Adverse Events Associated with Anti-EGFR Therapies for the Treatment of Metastatic Colorectal Cancer
Abstract
:1. INTRODUCTION
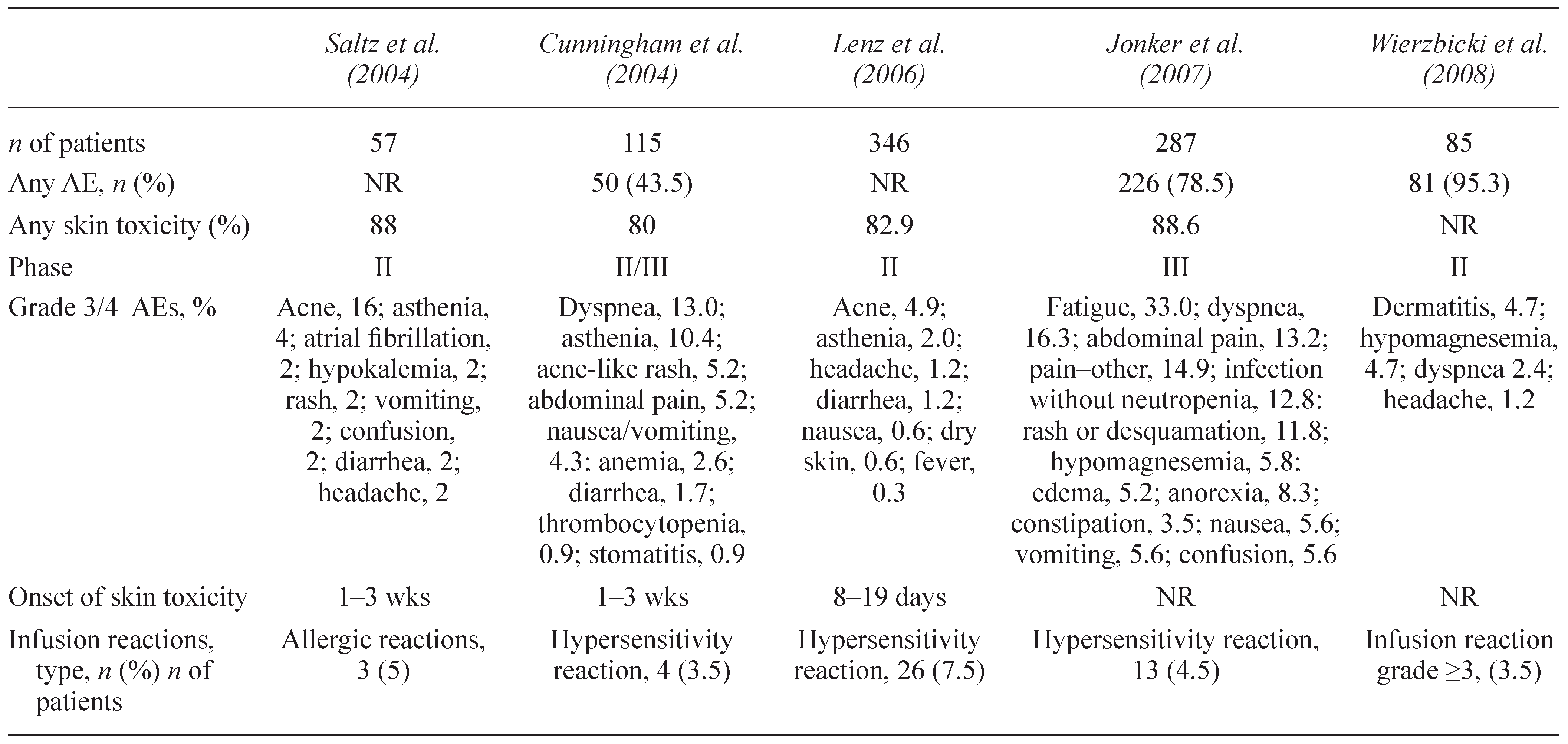
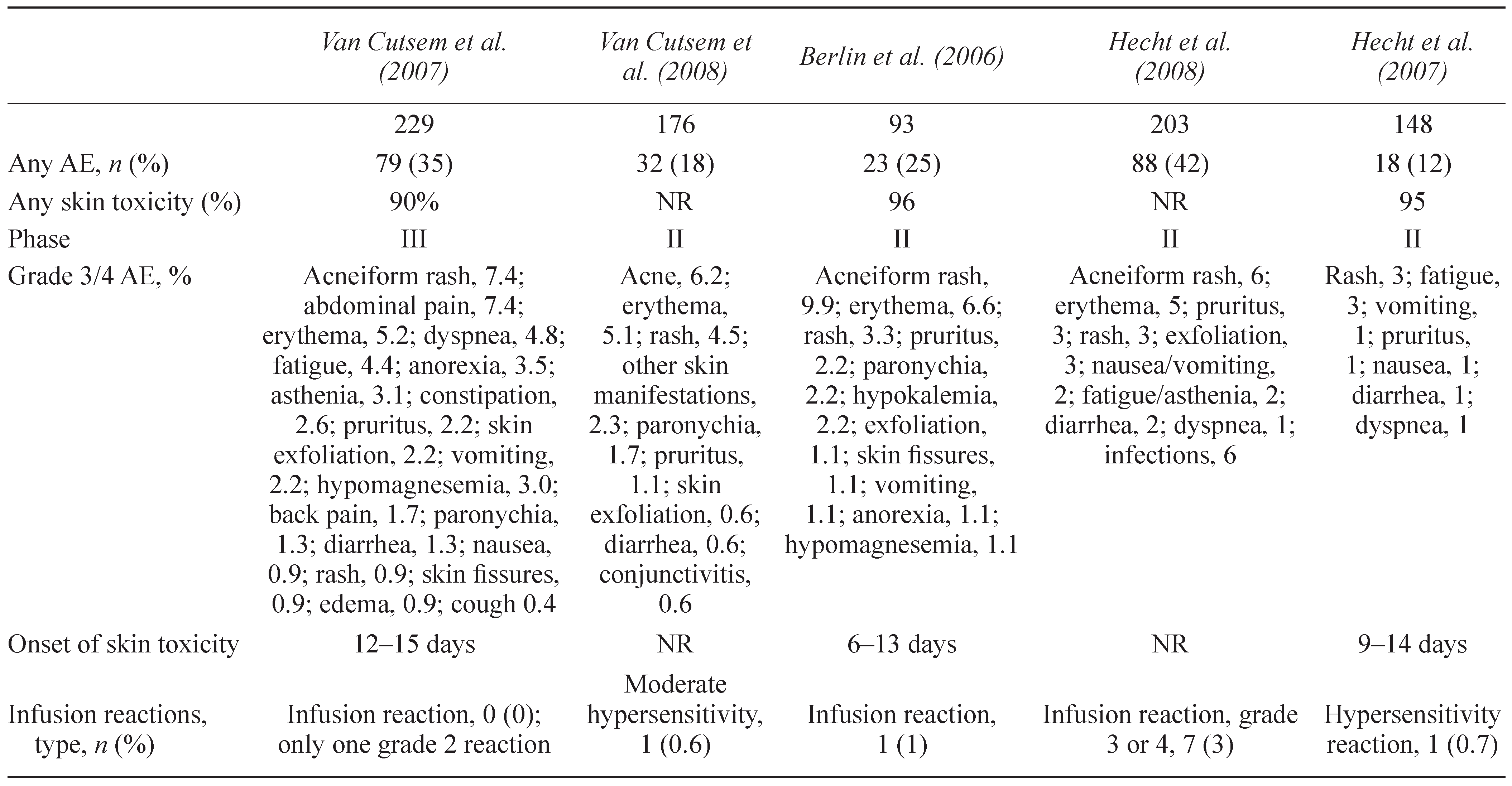
