy in Canada and Elsewhere
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaishnavi, A.; Le, A.T.; Doebele, R.C. TRKing Down an Old Oncogene in a New Era of Targeted Therapy. Cancer Discov. 2015, 5, 25–34. [Google Scholar] [CrossRef]
- Greco, A.; Miranda, C.; Pierotti, M.A. Rearrangements of NTRK1 Gene in Papillary Thyroid Carcinoma. Mol. Cell. Endocrinol. 2010, 321, 44–49. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and Their Receptors: A Convergence Point for Many Signalling Pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Pulciani, S.; Santos, E.; Lauver, A.V.; Long, L.K.; Aaronson, S.A.; Barbacid, M. Oncogenes in Solid Human Tumours. Nature 1982, 300, 539–542. [Google Scholar] [CrossRef]
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A Novel ETV6-NTRK3 Gene Fusion in Congenital Fibrosarcoma. Nat. Genet. 1998, 18, 184–187. [Google Scholar] [CrossRef]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 Gene Fusion as a Primary Event in Human Secretory Breast Carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef]
- Skalova, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.; Passador-Santos, F.; et al. Mammary Analogue Secretory Carcinoma of Salivary Glands, Containing the ETV6-NTRK3 Fusion Gene: A Hitherto Undescribed Salivary Gland Tumor Entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef]
- Stephens, R.M.; Loeb, D.M.; Copeland, T.D.; Pawson, T.; Greene, L.A.; Kaplan, D.R. Trk Receptors Use Redundant Signal Transduction Pathways Involving SHC and PLC-Gamma 1 to Mediate NGF Responses. Neuron 1994, 12, 691–705. [Google Scholar] [CrossRef]
- Holgado-Madruga, M.; Moscatello, D.K.; Emlet, D.R.; Dieterich, R.; Wong, A.J. Grb2-Associated Binder-1 Mediates Phosphatidylinositol 3-Kinase Activation and the Promotion of Cell Survival by Nerve Growth Factor. Proc. Natl. Acad. Sci. USA 1997, 94, 12419–12424. [Google Scholar] [CrossRef]
- Qian, X.; Riccio, A.; Zhang, Y.; Ginty, D.D. Identification and Characterization of Novel Substrates of Trk Receptors in Developing Neurons. Neuron 1998, 21, 1017–1029. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. NTRK1 Neurotrophic Receptor Tyrosine Kinase 1 [Homo sapiens (human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/4914 (accessed on 1 April 2020).
- U.S. National Library of Medicine. NTRK2 Neurotrophic Receptor Tyrosine Kinase 2 [Homo sapiens (human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/4915 (accessed on 1 April 2020).
- U.S. National Library of Medicine. NTRK3 Neurotrophic Receptor Tyrosine Kinase 3 [Homo sapiens (human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/4916 (accessed on 1 April 2020).
- Lassen, U. How I Treat NTRK Gene fusion-Positive Cancers. ESMO Open 2019, 4, e000612. [Google Scholar] [CrossRef]
- Rosen, E.Y.; Goldman, D.A.; Hechtman, J.F.; Benayed, R.; Schram, A.M.; Cocco, E.; Shifman, S.; Gong, Y.; Kundra, R.; Solomon, J.P.; et al. TRK Fusions Are Enriched in Cancers with Uncommon Histologies and the Absence of Canonical Driver Mutations. Clin. Cancer Res. 2020, 26, 1624–1632. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in Patients with TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Tao, J.J.; Schram, A.M.; Hyman, D.M. Basket Studies: Redefining Clinical Trials in the Era of Genome-Driven Oncology. Annu. Rev. Med. 2018, 69, 319–331. [Google Scholar] [CrossRef]
- Health Canada. Summary Basis of Decision—Vitrakvi—Health Canada. Available online: https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00455 (accessed on 9 April 2020).
- Hong, D.S.; Bauer, T.M.; Lee, J.J.; Dowlati, A.; Brose, M.S.; Farago, A.F.; Taylor, M.; Shaw, A.T.; Montez, S.; Meric-Bernstam, F.; et al. Larotrectinib in Adult Patients with Solid Tumours: A Multi-Centre, Open-Label, Phase I Dose-Escalation Study. Ann. Oncol. 2019, 30, 325–331. [Google Scholar] [CrossRef]
- Laetsch, T.W.; DuBois, S.G.; Mascarenhas, L.; Turpin, B.; Federman, N.; Albert, C.M.; Nagasubramanian, R.; Davis, J.L.; Rudzinski, E.; Feraco, A.M.; et al. Larotrectinib for Paediatric Solid Tumours Harbouring NTRK Gene Fusions: Phase 1 Results from a Multicentre, Open-Label, Phase 1/2 Study. Lancet Oncol. 2018, 19, 705–714. [Google Scholar] [CrossRef]
- Health Canada. Rozlytrek—Notice of Compliance with Conditions—Qualifying Notice. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/notice-compliance/conditions/rozlytrek-qualifying-notice.html (accessed on 9 April 2020).
