The Impact of Radiotherapy on the Incidence of Secondary Malignancies: A Pan-Cancer Study in the US SEER Cancer Registries
Abstract
1. Introduction
2. Material and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
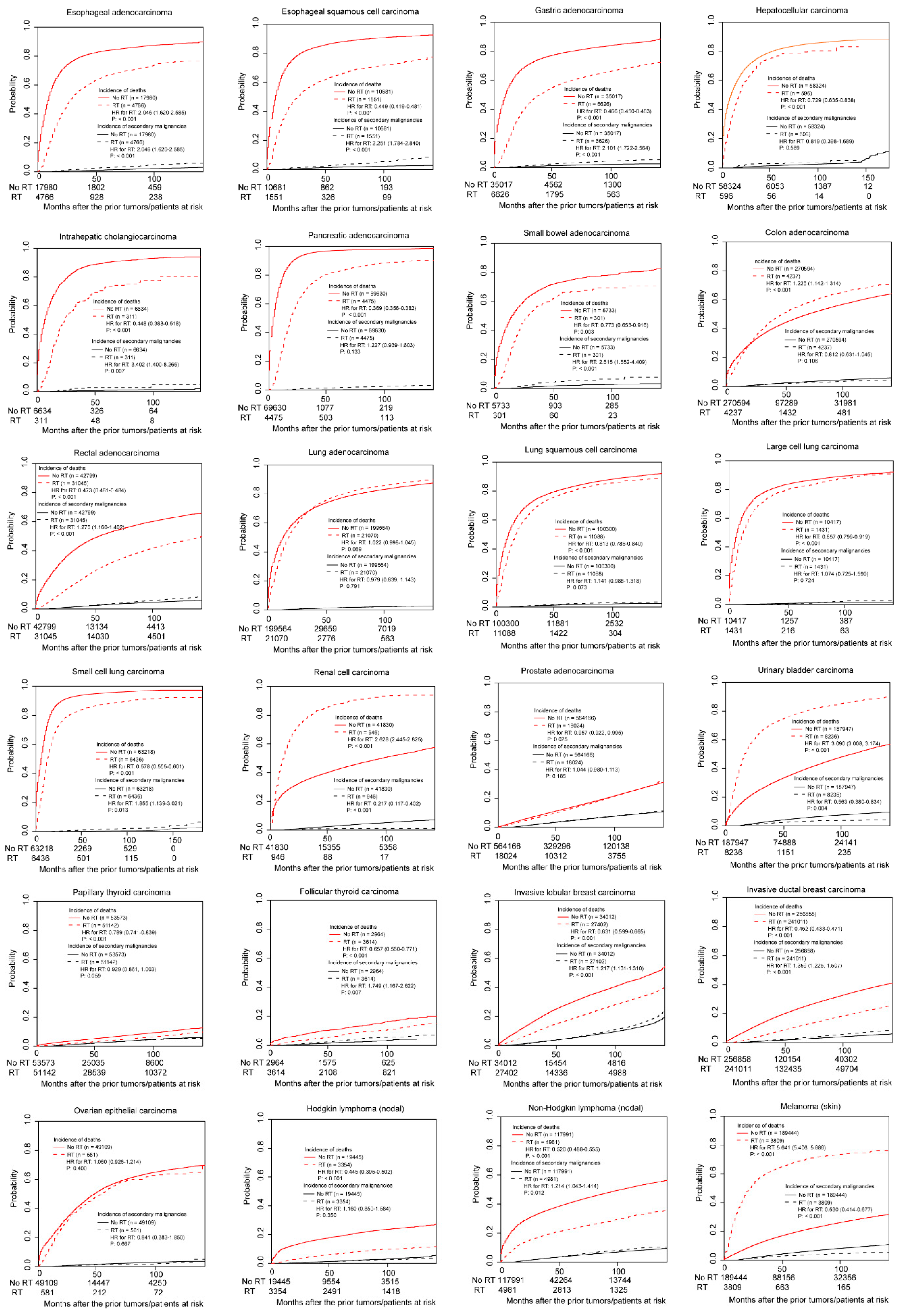
3.2. Association between Radiotherapy and Incidence of SPMs in Non-Adjusted Competing Risk Models
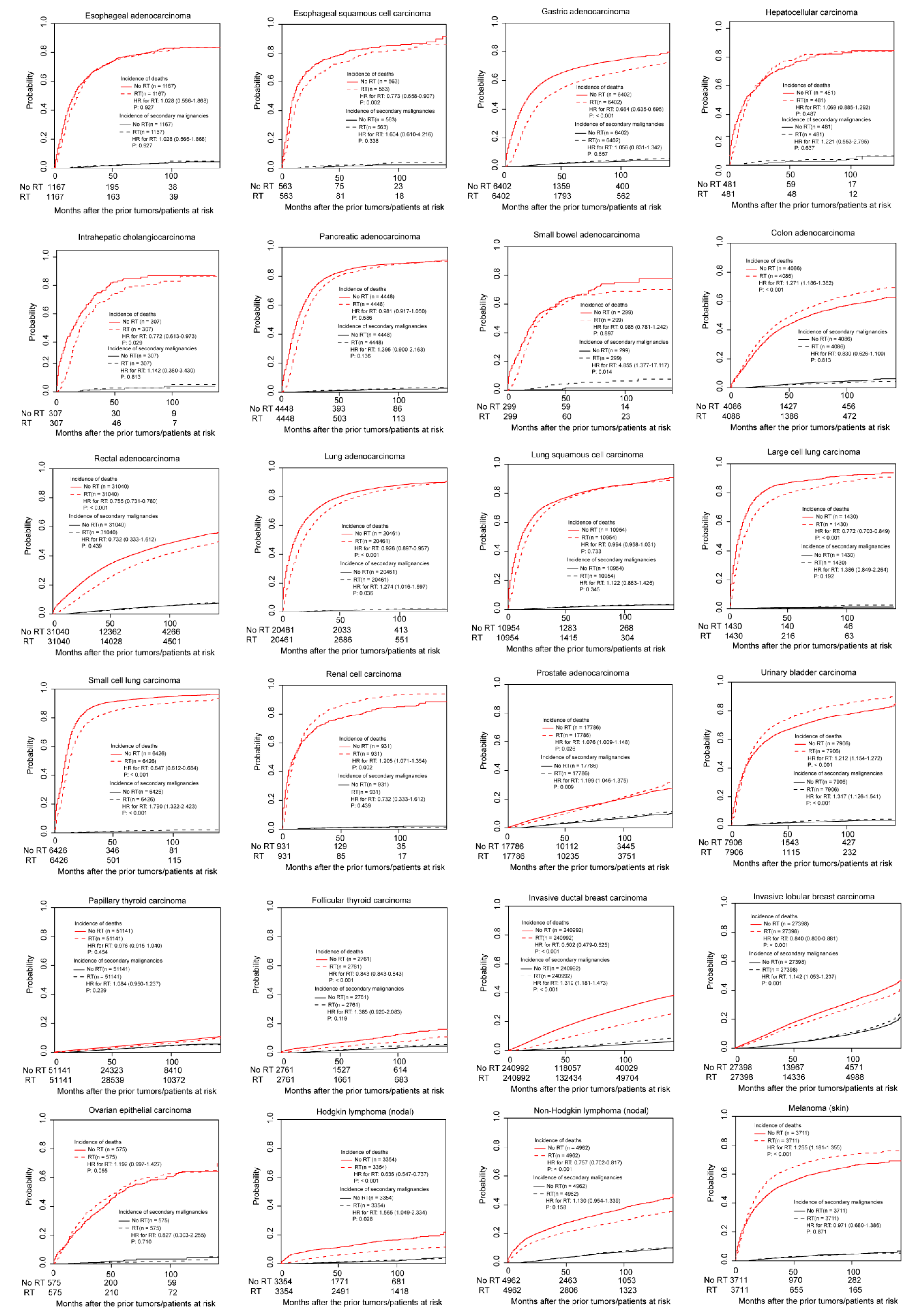
3.3. Association between Radiotherapy and Incidence of SPMs in PSM Cohorts
3.4. Association between Radiotherapy and Incidence of SPMs in Multivariable Competing Risk Models
3.5. Stratified Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018, 36, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Abou-Antoun, T.; Mikhael, R.; Massoud, M.; Chahine, G.; Saad, A. Effects of Radiotherapy on the Risk of Developing Secondary Malignant Neoplasms in Hodgkin’s Lymphoma Survivors. Asian Pac. J. Cancer Prev. 2016, 17, 749–754. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andrade-Campos, M.M.; Lievano, P.; Espinosa-Lara, N.; Soro-Alcubierre, G.; Grasa-Ulrich, J.M.; Lopez-Gomez, L.; Baringo, T.; Giraldo, P. Long-term complication in follicular lymphoma: Assessing the risk of secondary neoplasm in 242 patients treated or not with 90-yttrium-ibritumomab-tiuxetan. Eur. J. Haematol. 2016, 97, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Balcer-Kubiczek, E.K.; Eley, J.G. Secondary Malignancies in the Era of High-Precision Radiation Therapy. Crit. Rev. Oncog. 2018, 23, 93–112. [Google Scholar] [CrossRef]
- Kebudi, R.; Ozdemir, G.N. Secondary Neoplasms in Children Treated for Cancer. Curr. Pediatric Rev. 2017, 13, 34–41. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hsieh, C.C.; Li, C.Y.; Chuang, J.P.; Lee, J.C. Secondary Cancers after Radiation Therapy for Primary Prostate or Rectal Cancer. World J. Surg. 2016, 40, 895–905. [Google Scholar] [CrossRef]
- Mendes, B.M.; Trindade, B.M.; Fonseca, T.C.F.; de Campos, T.P.R. Assessment of radiation-induced secondary cancer risk in the Brazilian population from left-sided breast-3D-CRT using MCNPX. Br. J. Radiol. 2017, 90, 20170187. [Google Scholar] [CrossRef]
- Berrington de Gonzalez, A.; Curtis, R.E.; Kry, S.F.; Gilbert, E.; Lamart, S.; Berg, C.D.; Stovall, M.; Ron, E. Proportion of second cancers attributable to radiotherapy treatment in adults: A cohort study in the US SEER cancer registries. Lancet Oncol. 2011, 12, 353–360. [Google Scholar] [CrossRef]
- Kawada, K.; Hida, K.; Sakai, Y. Advantages of transperineal approach for secondary rectal cancer following prior radiotherapy to prostate cancer–video vignette. Colorectal Dis. 2017. [Google Scholar] [CrossRef]
