Gastric Cancer Treatments and Survival Trends in the United States
Abstract
1. Introduction
2. Experimental Section
3. Results
3.1. Patient Characteristics
3.2. Evaluation of Treatment Trends over Time
3.3. Multivariate Analyses of Predictors of the Receipt of Non-Guideline Concordant Care for Stage I and Stage II/III Gastric Cancer
3.4. Kaplan Meier Curves for 3-Year Overall Survival for All Stages of Disease
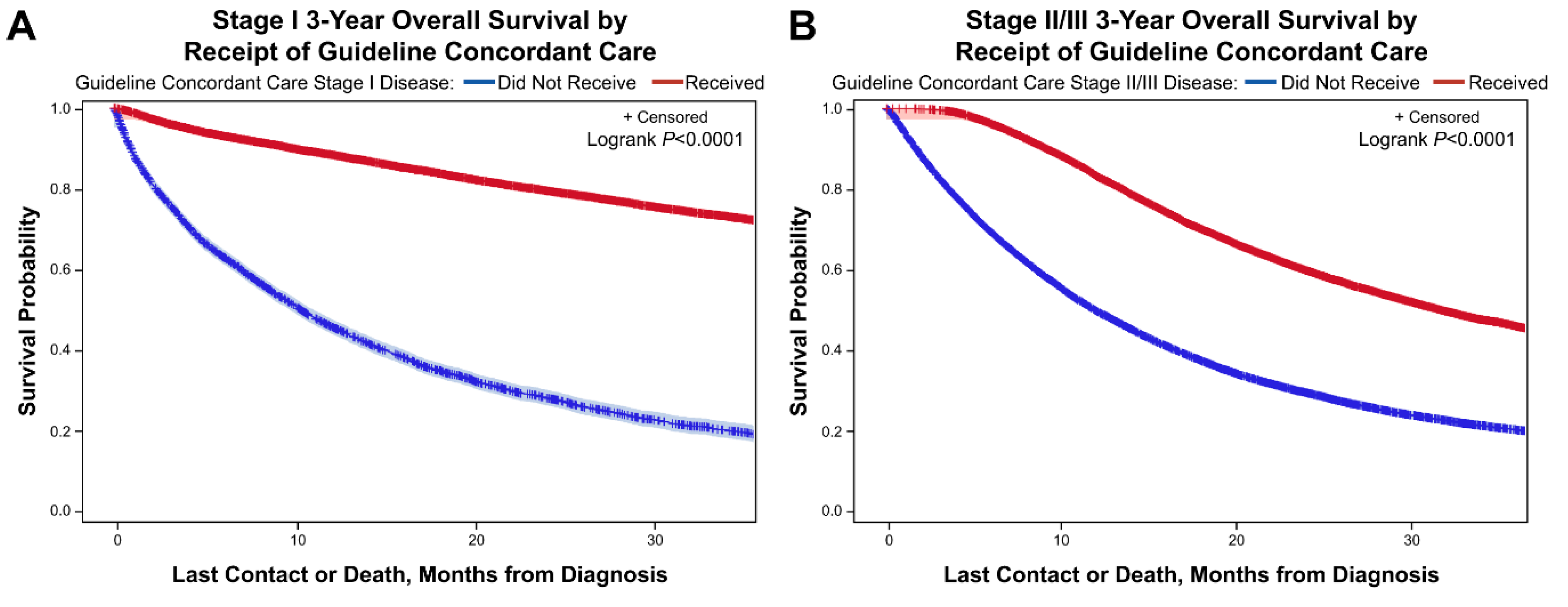
3.5. Kaplan Meier Curves for 3-Year Overall Survival for Stage I and Stage II/III Disease Based on Receipt of Guideline Concordant Care
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Jiang, L.; Lin, R.; Wei, K.; Gan, L.; Deng, C.; Guan, Q. Adjuvant chemoradiotherapy versus chemotherapy for gastric cancer: A meta-analysis of randomized controlled trials. J. Surg. Oncol. 2015, 111, 277–284. [Google Scholar] [CrossRef]
- Cats, A.; Jansen, E.P.M.; van Grieken, N.C.T.; Sikorska, K.; Lind, P.; Nordsmark, M.; Meershoek-Klein Kranenbarg, E.; Boot, H.; Trip, A.K.; Swellengrebel, H.A.; et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 616–628. [Google Scholar] [CrossRef]
- Zhu, W.G.; Xua, D.; Pu, J.; Zong, C.D.; Li, T.; Tao, G.Z.; Ji, F.Z.; Zhou, X.L.; Han, J.H.; Wang, C.S.; et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother. Oncol. 2012, 104, 361–366. [Google Scholar] [CrossRef]
- Slagter, A.E.; Jansen, E.P.M.; van Laarhoven, H.W.M.; van Sandick, J.W.; van Grieken, N.C.T.; Sikorska, K.; Cats, A.; Muller-Timmermans, P.; Hulshof, M.C.C.M.; Boot, H.; et al. CRITICS-II: A multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer 2018, 18, 877. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Gastric Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 18 November 2019).