- Desai, A.V.; Robinson, G.W.; Basu, E.M.; Foster, J.; Gauvain, K.; Sabnis, A.; Shusterman, S.; Macy, M.E.; Maese, L.; Yoon, J.; et al. Updated Entrectinib Data in Children and Adolescents with Recurrent or Refractory Solid tumors, Including Primary CNS Tumors. J. Clin. Oncol. 2020, 38, 107. [Google Scholar] [CrossRef]
- Drilon, A.; Nagasubramanian, R.; Blake, J.F.; Ku, N.; Tuch, B.B.; Ebata, K.; Smith, S.; Lauriault, V.; Kolakowski, G.R.; Brandhuber, B.J.; et al. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov. 2017, 7, 963–972. [Google Scholar] [CrossRef]
- Drilon, A.; Ou, S.I.; Cho, B.C.; Kim, D.W.; Lee, J.; Lin, J.J.; Zhu, V.W.; Ahn, M.J.; Camidge, D.R.; Nguyen, J.; et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent-Front Mutations. Cancer Discov. 2018, 8, 1227–1236. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Borazanci, E.; Shaw, A.T.; Katayama, R.; Shimizu, Y.; Zhu, V.W.; Sun, T.Y.; Wakelee, H.A.; Madison, R.; Schrock, A.B.; et al. US Phase 1 First-in-Human Study of Taletrectinib (DS-6051b/AB-106), a ROS1/TRK Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Cutz, J.C.; Craddock, K.J.; Torlakovic, E.; Brandao, G.; Carter, R.F.; Bigras, G.; Deschenes, J.; Izevbaye, I.; Xu, Z.; Greer, W.; et al. Canadian Anaplastic Lymphoma Kinase Study: A Model for Multicenter Standardization and Optimization of ALK Testing in Lung Cancer. J. Thorac. Oncol. 2014, 9, 1255–1263. [Google Scholar] [CrossRef]
- Conde, E.; Hernandez, S.; Martinez, R.; Angulo, B.; De Castro, J.; Collazo-Lorduy, A.; Jimenez, B.; Muriel, A.; Mate, J.L.; Moran, T.; et al. Assessment of a New ROS1 Immunohistochemistry Clone (SP384) for the Identification of ROS1 Rearrangements in Patients with Non-Small Cell Lung Carcinoma: The ROSING Study. J. Thorac. Oncol. 2019, 14, 2120–2132. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK Fusion Detection across Multiple Assays and 33,997 Cases: Diagnostic Implications and Pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef]
- Penault-Llorca, F.; Rudzinski, E.R.; Sepulveda, A.R. Testing Algorithm for Identification of Patients with TRK Fusion Cancer. J. Clin. Pathol. 2019, 72, 460–467. [Google Scholar] [CrossRef]
- Beadling, C.; Wald, A.I.; Warrick, A.; Neff, T.L.; Zhong, S.; Nikiforov, Y.E.; Corless, C.L.; Nikiforova, M.N. A Multiplexed Amplicon Approach for Detecting Gene Fusions by Next-Generation Sequencing. J. Mol. Diagn. 2016, 18, 165–175. [Google Scholar] [CrossRef]
- Tsao, M.S.; Torlakovic, E.; Stockley, T.; Lo, B. CANTRK: A Canadian Multi-Centre NTRK Gene Fusion Testing Validation in Solid Tumors Project. ST07. In Proceedings of the Association for Molecular Pathology Annual Meeting, 16–20 November 2020. [Google Scholar]
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected Estimates of Cancer in Canada in 2020. CMAJ. 2020, 192, E199–E205. [Google Scholar] [CrossRef]
- Younis, E. Oncogenesis of Thyroid Cancer. Asian Pac. J. Cancer Prev. 2017, 18, 1191–1199. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Chu, Y.H.; Dias-Santagata, D.; Farahani, A.A.; Boyraz, B.; Faquin, W.C.; Nosé, V.; Sadow, P.M. Clinicopathologic and Molecular Characterization of NTRK-Rearranged Thyroid Carcinoma (NRTC). Mod. Pathol. 2020. [Google Scholar] [CrossRef]
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. A National Cancer Data Base Report on 53,856 Cases of Thyroid Carcinoma Treated in the U.S., 1985–1995. Cancer 1998, 83, 2638–2648. [Google Scholar] [CrossRef]
- Pan-Canadian Oncology Drug Review. Final Recommendation Sorafenib (Nexavar). Available online: https://cadth.ca/sites/default/files/pcodr/pcodr_sorafenib_nexavar_dtc_fn_rec.pdf (accessed on 15 September 2020).