- Shiota, M.; Yokomizo, A.; Takeuchi, A.; Inokuchi, J.; Tatsugami, K.; Ohga, S.; Sasaki, T.; Nakamura, K.; Honda, H.; Eto, M. Smoking effect on secondary bladder cancer after external beam radiotherapy for prostate cancer. Jpn. J. Clin. Oncol. 2016, 46, 952–957. [Google Scholar] [CrossRef]
- Fogliata, A.; De Rose, F.; Franceschini, D.; Stravato, A.; Seppala, J.; Scorsetti, M.; Cozzi, L. Critical Appraisal of the Risk of Secondary Cancer Induction From Breast Radiation Therapy with Volumetric Modulated Arc Therapy Relative to 3D Conformal Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.J.; Lee, H.F.; Lan, J.H.; Guo, S.S.; Ting, H.M.; Huang, Y.J.; Chen, H.C.; Lee, T.F. Propensity-score-matched evaluation of the incidence of radiation pneumonitis and secondary cancer risk for breast cancer patients treated with IMRT/VMAT. Sci. Rep. 2017, 7, 13771. [Google Scholar] [CrossRef] [PubMed]
- Winaikosol, K.; Surakunprapha, P. Rapidly developed Secondary Cutaneous Squamous cell Carcinoma after Post-Surgical Radiation Therapy for Breast Cancer. J. Med. Assoc. Thail. 2016, 99, S173–S176. [Google Scholar]
- Warschkow, R.; Guller, U.; Cerny, T.; Schmied, B.M.; Plasswilm, L.; Putora, P.M. Secondary malignancies after rectal cancer resection with and without radiation therapy: A propensity-adjusted, population-based SEER analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2017, 123, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Gilbert, E.S.; Chen, B.E.; Storm, H.; Lynch, C.F.; Hall, P.; Langmark, F.; Pukkala, E.; Kaijser, M.; et al. Second cancers among 104,760 survivors of cervical cancer: Evaluation of long-term risk. J. Natl. Cancer Inst. 2007, 99, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Creutzberg, C.L.; Nout, R.A.; Lybeert, M.L.M.; Wárlám-Rodenhuis, C.C.; Jobsen, J.; Mens, J.M.; Lutgens, L.C.H.W.; Pras, E.; van de Poll-Franse, L.V.; van Putten, W.L.J. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e631–e638. [Google Scholar] [CrossRef]
- Journy, N.; Mansouri, I.; Allodji, R.S.; Demoor-Goldschmidt, C.; Ghazi, D.; Haddy, N.; Rubino, C.; Veres, C.; Zrafi, W.S.; Rivera, S.; et al. Volume effects of radiotherapy on the risk of second primary cancers: A systematic review of clinical and epidemiological studies. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 131, 150–159. [Google Scholar] [CrossRef]
- De Glas, N.A.; Kiderlen, M.; Vandenbroucke, J.P.; de Craen, A.J.; Portielje, J.E.; van de Velde, C.J.; Le Cessie, S. Performing Survival Analyses in the Presence of Competing Risks: A Clinical Example in Older Breast Cancer Patients. J. Natl. Cancer Inst. 2016, 108, djv366. [Google Scholar] [CrossRef]