- Thiels, C.A.; Hanson, K.T.; Habermann, E.B.; Boughey, J.C.; Grotz, T.E. Integrated cancer networks improve compliance with national guidelines and outcomes for resectable gastric cancer. Cancer 2020, 126. [Google Scholar] [CrossRef]
- Snyder, R.A.; Penson, D.F.; Ni, S.; Koyama, T.; Merchant, N.B. Trends in the use of evidence-based therapy for resectable gastric cancer. J. Surg. Oncol. 2014, 110. [Google Scholar] [CrossRef]
- Raigani, S.; Hardacre, J.M.; Kim, J.; Ammori, J.B. Trends in the Surgical Treatment of Gastric Adenocarcinoma. Ann. Surg. Oncol. 2014, 21. [Google Scholar] [CrossRef]
- American College of Surgeons. National Cancer Database. Available online: https://www.facs.org/quality-programs/cancer/ncdb (accessed on 2 July 2019).
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: The AIO-FLOT3 trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Park, S.R.; Yang, H.K.; Chung, H.C.; Chung, I.J.; Kim, S.W.; Kim, H.H.; Choi, J.H.; Kim, H.K.; Yu, W.; et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1389–1396. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Liu, N.; Xu, Y.; Rahnemai-Azar, A.A.; Abbott, D.E.; Weber, S.M.; Lidor, A.O. National Underutilization of Neoadjuvant Chemotherapy for Gastric Cancer. J. Gastrointest. Surg. 2019, 24, 949–958. [Google Scholar] [CrossRef]
- Russo, A.E.; Strong, V.E. Gastric Cancer Etiology and Management in Asia and the West. Annu. Rev. Med. 2019, 70, 353–367. [Google Scholar] [CrossRef]
- Irino, T.; Takeuchi, H.; Terashima, M.; Wakai, T.; Kitagawa, Y. Gastric Cancer in Asia: Unique Features and Management. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 279–291. [Google Scholar] [CrossRef]
- Gramaticu, I.M.; Croitoru, A.E. Current Management of Gastric Cancer in Europe. Chirurgia 2018, 113, 758–764. [Google Scholar] [CrossRef]
- Waddell, T.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi57–vi63. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Cunningham, D.; Oliveira, J. Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009, 20, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.; Smithers, B.M.; Haustermans, K.; Michael, M.; Gebski, V.; Miller, D.; Zalcberg, J.; Boussioutas, A.; Findlay, M.; O’Connell, R.L.; et al. TOPGEAR: A randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: Interim results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann. Surg. Oncol. 2017, 24, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Al-Refaie, W.B.; Gay, G.; Virnig, B.A.; Tseng, J.F.; Stewart, A.; Vickers, S.M.; Tuttle, T.M.; Feig, B.W. Variations in gastric cancer care: A trend beyond racial disparities. Cancer 2010, 116, 465–475. [Google Scholar] [CrossRef]
- Ikoma, N.; Cormier, J.N.; Feig, B.; Du, X.L.; Yamal, J.; Hofstetter, W.; Das, P.; Ajani, J.A.; Roland, C.L.; Fournier, K.; et al. Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: Analysis of the National Cancer Data Base, 2006–2014. Cancer 2018, 124, 998–1007. [Google Scholar] [CrossRef]
- Howlander, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2016. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 10 December 2019).