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib Versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Muller, S.P.; Schoffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef]
- Waguespack, S.G.; Drilon, A.; Farago, A.F.; Sohal, D.; Oh, D.; Ma, P.; McDermott, R.; Nanda, S.; Kummar, S.; Lee, J.L.; et al. Treatment of Advanced TRK Fusion Thyroid Cancer with Larotrectinib. OP-01-01. In Proceedings of the 42nd Annual Meeting of the European Thyroid Association, Budapest, Hungary, 7–10 September 2019. [Google Scholar]
- Chou, A.; Fraser, T.; Ahadi, M.; Fuchs, T.; Sioson, L.; Clarkson, A.; Sheen, A.; Singh, N.; Corless, C.L.; Gill, A.J. NTRK Gene Rearrangements Are Highly Enriched in MLH1/PMS2 Deficient, BRAF Wild-Type Colorectal Carcinomas—A Study of 4569 Cases. Mod. Pathol. 2019. [Google Scholar] [CrossRef]
- Lasota, J.; Chłopek, M.; Lamoureux, J.; Christiansen, J.; Kowalik, A.; Wasąg, B.; Felisiak-Gołąbek, A.; Agaimy, A.; Biernat, W.; Canzonieri, V.; et al. Colonic Adenocarcinomas Harboring NTRK Fusion Genes: A Clinicopathologic and Molecular Genetic Study of 16 Cases and Review of the Literature. Am. J. Surg. Pathol. 2020, 44, 162–173. [Google Scholar] [CrossRef]
- Koopman, M.; Kortman, G.A.; Mekenkamp, L.; Ligtenberg, M.J.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.; van Krieken, J.H. Deficient Mismatch Repair System in Patients with Sporadic Advanced Colorectal Cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef]
- Lothe, R.A.; Peltomäki, P.; Meling, G.I.; Aaltonen, L.A.; Nyström-Lahti, M.; Pylkkänen, L.; Heimdal, K.; Andersen, T.I.; Møller, P.; Rognum, T.O.; et al. Genomic Instability in Colorectal Cancer: Relationship to Clinicopathological Variables and Family History. Cancer Res. 1993, 53, 5849–5852. [Google Scholar] [PubMed]
- Kane, M.F.; Loda, M.; Gaida, G.M.; Lipman, J.; Mishra, R.; Goldman, H.; Jessup, J.M.; Kolodner, R. Methylation of the hMLH1 Promoter Correlates with Lack of Expression of hMLH1 in Sporadic Colon Tumors and Mismatch Repair-Defective Human Tumor Cell Lines. Cancer Res. 1997, 57, 808–811. [Google Scholar] [PubMed]
- Cunningham, J.M.; Christensen, E.R.; Tester, D.J.; Kim, C.Y.; Roche, P.C.; Burgart, L.J.; Thibodeau, S.N. Hypermethylation of the hMLH1 Promoter in Colon Cancer with Microsatellite Instability. Cancer Res. 1998, 58, 3455–3460. [Google Scholar] [PubMed]
- Peltomäki, P. Role of DNA Mismatch Repair Defects in the Pathogenesis of Human Cancer. J. Clin. Oncol. 2003, 21, 1174–1179. [Google Scholar] [CrossRef]
- Wang, J.; Yi, Y.; Xiao, Y.; Dong, L.; Liang, L.; Teng, L.; Ying, J.M.; Lu, T.; Liu, Y.; Guan, Y.; et al. Prevalence of Recurrent Oncogenic Fusion in Mismatch Repair-Deficient Colorectal Carcinoma with Hypermethylated MLH1 and Wild-Type BRAF and KRAS. Mod. Pathol. 2019, 32, 1053–1064. [Google Scholar] [CrossRef]
- Berlin, J.; Hong, D.S.; Deeken, J.F.; Boni, V.; Oh, D.; Patel, J.D.; Nanda, S.; Brega, N.; Childs, B.H.; Hyman, D.M.; et al. Efficacy and Safety of Larotrectinib in Patients with TRK Fusion Gastrointestinal Cancer. J. Clin. Oncol. 2020, 38, 824. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Di Nicolantonio, F.; Schrock, A.B.; Lee, J.; Tejpar, S.; Sartore-Bianchi, A.; Hechtman, J.F.; Christiansen, J.; Novara, L.; Tebbutt, N.; et al. ALK, ROS1, and NTRK Rearrangements in Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Cocco, E.; Benhamida, J.; Middha, S.; Zehir, A.; Mullaney, K.; Shia, J.; Yaeger, R.; Zhang, L.; Wong, D.; Villafania, L.; et al. Colorectal Carcinomas Containing Hypermethylated MLH1 Promoter and Wild-Type BRAF/KRAS Are Enriched for Targetable Kinase Fusions. Cancer Res. 2019, 79, 1047–1053. [Google Scholar] [CrossRef]
- Abrahao, A.B.K.; Karim, S.; Colwell, B.; Berry, S.; Biagi, J. The Predictive Effect of Primary Tumour Location in the Treatment of Metastatic Colorectal Cancer: A Canadian Consensus Statement. Curr. Oncol. 2017, 24, 390–400. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and Predictive Value of Primary Tumour Side in Patients with RAS Wild-Type Metastatic Colorectal Cancer Treated with Chemotherapy and EGFR Directed Antibodies in Six Randomized Trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Merck Canada Inc. Keytruda (Pembrolizumab) Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00058310.PDF (accessed on 9 October 2020).