- Wissing, M.D.; Azoulay, L. Postoperative Pelvic Radiotherapy in Patients With Endometrial Cancer May Increase the Risk for Secondary Pelvic Cancers: A Post Hoc Analysis of Results From the TME, PORTEC-1, and PORTEC-2 Trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1861–1862. [Google Scholar] [CrossRef]
- Abraha, I.; Aristei, C.; Palumbo, I.; Lupattelli, M.; Trastulli, S.; Cirocchi, R.; de Florio, R.; Valentini, V. Preoperative radiotherapy and curative surgery for the management of localised rectal carcinoma. Cochrane Database Syst. Rev. 2018, 10, CD002102. [Google Scholar] [CrossRef]
- Zhang, N.; Fei, Q.; Gu, J.; Yin, L.; He, X. Progress of preoperative and postoperative radiotherapy in gastric cancer. World J. Surg. Oncol. 2018, 16, 187. [Google Scholar] [CrossRef] [PubMed]
- Crehange, G.; Mabrut, J.Y.; Rouffiac, M. Surgery after upfront radiochemotherapy for locally advanced esophageal cancer: To do or not to do? Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2018, 22, 540–545. [Google Scholar]
- Vyfhuis, M.A.L.; Rice, S.; Remick, J.; Mossahebi, S.; Badiyan, S.; Mohindra, P.; Simone, C.B., 2nd. Reirradiation for locoregionally recurrent non-small cell lung cancer. J. Thorac. Dis. 2018, 10, S2522–S2536. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.Y.; Wang, W.P.; Wang, Y.C.; Hu, W.P.; Ni, P.Z.; Lin, Y.D.; Chen, L.Q. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur. J. Cardio Thorac. Surg. Off. J. Eur. Assoc. Cardio Thorac. Surg. 2017, 51, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Horner-Rieber, J.; Bernhardt, D.; Dern, J.; Konig, L.; Adeberg, S.; Paul, A.; Heussel, C.P.; Kappes, J.; Hoffmann, H.; Herth, F.J.P.; et al. Histology of non-small cell lung cancer predicts the response to stereotactic body radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2017, 125, 317–324. [Google Scholar] [CrossRef]
- Lee, H.F.; Lan, J.H.; Chao, P.J.; Ting, H.M.; Chen, H.C.; Hsu, H.C.; Lee, T.F. Radiation-induced secondary malignancies for nasopharyngeal carcinoma: A pilot study of patients treated via IMRT or VMAT. Cancer Manag. Res. 2018, 10, 131–141. [Google Scholar] [CrossRef]
- Yamanaka, R.; Abe, E.; Sato, T.; Hayano, A.; Takashima, Y. Secondary Intracranial Tumors Following Radiotherapy for Pituitary Adenomas: A Systematic Review. Cancers 2017, 9, 103. [Google Scholar] [CrossRef]
- Conway, J.L.; Connors, J.M.; Tyldesley, S.; Savage, K.J.; Campbell, B.A.; Zheng, Y.Y.; Hamm, J.; Pickles, T. Secondary Breast Cancer Risk by Radiation Volume in Women with Hodgkin Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 35–41. [Google Scholar] [CrossRef]