| Stage I (n = 23,584) | Stage II/III (n = 40,216) | Stage IV (n = 44,350) | p Value | |
|---|---|---|---|---|
| Sex | <0.001 | |||
| Male | 15,242 (64.6%) | 27,772 (69.1%) | 29,067 (65.5%) | |
| Female | 8342 (35.4%) | 12,444 (30.9%) | 15,283 (34.5%) | |
| Age | <0.001 | |||
| <50 | 1149 (4.9%) | 2973 (7.4%) | 4315 (9.7%) | |
| 50–69 | 9179 (38.9%) | 18,786 (46.7%) | 21,407 (48.3%) | |
| ≥70 | 13,256 (56.2%) | 18,457 (45.9%) | 18,628 (42%) | |
| Race | <0.001 | |||
| White non-Hispanic | 16,593 (70.4%) | 28,616 (71.2%) | 30,397 (68.5%) | |
| White Hispanic | 1735 (7.4%) | 3273 (8.1%) | 4363 (9.8%) | |
| African American | 3215 (13.6%) | 5591 (13.9%) | 6859 (15.5%) | |
| Asian Pacific Islander | 2041 (8.7%) | 2736 (6.8%) | 2731 (6.2%) | |
| CDCI | <0.001 | |||
| None/few comorbidities | 20,520 (87%) | 36,385 (90.5%) | 40,316 (90.9%) | |
| Multiple comorbidities | 3064 (13%) | 3831 (9.5%) | 4034 (9.1%) | |
| Year of diagnosis | <0.001 | |||
| 2004–2008 | 8076 (34.2%) | 11,165 (27.8%) | 15,092 (34%) | |
| 2009–2012 | 7533 (31.9%) | 13549 (33.7%) | 13,716 (30.9%) | |
| 2013–2016 | 7975 (33.8%) | 15,502 (38.5%) | 15,542 (35%) | |
| Insurance status | <0.001 | |||
| Not Insured | 459 (1.9%) | 1321 (3.3%) | 2444 (5.5%) | |
| Insured | 23,125 (98.1%) | 38,895 (96.7%) | 41,906 (94.5%) | |
| Median income quartile 2008–2012 | <0.001 | |||
| $38,000 | 4395 (18.6%) | 7627 (19%) | 8825 (19.9%) | |
| $38,000–47,999 | 5348 (22.7%) | 9278 (23.1%) | 10,123 (22.8%) | |
| $480,000–62,999 | 6209 (26.3%) | 10,649 (26.5%) | 11,800 (26.6%) | |
| ≥$63,000 | 7632 (32.4%) | 12,662 (31.5%) | 13,602 (30.7%) | |
| Urban/rural status | <0.001 | |||
| Metropolitan | 20,491 (86.9%) | 34,282 (85.2%) | 38,201 (86.1%) | |
| Rural Adjacent to metropolitan area | 2161 (9.2%) | 4137 (10.3%) | 4264 (9.6%) | |
| Rural | 932 (4%) | 1797 (4.5%) | 1885 (4.3%) | |
| Facility type | <0.001 | |||
| Community cancer program | 1945 (8.2%) | 3338 (8.3%) | 4554 (10.3%) | |
| Comprehensive community cancer program | 8112 (34.4%) | 14,965 (37.2%) | 17,590 (39.7%) | |
| Academic/research cancer program | 13,527 (57.4%) | 21,913 (54.5%) | 22,206 (50.1%) | |
| Facility location | <0.001 | |||
| Northeast and Atlantic | 6085 (25.8%) | 9437 (23.5%) | 10,326 (23.3%) | |
| South Atlantic and South East | 6528 (27.7%) | 11,123 (27.7%) | 11,931 (26.9%) | |
| Midwest | 7137 (30.3%) | 12,732 (31.7%) | 14,280 (32.2%) | |
| West and Pacific | 3834 (16.3%) | 6924 (17.2%) | 7813 (17.6%) | |
| Facility case volume by quartile | <0.001 | |||
| 0th–25th | 963 (4.1%) | 1673 (4.2%) | 2501 (5.6%) | |
| 26th–49th | 2515 (10.7%) | 4752 (11.8%) | 6144 (13.9%) | |
| 50th–74th | 4855 (20.6%) | 8769 (21.8%) | 10,981 (24.8%) | |
| 75th–100th | 15,251 (64.7%) | 25,022 (62.2%) | 24,724 (55.7%) | |
| Treatment received | <0.001 | |||
| No therapy | 3604 (15.3%) | 2745 (6.8%) | 13,331 (30.1%) | |