- Andre, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.M.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab Versus Chemotherapy for Microsatellite Instability-High/Mismatch Repair Deficient Metastatic Colorectal Cancer: The Phase 3 KEYNOTE-177 Study. LBA4. In Proceedings of the American Society of Clinical Oncology, 29 May–2 June 2020. [Google Scholar]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jager, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Kennecke, H.; Berry, S.; Maroun, J.; Kavan, P.; Aucoin, N.; Couture, F.; Poulin-Costello, M.; Gillesby, B. A Retrospective Observational Study to Estimate the Attrition of Patients across Lines of Systemic Treatment for Metastatic Colorectal Cancer in Canada. Curr. Oncol. 2019, 26, e748–e754. [Google Scholar] [CrossRef]
- Farago, A.F.; Taylor, M.S.; Doebele, R.C.; Zhu, V.W.; Kummar, S.; Spira, A.I.; Boyle, T.A.; Haura, E.B.; Arcila, M.E.; Benayed, R.; et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis. Oncol. 2018, 2018. [Google Scholar] [CrossRef]
- Hanna, N.H.; Temin, S.; Masters, G. Therapy for Stage IV Non-Small-Cell Lung Cancer without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update Summary. JCO Oncol. Pract. 2020, 16, e844–e848. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodriguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Domine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis from KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab Plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 24 June 2020).
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib Versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib Versus Gefitinib as First-Line Treatment of Patients with EGFR Mutation-Positive Non-Small-Cell Lung Cancer (LUX-Lung 7): A Phase 2B, Open-Label, Randomised Controlled Trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Wu, Y.L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib Versus Gefitinib as First-Line Treatment for Patients with EGFR-Mutation-Positive Non-Small-Cell Lung Cancer (ARCHER 1050): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib Versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib Versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.; Han, J.Y.; Lee, J.S.; Hochmair, M.J.; Li, J.Y.; Chang, G.C.; Lee, K.H.; et al. Brigatinib Versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib Versus Crizotinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer (J-ALEX): An Open-Label, Randomised Phase 3 Trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.J.; et al. First-Line Ceritinib Versus Platinum-Based Chemotherapy in Advanced ALK-Rearranged Non-Small-Cell Lung Cancer (ASCEND-4): A Randomised, Open-Label, Phase 3 Study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Chu, Q.S. Targeting Non-Small Cell Lung Cancer: Driver Mutation Beyond Epidermal Growth Factor Mutation and Anaplastic Lymphoma Kinase Fusion. Ther. Adv. Med. Oncol. 2020, 12, 1758835919895756. [Google Scholar] [CrossRef]
- Farago, A.; Kummar, S.; Moreno, V.; Patel, J.; Lassen, U.; Rosen, L.; Ku, N.; Cox, M.; Nanda, S.; Childs, B.; et al. MA09.07 Activity of Larotrectinib in TRK Fusion Lung Cancer. J. Thorac. Oncol. 2019, 14, S283–S284. [Google Scholar] [CrossRef]
- Drilon, A.E.; DuBois, S.G.; Farago, A.F.; Geoerger, B.; Grilley-Olson, J.E.; Hong, D.S.; Sohal, D.; van Tilburg, C.M.; Ziegler, D.S.; Ku, N.; et al. Activity of Larotrectinib in TRK Fusion Cancer Patients with Brain Metastases or Primary Central Nervous System Tumors. J. Clin. Oncol. 2019, 37, 2006. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Doebele, R.C.; Farago, A.F.; Liu, S.V.; Chawla, S.P.; Tosi, D.; Blakely, C.M.; Krauss, J.C.; Sigal, D.; Bazhenova, L.; et al. Entrectinib in NTRK Fusion-Positive Non-Small Cell Lung Cancer (NSCLC): Integrated Analysis of Patients (pts) Enrolled in STARTRK-2, STARTRK-1 and ALKA-372-001. Ann. Oncol. 2019, 30 (Suppl. 2), ii48–ii49. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients with Lung Cancer for Treatment with Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef]
- Provencio, M.; Isla, D.; Sánchez, A.; Cantos, B. Inoperable Stage III Non-Small Cell Lung Cancer: Current Treatment and Role of Vinorelbine. J. Thorac. Dis. 2011, 3, 197–204. [Google Scholar] [CrossRef]
- Solomon, J.P.; Benayed, R.; Hechtman, J.F.; Ladanyi, M. Identifying Patients with NTRK Fusion Cancer. Ann. Oncol. 2019, 30, viii16–viii22. [Google Scholar] [CrossRef]
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with no Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019. Available online: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2019-EN.pdf?la=en (accessed on 7 May 2020).