- Tulchinsky, M.; Binse, I.; Campenni, A.; Dizdarevic, S.; Giovanella, L.; Jong, I.; Kairemo, K.; Kim, C.K. Radioactive Iodine Therapy for Differentiated Thyroid Cancer: Lessons from Confronting Controversial Literature on Risks for Secondary Malignancy. J. Nucl. Med. 2018, 59, 723–725. [Google Scholar] [CrossRef]
- Gillis, C.C.; Chang, E.H.; Al-Kharazi, K.; Pickles, T. Secondary malignancy following radiotherapy for thyroid eye disease. Rep. Pract. Oncol. Radiother. 2016, 21, 156–161. [Google Scholar] [CrossRef][Green Version]
- Elson, J.K.; Kachnic, L.A.; Kharofa, J.R. Intensity-modulated radiotherapy improves survival and reduces treatment time in squamous cell carcinoma of the anus: A National Cancer Data Base study. Cancer 2018, 124, 4383–4392. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Kilic, S.S.; Hsueh, W.D.; Eloy, J.A.; Baredes, S.; Woo Park, R.C.; Mahmoud, O. Radiotherapy modality as a predictor of survival in hypopharyngeal cancer. Head Neck 2018, 40, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hu, H.; Tang, L.Q.; You, R.; Zhao, J.J.; Weng, D.S.; Pan, Q.Z.; Chen, C.L.; Zhou, Z.Q.; Tang, Y.; et al. Weekly versus triweekly cisplatin plus intensity-modulated radiotherapy in locally advanced nasopharyngeal carcinoma: A propensity score analysis with a large cohort. J. Cancer 2018, 9, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Gensheimer, M.F.; Loo, B.W., Jr. Optimal Radiation Therapy for Small Cell Lung Cancer. Curr. Treat. Opt. Oncol. 2017, 18, 21. [Google Scholar] [CrossRef]

| Prior Cancer | Number of Patients with FPMs | Number of Patients with SPMs | Time to a Secondary Malignancy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Number | Radiation * | No Radiation * | Total Number | Radiation * | No Radiati on * | Total Cohort ** | Radiation ** | No Radiation ** | |
| Esophagus | |||||||||
| Adenocarcinoma | 22,746 | 4766 (21.0) | 17,980 (79.0) | 390 | 137 (35.1) | 253 (64.9) | 47.6 (40.5) | 44.8 (37.0) | 49.1 (42.0) |
| Squamous cell carcinoma | 12,232 | 1551 (12.7) | 10,681 (87.3) | 230 | 58 (25.2) | 172 (74.8) | 45.9 (37.0) | 53.9 (47.5) | 43.2 (36.0) |
| Gastric adenocarcinoma | 41,643 | 6626 (15.9) | 35,017 (84.1) | 719 | 204 (28.4) | 515 (71.6) | 43.7 (36.0) | 45.6 (40.5) | 42.9 (34.0) |
| Liver | |||||||||
| Hepatocellular carcinoma | 58,920 | 596 (1.0) | 58,324 (99.0) | 987 | 16 (1.6) | 971 (98.4) | 46.0 (34.0) | 28.7 (20.0) | 46.3 (34.0) |
| Intrahepatic cholangiocarcinoma | 6945 | 311 (4.5) | 6634 (95.5) | 51 | 7 (13.7) | 44 (86.3) | 42.9 (34.0) | 38.1 (29.0) | 43.6 (35.5) |