| Chemotherapy only | 748 (3.2%) | 2625 (6.5%) | 17,728 (40%) | |
| Radiation only | 546 (2.3%) | 784 (1.9%) | 1924 (4.3%) | |
| Chemoradiation | 1323 (5.6%) | 5464 (13.6%) | 5007 (11.3%) | |
| Surgery alone | 12,655 (53.7%) | 7752 (19.3%) | 2553 (5.8%) | |
| Surgery and chemotherapy | 1603 (6.8%) | 6477 (16.1%) | 2399 (5.4%) | |
| Surgery and radiation | 121 (0.5%) | 396 (1%) | 84 (0.2%) | |
| Trimodality | 2984 (12.7%) | 13,973 (34.7%) | 1324 (3%) | |
| Location of tumor within stomach | <0.001 | |||
| Proximal | 10,394 (44.1%) | 20,020 (49.8%) | 17,785 (40.1%) | |
| Body | 6916 (29.3%) | 10,293 (25.6%) | 16,352 (36.9%) | |
| Distal | 5344 (22.7%) | 7411 (18.4%) | 6665 (15%) | |
| Unknown | 930 (3.9%) | 2492 (6.2%) | 3548 (8%) | |
| Histologic grade | <0.001 | |||
| Low grade | 10,740 (45.5%) | 11,461 (28.5%) | 9088 (20.5%) | |
| High grade | 9200 (39%) | 24,567 (61.1%) | 25,265 (57%) | |
| Unknown | 3644 (15.5%) | 4188 (10.4%) | 9997 (22.5%) |
| Stage I (n = 17,363) | Stage II/III (n = 28,598) | p Value | |
|---|---|---|---|
| No. of lymph nodes examined | <0.001 | ||
| None | 3292 (19%) | 1049 (3.7%) | |
| <15 | 8126 (46.8%) | 12,835 (44.9%) | |
| 15–25 | 4062 (23.4%) | 9696 (33.9%) | |
| >25 | 1836 (10.6%) | 4943 (17.3%) | |
| Unknown | 47 (0.3%) | 75 (0.3%) | |
| Surgical margins | <0.001 | ||
| R0 resection | 15,923 (91.7%) | 23,300 (81.5%) | |
| R1 resection | 404 (2.3%) | 2613 (9.1%) | |
| R2 resection | 311 (1.8%) | 1985 (6.9%) | |
| Not applicable/unknown | 725 (4.2%) | 700 (2.4%) |
| Stage 1 (n = 9659) | Stage II/III (n = 18,021) | p Value | |
|---|---|---|---|
| Surgical Approach | <0.001 | ||
| Robotic Assisted | 442 (4.6%) | 718 (4%) | |
| Robotic to Open | 27 (0.3%) | 59 (0.3%) | |
| Endoscopic or Laparoscopic | 3292 (34.1%) | 2880 (16%) | |
| Endo/Lap to Open | 265 (2.7%) | 614 (3.4%) | |
| Open | 4776 (49.4%) | 10,794 (59.9%) |
| OR | Lower CL | Upper CL | p Value | |
|---|---|---|---|---|
| Race | ||||
| White non-Hispanic | Reference | |||
| African American | 1.384 | 1.259 | 1.521 | <0.0001 |
| Asian Pacific Islander | 0.744 | 0.651 | 0.850 | <0.0001 |
| White Hispanic | 1.042 | 0.917 | 1.184 | 0.6325 |
| Age | ||||
| <50 | Reference | |||
| 50–69 | 1.015 | 0.855 | 1.206 | <0.0001 |
| ≥70 | 2.897 | 2.451 | 3.423 | <0.0001 |
| Gender | ||||
| Male | Reference | |||
| Female | 1.022 | 0.957 | 1.093 | 0.5133 |
| Year of diagnosis | ||||
| 2004–2008 | 0.727 | 0.650 | .848 | <0.0001 |
| 2009–2012 | 1.014 | 0.940 | 1.093 | <0.0001 |
| 2013–2016 | Reference | |||
| Facility location | ||||
| Northeast & Atlantic | Reference | |||
| Midwest | 0.957 | 0.879 | 1.042 | 0.0622 |
| South Atlantic and South East | 1.079 | 0.990 | 1.177 | 0.0091 |
| West and Pacific | 0.988 | 0.892 | 1.096 | 0.6222 |
| Facility case volume by quartile | ||||
| 75th–100th | Reference | |||
| 0th–25th | 3.132 | 2.716 | 3.611 | <0.0001 |
| 26th–49th | 2.370 | 2.153 | 2.608 | <0.0001 |