- National Cancer Institute. Cancer Stat Facts: Soft Tissue Including Heart Cancer. Available online: https://seer.cancer.gov/statfacts/html/soft.html (accessed on 20 August 2020).
- Smrke, A.; Wang, Y.; Simmons, C. Update on Systemic Therapy for Advanced Soft-Tissue Sarcoma. Curr. Oncol. 2020, 27, 25–33. [Google Scholar] [CrossRef]
- Ducimetière, F.; Lurkin, A.; Ranchère-Vince, D.; Decouvelaere, A.V.; Péoc’h, M.; Istier, L.; Chalabreysse, P.; Muller, C.; Alberti, L.; Bringuier, P.P.; et al. Incidence of Sarcoma Histotypes and Molecular Subtypes in a Prospective Epidemiological Study with Central Pathology Review and Molecular Testing. PLoS ONE 2011, 6, e20294. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Coindre, J.M.; Ducimetière, F.; Dei Tos, A.P.; Fadda, E.; Blay, J.Y.; Buja, A.; Fedeli, U.; Cegolon, L.; Frasson, A.; et al. Incidence of Soft Tissue Sarcoma and Beyond: A Population-Based Prospective Study in 3 European Regions. Cancer 2012, 118, 5339–5348. [Google Scholar] [CrossRef]
- Canadian Cancer Society. Bone Cancer Statistics. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/bone/statistics/?region=on (accessed on 14 May 2020).
- Canadian Cancer Society. Soft Tissue Sarcoma Statistics. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/soft-tissue-sarcoma/statistics/?region=bc (accessed on 22 April 2020).
- Wong, D.D.; Vargas, A.C.; Bonar, F.; Maclean, F.; Kattampallil, J.; Stewart, C.; Sulaiman, B.; Santos, L.; Gill, A.J. NTRK-Rearranged Mesenchymal Tumours: Diagnostic Challenges, Morphological Patterns and Proposed Testing Algorithm. Pathology 2020. [Google Scholar] [CrossRef]
- Italiano, A. KIT and PDGFRA Wild-Type Gastrointestinal Stromal Tumours (GISTS): ESMO Biomarker Factsheet. Available online: https://oncologypro.esmo.org/education-library/factsheets-on-biomarkers/kit-and-pdgfra-wild-type-gastrointestinal-stromal-tumours-gists (accessed on 23 April 2020).
- Szucs, Z.; Thway, K.; Fisher, C.; Bulusu, R.; Constantinidou, A.; Benson, C.; van der Graaf, W.T.; Jones, R.L. Molecular Subtypes of Gastrointestinal Stromal Tumors and Their Prognostic and Therapeutic Implications. Future Oncol. 2017, 13, 93–107. [Google Scholar] [CrossRef]
- Von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143. [Google Scholar] [CrossRef]
- Boikos, S.A.; Pappo, A.S.; Killian, J.K.; LaQuaglia, M.P.; Weldon, C.B.; George, S.; Trent, J.C.; von Mehren, M.; Wright, J.A.; Schiffman, J.D.; et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016, 2, 922–928. [Google Scholar] [CrossRef]
- Agaimy, A.; Terracciano, L.M.; Dirnhofer, S.; Tornillo, L.; Foerster, A.; Hartmann, A.; Bihl, M.P. V600E BRAF Mutations Are Alternative Early Molecular Events in a Subset of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumours. J. Clin. Pathol. 2009, 62, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Agaram, N.P.; Wong, G.C.; Guo, T.; Maki, R.G.; Singer, S.; Dematteo, R.P.; Besmer, P.; Antonescu, C.R. Novel V600E BRAF Mutations in Imatinib-Naive and Imatinib-Resistant Gastrointestinal Stromal Tumors. Genes Chromosomes Cancer 2008, 47, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Gasparotto, D.; Miceli, R.; Toffolatti, L.; Gallina, G.; Scaramel, E.; Marzotto, A.; Boscato, E.; Messerini, L.; Bearzi, I.; et al. KIT, PDGFRA, and BRAF Mutational Spectrum Impacts on the Natural History of Imatinib-Naive Localized GIST: A Population-Based Study. Am. J. Surg. Pathol. 2015, 39, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Atiq, M.A.; Davis, J.L.; Hornick, J.L.; Dickson, B.C.; Fletcher, C.D.M.; Fletcher, J.A.; Folpe, A.L.; Mariño-Enríquez, A. Mesenchymal Tumors of the Gastrointestinal Tract with NTRK Rearrangements: A Clinicopathological, Immunophenotypic, and Molecular Study of Eight Cases, Emphasizing Their Distinction from Gastrointestinal Stromal Tumor (GIST). Mod. Pathol. 2020. [Google Scholar] [CrossRef]