| Pancreatic adenocarcinoma | 74,105 | 4475 (6.0) | 69,630 (94.0) | 244 | 81 (33.2) | 163 (66.8) | 38.8 (29.0) | 43.1 (31.0) | 36.6 (28.0) |
| Small bowel adenocarcinoma | 6034 | 301 (5.0) | 5733 (95.0) | 117 | 14 (12.0) | 103 (88.0) | 43.0 (36.0) | 42.7 (34.5) | 43.0 (36.0) |
| Colon adenocarcinoma | |||||||||
| Cecum | 62,641 | 827 (1.3) | 61,594 (98.7) | 2006 | 26 (1.3) | 1980 (98.7) | 47.7 (41.0) | 39.2 (22.0) | 47.9 (42.0) |
| Appendix | 6382 | 118 (1.8) | 6264 (98.2) | 106 | 6 (5.7) | 102 (94.3) | 43.3 (35.5) | 30.2 (27.5) | 43.8 (35.5) |
| Ascending colon | 54,343 | 456 (0.8) | 53,887 (99.2) | 1967 | 8 (0.4) | 1959 (99.6) | 48.5 (41.0) | 60.2 (36.2) | 48.5 (41.0) |
| Transverse colon | 26,637 | 189 (0.7) | 26,448 (99.3) | 901 | 6 (0.7) | 895 (99.3) | 48.2 (40.0) | 64.7 (32.1) | 48.1 (40.0) |
| Descending colon | 16,721 | 225 (1.3) | 16,496 (98.7) | 526 | 4 (0.8) | 522 (99.2) | 50.4 (43.0) | 51.0 (45.0) | 50.4 (53.0) |
| Sigmoid colon | 74,642 | 2099 (2.8) | 72,543 (97.2) | 2567 | 59 (2.3) | 2508 (97.7) | 50.4 (44.0) | 48.5 (38.0) | 50.4 (44.0) |
| Rectal adenocarcinoma | 73,835 | 31,045 (42.0) | 42,790 (58.0) | 2495 | 1183 (47.4) | 1312 (52.6) | 50.6 (45.0) | 52.9 (47.0) | 48.5 (42.0) |
| Lung | |||||||||
| Adenocarcinoma | 220,634 | 21,070 (9.5) | 199,564 (90.5) | 3046 | 182 (6.0) | 2764 (94.0) | 44.6 (37.0) | 41.3 (32.5) | 44.9 (38.0) |
| Squamous cell carcinoma | 111,388 | 11,088 (10.0) | 100,300 (90.0) | 1669 | 211 (12.6) | 1458 (87.4) | 41.6 (35.0) | 41.3 (36.0) | 41.6 (35.0) |
| Small cell carcinoma | 69,654 | 6436 (9.2) | 63,218 (90.8) | 332 | 70 (21.1) | 262 (78.9) | 52.3 (42.0) | 51.7 (41.0) | 52.4 (42.0) |
| Large cell carcinoma | 11,848 | 1431 (12.1) | 10,417 (87.9) | 155 | 23 (14.8) | 132 (85.2) | 48.6 (45.0) | 65.4 (75.0) | 45.6 (42.0) |
| Renal cell carcinoma | 42,776 | 946 (2.2) | 41,830 (97.8) | 1474 | 8 (0.5) | 1466 (99.5) | 51.1 (45.0) | 31.6 (28.0) | 51.2 (45.0) |
| Prostate adenocarcinoma | 582,190 | 18,024 (3.1) | 564,166 (96.9) | 30,836 | 976 (3.2) | 29860 (96.8) | 62.2 (59.0) | 61.7 (58.0) | 62.2 (59.0) |
| Urinary bladder transitional cell carcinoma | 196,183 | 8236 (4.2) | 187,947 (95.8) | 9969 | 214 (2.1) | 9755 (97.9) | 46.5 (39.0) | 38.8 (34.5) | 46.7 (40.0) |
| Thyroid | |||||||||
| Papillary thyroid carcinoma | 104,715 | 51,142 † (48.8) | 53,573 (51.2) | 2626 | 1342 (51.1) | 1284 (48.9) | 48.6 (43.0) | 48.8 (44.0) | 48.4 (43.0) |
| Follicular thyroid carcinoma | 6578 | 3614 ‡ (54.9) | 2964 (45.1) | 191 | 129 (67.5) | 62 (32.5) | 51.6 (47.0) | 50.9 (47.0) | 53.0 (47.5) |
| Breast | |||||||||