| 50th–74th | 1.834 | 1.699 | 1.981 | 0.1536 |
| Location of tumor within stomach | ||||
| Distal | Reference | |||
| Body | 1.460 | 1.335 | 1.597 | 0.2698 |
| Proximal | 1.806 | 1.651 | 1.976 | <0.0001 |
| Unknown | 1.503 | 1.271 | 1.778 | 0.2926 |
| Histologic grade | ||||
| Low Grade | Reference | |||
| High Grade | 1.845 | 1.718 | 1.981 | <0.0001 |
| Unknown | 4.528 | 4.154 | 4.935 | <0.0001 |
| OR | Lower CL | Upper CL | p Value | |
|---|---|---|---|---|
| Race | ||||
| White non-Hispanic | Reference | |||
| African American | 1.161 | 1.088 | 1.239 | <0.001 |
| Asian Pacific Islander | 0.864 | 0.791 | 0.944 | <0.001 |
| White Hispanic | 1.025 | 0.946 | 1.110 | 0.5732 |
| Age | ||||
| <50 | Reference | |||
| 50–69 | 1.498 | 1.373 | 1.634 | <0.001 |
| ≥70 | 5.026 | 4.604 | 5.486 | <0.001 |
| Gender | ||||
| Male | Reference | |||
| Female | 1.175 | 1.121 | 1.230 | <0.001 |
| Year of diagnosis | ||||
| 2004–2008 | 1.219 | 1.157 | 1.284 | <0.001 |
| 2009–2012 | 1.124 | 1.070 | 1.181 | 0.4224 |
| 2013–2016 | Reference | |||
| Facility location | ||||
| Northeast & Atlantic | Reference | |||
| Midwest | 0.988 | 0.933 | 1.046 | <0.001 |
| South Atlantic and South East | 1.078 | 1.016 | 1.143 | 0.1170 |
| West and Pacific | 1.130 | 1.056 | 1.209 | <0.001 |
| Facility case volume by quartile | ||||
| 75th–100th | Reference | |||
| 0th–25th | 1.604 | 1.441 | 1.784 | <0.001 |
| 26th–49th | 1.452 | 1.359 | 1.552 | 0.0010 |
| 50th–74th | 1.331 | 1.263 | 1.402 | 0.8965 |
| Location of tumor within stomach | ||||
| Distal | Reference | |||
| Body | 1.137 | 1.067 | 1.212 | 0.6917 |
| Proximal | 1.278 | 1.201 | 1.359 | <0.001 |
| Unknown | 1.188 | 1.079 | 1.309 | 0.2800 |
| Histologic grade | ||||
| Low Grade | Reference | |||
| High Grade | 0.953 | 0.909 | 1.000 | <0.001 |
| Unknown | 2.169 | 2.007 | 2.343 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stahl, K.A.; Olecki, E.J.; Dixon, M.E.; Peng, J.S.; Torres, M.B.; Gusani, N.J.; Shen, C. Gastric Cancer Treatments and Survival Trends in the United States. Curr. Oncol. 2021, 28, 138-151. https://doi.org/10.3390/curroncol28010017
Stahl KA, Olecki EJ, Dixon ME, Peng JS, Torres MB, Gusani NJ, Shen C. Gastric Cancer Treatments and Survival Trends in the United States. Current Oncology. 2021; 28(1):138-151. https://doi.org/10.3390/curroncol28010017
Chicago/Turabian StyleStahl, Kelly A., Elizabeth J. Olecki, Matthew E. Dixon, June S. Peng, Madeline B. Torres, Niraj J. Gusani, and Chan Shen. 2021. "Gastric Cancer Treatments and Survival Trends in the United States" Current Oncology 28, no. 1: 138-151. https://doi.org/10.3390/curroncol28010017
APA StyleStahl, K. A., Olecki, E. J., Dixon, M. E., Peng, J. S., Torres, M. B., Gusani, N. J., & Shen, C. (2021). Gastric Cancer Treatments and Survival Trends in the United States. Current Oncology, 28(1), 138-151. https://doi.org/10.3390/curroncol28010017