- Debiec-Rychter, M.; Sciot, R.; Le Cesne, A.; Schlemmer, M.; Hohenberger, P.; van Oosterom, A.T.; Blay, J.Y.; Leyvraz, S.; Stul, M.; Casali, P.G.; et al. KIT Mutations and Dose Selection for Imatinib in Patients with Advanced Gastrointestinal Stromal Tumours. Eur. J. Cancer 2006, 42, 1093–1103. [Google Scholar] [CrossRef]
- Bramwell, V.H.; Anderson, D.; Charette, M.L. Doxorubicin-Based Chemotherapy for the Palliative Treatment of Adult Patients with Locally Advanced or Metastatic Soft Tissue Sarcoma. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef]
- Demetri, G.D.; Le Cesne, A.; Chawla, S.P.; Brodowicz, T.; Maki, R.G.; Bach, B.A.; Smethurst, D.P.; Bray, S.; Hei, Y.J.; Blay, J.Y. First-Line Treatment of Metastatic or Locally Advanced Unresectable Soft Tissue Sarcomas with Conatumumab in Combination with Doxorubicin or Doxorubicin Alone: A Phase I/II Open-Label and Double-Blind Study. Eur. J. Cancer 2012, 48, 547–563. [Google Scholar] [CrossRef]
- Blay, J.Y.; Leahy, M.G.; Nguyen, B.B.; Patel, S.R.; Hohenberger, P.; Santoro, A.; Staddon, A.P.; Penel, N.; Piperno-Neumann, S.; Hendifar, A.; et al. Randomised Phase III Trial of Trabectedin Versus Doxorubicin-Based Chemotherapy as First-Line Therapy in Translocation-Related Sarcomas. Eur. J. Cancer 2014, 50, 1137–1147. [Google Scholar] [CrossRef]
- Gelderblom, H.; Blay, J.Y.; Seddon, B.M.; Leahy, M.; Ray-Coquard, I.; Sleijfer, S.; Kerst, J.M.; Rutkowski, P.; Bauer, S.; Ouali, M.; et al. Brostallicin Versus Doxorubicin as First-Line Chemotherapy in Patients with Advanced or Metastatic Soft Tissue Sarcoma: An European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Randomised Phase II and Pharmacogenetic Study. Eur. J. Cancer 2014, 50, 388–396. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schoffski, P.; Blay, J.Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin Alone Versus Intensified Doxorubicin Plus Ifosfamide for First-Line Treatment of Advanced or Metastatic Soft-Tissue Sarcoma: A Randomised Controlled Phase 3 Trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Bui-Nguyen, B.; Butrynski, J.E.; Penel, N.; Blay, J.Y.; Isambert, N.; Milhem, M.; Kerst, J.M.; Reyners, A.K.; Litiere, S.; Marreaud, S.; et al. A Phase IIb Multicentre Study Comparing the Efficacy of Trabectedin to Doxorubicin in Patients with Advanced or Metastatic Untreated Soft Tissue Sarcoma: The TRUSTS Trial. Eur. J. Cancer 2015, 51, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Jones, R.L.; Van Tine, B.A.; Chmielowski, B.; Elias, A.D.; Adkins, D.; Agulnik, M.; Cooney, M.M.; Livingston, M.B.; Pennock, G.; et al. Olaratumab and Doxorubicin Versus Doxorubicin Alone for Treatment of Soft-Tissue Sarcoma: An Open-Label Phase 1b and Randomised Phase 2 Trial. Lancet 2016, 388, 488–497. [Google Scholar] [CrossRef]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and Docetaxel Versus Doxorubicin as First-Line Treatment in Previously Untreated Advanced Unresectable or Metastatic Soft-Tissue Sarcomas (GeDDiS): A Randomised Controlled Phase 3 Trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef]
- Demetri, G.D.; Albert, C.M.; Daniel, S.W.; Stefan, B.; Orbach, D.; DuBois, S.G.; Federman, N.; Geoerger, B.; Kummar, S.; Laetsch, T.W.; et al. Larotrectinib Efficacy and Safety in Patients with TRK Fusion Sarcomas. In Proceedings of the CTOS Annual Meeting, Tokyo, Japan, 13–16 November 2019. [Google Scholar]
- Drilon, A. TRK Fusion-Positive Cancer and TRK Inhibitor Therapy. Available online: https://www.uptodate.com/contents/trk-fusion-positive-cancers-and-trk-inhibitor-therapy/print?search=pediatric (accessed on 14 May 2020).