| Invasive ductal carcinoma | 497,869 | 241,011 (48.4) | 286,858 (51.6) | 15,236 | 8749 (57.4) | 6487 (42.6) | 55.0 (50.0) | 56.4 (52.0) | 53.0 (47.0) |
| Invasive lobular carcinoma | 61,414 | 27,402 (44.6) | 34,012 (55.4) | 4450 | 2139 (48.1) | 2311 (51.9) | 63.7 (60.0) | 66.9 (64.0) | 60.8 (55.0) |
| Ovarian epithelial carcinoma | 49,690 | 581 (1.2) | 49,109 (98.8) | 932 | 10 (1.1) | 922 (98.9) | 46.9 (40.0) | 58.9 (43.0) | 46.8 (40.0) |
| Lymphoma | |||||||||
| Hodgkin lymphoma (nodal) | 22,799 | 3354 (14.7) | 19,445 (85.3) | 455 | 75 (16.5) | 380 (83.5) | 55.7 (51.0) | 56.8 (52.0) | 55.5 (50.5) |
| Non-Hodgkin lymphoma (nodal) | 122,972 | 4981 (4.1) | 117,991 (95.9) | 5652 | 320 (5.7) | 5332 (94.3) | 49.9 (43.0) | 55.7 (53.0) | 49.6 (43.0) |
| Melanoma (skin) | 193,253 | 3809 (2.0) | 189,444 (98.0) | 9865 | 109 (1.1) | 9756 (98.9) | 47.6 (40.0) | 38.4 (31.0) | 47.7 (41.0) |
| Prior Cancer | Fine and Gray Models in PSM Cohorts | |||
|---|---|---|---|---|
| Number | Events | HR (95%CI) | p Values | |
| Esophagus | ||||
| Adenocarcinoma | 2334 | 57 | 1.028 (0.566–1.868) | 0.927 |
| Squamous cell carcinoma | 1126 | 21 | 1.604 (0.610–4.216) | 0.338 |
| Gastric adenocarcinoma | 12,804 | 371 | 1.056 (0.831–1.342) | 0.657 |
| Hepatocellular carcinoma | 962 | 24 | 1.221 (0.553–2.795) | 0.637 |
| Intrahepatic cholangiocarcinoma | 614 | 13 | 1.142 (0.380–3.430) | 0.813 |
| Pancreatic adenocarcinoma | 8896 | 134 | 1.395 (0.900–2.163) | 0.136 |
| Small bowel adenocarcinoma | 598 | 17 | 4.855 (1.377–17.117) | 0.014 |
| Colon adenocarcinoma | ||||
| Cecum | 1562 | 47 | 1.507 (0.944–2.406) | 0.086 |
| Appendix | 214 | 6 | 1.878 (0.333–10.582) | 0.475 |
| Ascending colon | 852 | 17 | 0.660 (0.248–1.758) | 0.406 |
| Transverse colon | 362 | 19 | 0.478 (0.177–1.293) | 0.146 |
| Descending colon | 422 | 10 | 0.652 (0.175–2.428) | 0.523 |
| Sigmoid colon | 3890 | 99 | 1.209 (0.782–1.870) | 0.393 |
| Rectal adenocarcinoma | 62,080 | 2404 | 0.984 (0.904–1.071) | 0.706 |
| Lung | ||||
| Adenocarcinoma | 40,922 | 452 | 1.179 (0.933–1.490) | 0.169 |
| Squamous carcinoma | 21,908 | 391 | 1.122 (0.883–1.426) | 0.345 |
| Small cell carcinoma | 12,852 | 109 | 1.790 (1.322–2.423) | <0.001 |
| Large cell carcinoma | 2860 | 37 | 1.386 (0.849–2.264) | 0.192 |
| Renal cell carcinoma | 1862 | 20 | 0.732 (0.333–1.612) | 0.439 |
| Prostate adenocarcinoma | 35572 | 1783 | 1.199 (1.046–1.375) | 0.009 |
| Urinary bladder transitional cell carcinoma | 15,812 | 405 | 1.317 (1.126–1.541) | <0.001 |
| Thyroid | ||||
| Papillary thyroid carcinoma | 102,282 | 2591 | 1.084 (0.950–1.237) | 0.229 |
| Follicular thyroid carcinoma | 5522 | 140 | 1.385 (0.920–2.083) | 0.119 |
| Breast | ||||