- Canadian Cancer Society. Salivary Gland Cancer Statistics. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/salivary-gland/statistics/?region=on (accessed on 23 April 2020).
- Luk, P.P.; Selinger, C.I.; Eviston, T.J.; Lum, T.; Yu, B.; O’Toole, S.A.; Clark, J.R.; Gupta, R. Mammary Analogue Secretory Carcinoma: An Evaluation of Its Clinicopathological and Genetic Characteristics. Pathology 2015, 47, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Majewska, H.; Skálová, A.; Stodulski, D.; Klimková, A.; Steiner, P.; Stankiewicz, C.; Biernat, W. Mammary Analogue Secretory Carcinoma of Salivary Glands: A New Entity Associated with ETV6 Gene Rearrangement. Virchows Arch. 2015, 466, 245–254. [Google Scholar] [CrossRef]
- Black, M.; Liu, C.Z.; Onozato, M.; Iafrate, A.J.; Darvishian, F.; Jour, G.; Cotzia, P. Concurrent Identification of Novel EGFR-SEPT14 Fusion and ETV6-RET Fusion in Secretory Carcinoma of the Salivary Gland. Head Neck Pathol. 2019. [Google Scholar] [CrossRef]
- Ito, Y.; Ishibashi, K.; Masaki, A.; Fujii, K.; Fujiyoshi, Y.; Hattori, H.; Kawakita, D.; Matsumoto, M.; Miyabe, S.; Shimozato, K.; et al. Mammary Analogue Secretory Carcinoma of Salivary Glands: A Clinicopathologic and Molecular Study Including 2 Cases Harboring ETV6-X Fusion. Am. J. Surg. Pathol. 2015, 39, 602–610. [Google Scholar] [CrossRef]
- Skalova, A.; Vanecek, T.; Martinek, P.; Weinreb, I.; Stevens, T.M.; Simpson, R.H.W.; Hyrcza, M.; Rupp, N.J.; Baneckova, M.; Michal, M., Jr.; et al. Molecular Profiling of Mammary Analog Secretory Carcinoma Revealed a Subset of Tumors Harboring a Novel ETV6-RET Translocation: Report of 10 Cases. Am. J. Surg. Pathol. 2018, 42, 234–246. [Google Scholar] [CrossRef]
- Skálová, A.; Banečkova, M.; Thompson, L.D.R.; Ptáková, N.; Stevens, T.M.; Brcic, L.; Hyrcza, M.; Michal, M., Jr.; Simpson, R.H.W.; Santana, T.; et al. Expanding the Molecular Spectrum of Secretory Carcinoma of Salivary Glands with a Novel VIM-RET FUSION. Am. J. Surg. Pathol. 2020, 44, 1295–1307. [Google Scholar] [CrossRef]
- Lin, H.H.; Limesand, K.H.; Ann, D.K. Current State of Knowledge on Salivary Gland Cancers. Crit. Rev. Oncog. 2018, 23, 139–151. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Head and Neck Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 13 June 2020).