| Invasive ductal carcinoma | 481,984 | 15070 | 1.319 (1.181–1.473) | <0.001 |
| Invasive lobular carcinoma | 54,796 | 4074 | 1.142 (1.053–1.237) | 0.001 |
| Ovarian epithelial cancer | 1150 | 24 | 0.827 (0.303–2.255) | 0.710 |
| Lymphoma | ||||
| Hodgkin lymphoma (nodal) | 6708 | 124 | 1.565 (1.049–2.334) | 0.028 |
| Non-Hodgkin lymphoma (nodal) | 9924 | 608 | 1.130 (0.954–1.339) | 0.158 |
| Melanoma (skin) | 7422 | 228 | 0.971 (0.680–1.386) | 0.871 |
| Prior Cancer | Fine and Gray Models | |||||||
|---|---|---|---|---|---|---|---|---|
| Model I | Model II | |||||||
| Number | Events | HR (95%CI) | p Value | Number | Events | HR (95%CI) | p Value | |
| Esophagus | ||||||||
| Adenocarcinoma | 22,746 | 390 | 1.895 (1.516–2.369) | <0.001 | 22,650 | 388 | 0.856 (0.524–1.399) | 0.535 |
| Squamous cell carcinoma | 12,232 | 230 | 2.085 (1.668–2.607) | <0.001 | 12,194 | 230 | 0.694 (0.458–1.053) | 0.086 |
| Gastric adenocarcinoma | 41,643 | 719 | 1.898 (1.547–2.329) | <0.001 | 41,523 | 718 | 0.954 (0.764–1.190) | 0.674 |
| Hepatocellular carcinoma | 58,920 | 987 | 0.494 (0.186–1.316) | 0.159 | 48,292 | 674 | 1.056 (0.555–2.008) | 0.869 |
| Intrahepatic cholangiocarcinoma | 6945 | 51 | 4.165 (1.255–13.828) | 0.020 | 6894 | 50 | 0.700 (0.203–2.415) | 0.572 |
| Pancreatic adenocarcinoma | 74,105 | 244 | 1.274 (0.963–1.684) * | 0.090 | 73,837 | 243 | 1.479 (1.067–2.048) * | 0.019 |
| Small bowel adenocarcinoma | 6033 | 117 | 2.511 (1.310–4.813) | 0.006 | 6021 | 117 | 2.669 (1.369–5.206) | 0.004 |
| Colon adenocarcinoma | ||||||||
| Cecum | 62,641 | 2006 | 0.955 (0.659–1.385) | 0.809 | 60,367 | 1988 | 1.168 (0.790–1.727) | 0.436 |
| Appendix | 6382 | 106 | 2.199 (0.854–5.666) | 0.103 | 4789 | 105 | 2.008 (0.732–5.509) | 0.176 |
| Ascending colon | 54,343 | 1967 | 0.443 (0.201–0.975) | 0.043 | 52,517 | 1946 | 0.625 (0.284–1.374) | 0.242 |
| Transverse colon | 26,637 | 901 | 0.987 (0.451–2.163) | 0.974 | 25,753 | 886 | 1.049 (0.484–2.274) | 0.904 |
| Descending colon | 16,721 | 526 | 0.548 (0.148–2.021) | 0.366 | 16,084 | 518 | 0.591 (0.171–2.040) | 0.406 |
| Sigmoid colon | 74,642 | 2567 | 0.834 (0.605–1.450) | 0.268 | 71,723 | 2499 | 0.956 (0.682–1.339) | 0.791 |
| Rectal adenocarcinoma | 73,835 | 2495 | 0.957 (0.883–1.037) * | 0.285 | 73,654 | 2491 | 1.070 (0.942–1.216) * | 0.298 |
| Lung | ||||||||
| Adenocarcinoma | 220,634 | 3046 | 1.163 (1.026–1.318) * | 0.018 | 220,107 | 3045 | 1.076 (0.943–1.227) * | 0.277 |
| Squamous carcinoma | 111,388 | 1669 | 1.232 (1.047–1.450) | 0.012 | 110,971 | 1665 | 1.077 (0.877–1.322) | 0.480 |
| Small cell carcinoma | 69,654 | 332 | 1.776 (1.016–3.106) | 0.044 | 69,352 | 331 | 1.459 (1.013–2.103) | 0.043 |
| Large cell carcinoma | 11,848 | 155 | 1.000 (0.638–1.568) | 0.998 | 11,804 | 154 | 0.836 (0.520–1.344) | 0.460 |
| Renal cell carcinoma | 42,776 | 1474 | 0.242 (0.120–0.489) | <0.001 | 42,630 | 1474 | 0.502 (0.200–1.261) | 0.143 |
| Prostate adenocarcinoma | 582,190 | 30836 | 1.110 (1.041–1.183) * | 0.001 | 579,521 | 30712 | 1.216 (1.138–1.300) * | <0.001 |
| Urinary bladder transitional cell carcinoma | 196,183 | 9969 | 1.477 (1.288–1.694) * | <0.001 | 195,777 | 9948 | 1.757 (1.516–2.037) * | <0.001 |
| Thyroid | ||||||||
| Papillary thyroid carcinoma | 104,715 | 2626 | 1.067 (0.987–1.152) * | 0.102 | 104,680 | 2626 | 1.028 (0.948–1.114) * | 0.504 |
| Follicular thyroid carcinoma | 6578 | 191 | 1.566 (1.155–2.123) * | 0.004 | 6574 | 191 | 1.553 (1.134–2.127) * | 0.006 |
| Breast (women) | ||||||||
| Invasive ductal carcinoma | 497,869 | 15236 | / | 497,312 | 15229 | / | ||
| Invasive lobular carcinoma | 61,414 | 4450 | 1.373 (1.218–1.547) | <0.001 | 61,336 | 4444 | 1.108 (1.108–1.108) | <0.001 |
| Ovarian epithelial cancer | 49,690 | 932 | 0.841 (0.389–1.819) | 0.660 | 49,636 | 931 | 0.744 (0.330–1.677) | 0.475 |
| Lymphoma | ||||||||
| Hodgkin lymphoma (nodal) | 22,799 | 455 | 0.824 (0.642–1.057) * | 0.128 | 22,799 | 455 | 0.872 (0.653–1.164) * | 0.351 |
| Non-Hodgkin lymphoma (nodal) | 122,972 | 5652 | 1.230 (1.058–1.430) | 0.007 | 122,972 | 5652 | 1.090 (0.948–1.254) | 0.228 |
| Melanoma (skin) | 193,253 | 9865 | 1.425 (1.180–1.722) * | < 0.001 | 192,954 | 9855 | 1.109 (0.912–1.348) * | 0.302 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xiao, H.; Xu, X.; Zhang, Y. The Impact of Radiotherapy on the Incidence of Secondary Malignancies: A Pan-Cancer Study in the US SEER Cancer Registries. Curr. Oncol. 2021, 28, 301-316. https://doi.org/10.3390/curroncol28010035
Li W, Xiao H, Xu X, Zhang Y. The Impact of Radiotherapy on the Incidence of Secondary Malignancies: A Pan-Cancer Study in the US SEER Cancer Registries. Current Oncology. 2021; 28(1):301-316. https://doi.org/10.3390/curroncol28010035
Chicago/Turabian StyleLi, Wei, Haitao Xiao, Xuewen Xu, and Yange Zhang. 2021. "The Impact of Radiotherapy on the Incidence of Secondary Malignancies: A Pan-Cancer Study in the US SEER Cancer Registries" Current Oncology 28, no. 1: 301-316. https://doi.org/10.3390/curroncol28010035
APA StyleLi, W., Xiao, H., Xu, X., & Zhang, Y. (2021). The Impact of Radiotherapy on the Incidence of Secondary Malignancies: A Pan-Cancer Study in the US SEER Cancer Registries. Current Oncology, 28(1), 301-316. https://doi.org/10.3390/curroncol28010035