- Gilbert, J.; Li, Y.; Pinto, H.A.; Jennings, T.; Kies, M.S.; Silverman, P.; Forastiere, A.A. Phase II Trial of Taxol in Salivary Gland Malignancies (E1394): A Trial of the Eastern Cooperative Oncology Group. Head Neck 2006, 28, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Licitra, L.; Cavina, R.; Grandi, C.; Palma, S.D.; Guzzo, M.; Demicheli, R.; Molinari, R. Cisplatin, Doxorubicin and Cyclophosphamide in Advanced Salivary Gland Carcinoma. A Phase II Trial of 22 Patients. Ann. Oncol. 1996, 7, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyian, V.; Sherman, E.J.; Dunn, L.; Tran, C.; Baxi, S.; Katabi, N.; Antonescu, C.R.; Ostrovnaya, I.; Haque, S.S.; Pfister, D.G.; et al. Phase II Study of Lenvatinib in Patients with Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma. J. Clin. Oncol. 2019, 37, 1529–1537. [Google Scholar] [CrossRef]
- Keam, B.; Kang, E.J.; Ahn, M.-J.; Ock, C.-Y.; Lee, K.W.; Kwon, J.H.; Yang, Y.; Choi, Y.H.; Kim, M.K.; Ji, J.H.; et al. Randomized Phase II Study of Axitinib Versus Observation in Patients with Recurred or Metastatic Adenoid Cystic Carcinoma. J. Clin. Oncol. 2020, 38, 6503. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK Fusion-Positive Cancers and TRK Inhibitor Therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Stransky, N.; Cerami, E.; Schalm, S.; Kim, J.L.; Lengauer, C. The Landscape of Kinase Fusions in Cancer. Nat. Commun. 2014, 5, 4846. [Google Scholar] [CrossRef]
- Greco, A.; Mariani, C.; Miranda, C.; Lupas, A.; Pagliardini, S.; Pomati, M.; Pierotti, M.A. The DNA Rearrangement That Generates the TRK-T3 Oncogene Involves a Novel Gene on Chromosome 3 Whose Product Has a Potential Coiled-Coil Domain. Mol. Cell. Biol. 1995, 15, 6118–6127. [Google Scholar] [CrossRef]
- Pan-Canadian Oncology Drug Review. Final Recommendation Larotrectinib (Vitrakvi). Available online: https://cadth.ca/sites/default/files/pcodr/Reviews2019/10159LarotrectinibNTRK%2BSolidTumours_fnRec_REDACT_31Oct201_ChairApproved_final.pdf (accessed on 9 April 2020).
- Institut National D’excellence en Santé et en Services Sociaux. VITRAKVI—Tumeurs Solides Porteuses d’une Fusion d’un Gène NTRK Avis Transmis à la Ministre en Octobre 2019. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Novembre_2019/Vitrakvi_2019_10.pdf (accessed on 9 April 2020).
- Pan-Canadian Oncology Drug Review. Entrectinib (TBD) for Neurotrophic Tyrosine Receptor Kinase (NTRK) Fusion-Positive Solid Tumours. Available online: https://www.cadth.ca/entrectinib-tbd-neurotrophic-tyrosine-receptor-kinase-ntrk-fusion-positive-solid-tumours (accessed on 30 July 2020).
- National Institute for Health and Care Excellence. Larotrectinib for Treating NTRK Fusion-Positive Solid Tumours. Available online: https://www.nice.org.uk/guidance/ta630 (accessed on 30 July 2020).
- National Institute for Health and Care Excellence. Entrectinib for Treating NTRK Fusion-Positive Solid Tumours. Available online: https://www.nice.org.uk/guidance/gid-ta10414/documents/html-content-2 (accessed on 30 July 2020).

| Government-funded |
|
| Industry-sponsored |
| • FastTRK (fasttrk.ca) |
| Private, direct to consumer (out of pocket cost for patient) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebb, D.G.; Banerji, S.; Blais, N.; Desmeules, P.; Gill, S.; Grin, A.; Feilotter, H.; Hansen, A.R.; Hyrcza, M.; Krzyzanowska, M.; et al. Canadian Consensus for Biomarker Testing and Treatment of TRK Fusion Cancer in Adults. Curr. Oncol. 2021, 28, 523-548. https://doi.org/10.3390/curroncol28010053
Bebb DG, Banerji S, Blais N, Desmeules P, Gill S, Grin A, Feilotter H, Hansen AR, Hyrcza M, Krzyzanowska M, et al. Canadian Consensus for Biomarker Testing and Treatment of TRK Fusion Cancer in Adults. Current Oncology. 2021; 28(1):523-548. https://doi.org/10.3390/curroncol28010053
Chicago/Turabian StyleBebb, D. Gwyn, Shantanu Banerji, Normand Blais, Patrice Desmeules, Sharlene Gill, Andrea Grin, Harriet Feilotter, Aaron R. Hansen, Martin Hyrcza, Monika Krzyzanowska, and et al. 2021. "Canadian Consensus for Biomarker Testing and Treatment of TRK Fusion Cancer in Adults" Current Oncology 28, no. 1: 523-548. https://doi.org/10.3390/curroncol28010053
APA StyleBebb, D. G., Banerji, S., Blais, N., Desmeules, P., Gill, S., Grin, A., Feilotter, H., Hansen, A. R., Hyrcza, M., Krzyzanowska, M., Melosky, B., Noujaim, J., Purgina, B., Ruether, D., Simmons, C. E., Soulieres, D., Torlakovic, E. E., & Tsao, M.-S. (2021). Canadian Consensus for Biomarker Testing and Treatment of TRK Fusion Cancer in Adults. Current Oncology, 28(1), 523-548. https://doi.org/10.3390/curroncol28010053