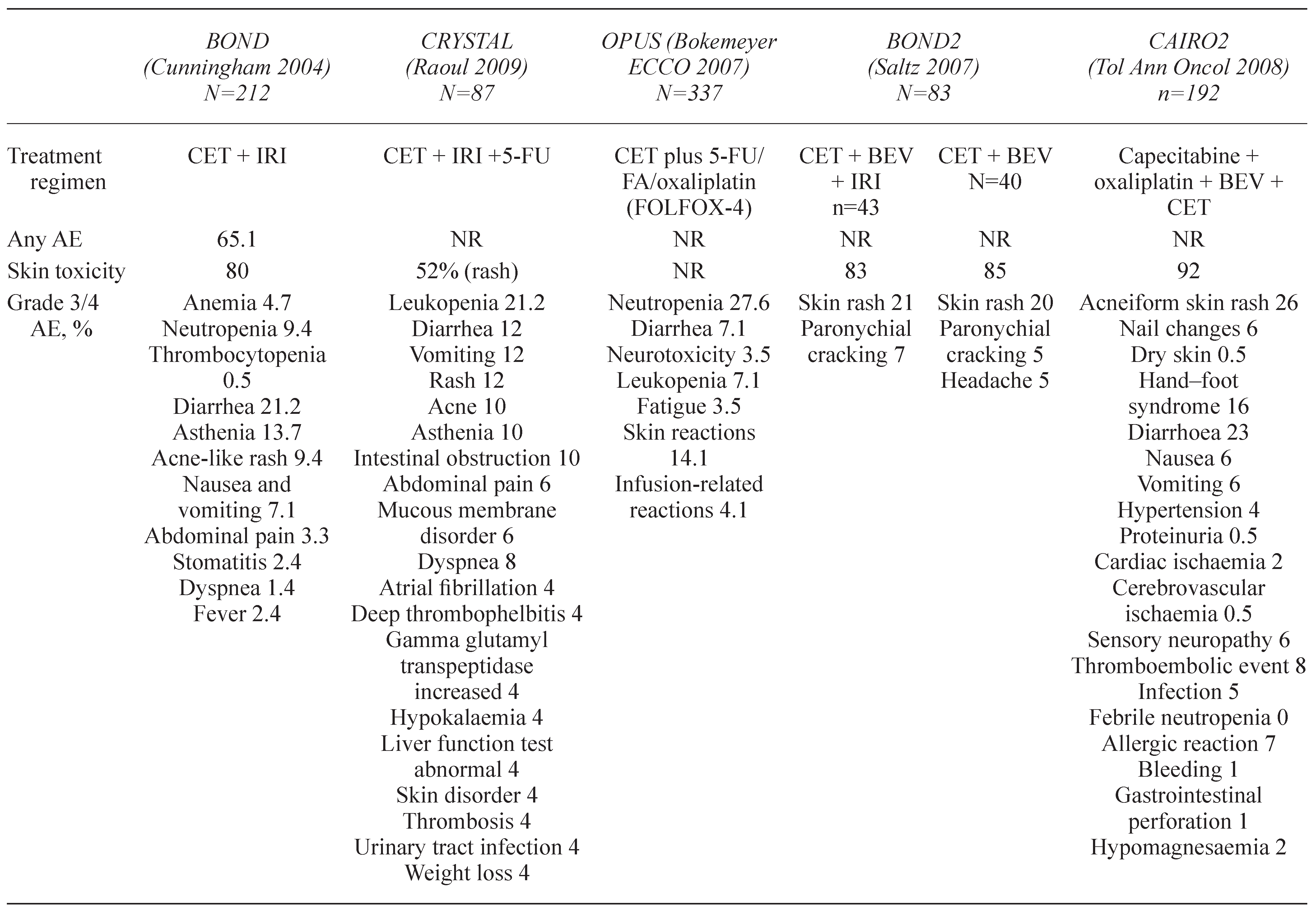
2. DERMATOLOGICAL TOXICITIES
2.1. Papulopustular rash
2.1.1. Correlation between the efficacy of EGFR inhibitors and the occurrence of rash
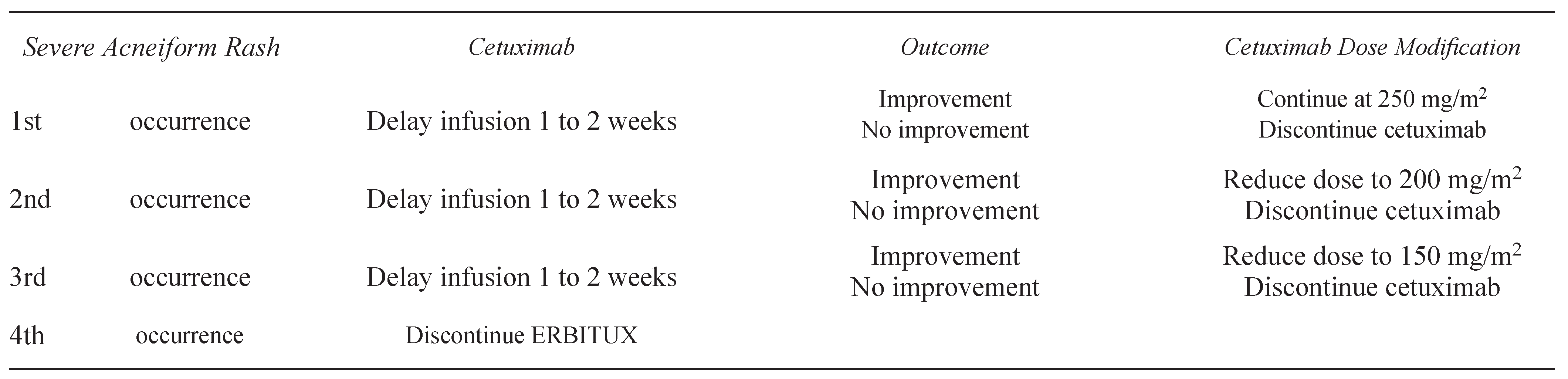
2.1.2. Management of skin rash associated with cetuximab and panitumumab
2.2. Xerosis
2.3. Paronychia
2.4. Hair changes
2.5. Telangiectasias and hyperpigmentation
2.6. Radiation dermatitis
3. OCULAR TOXICITIES
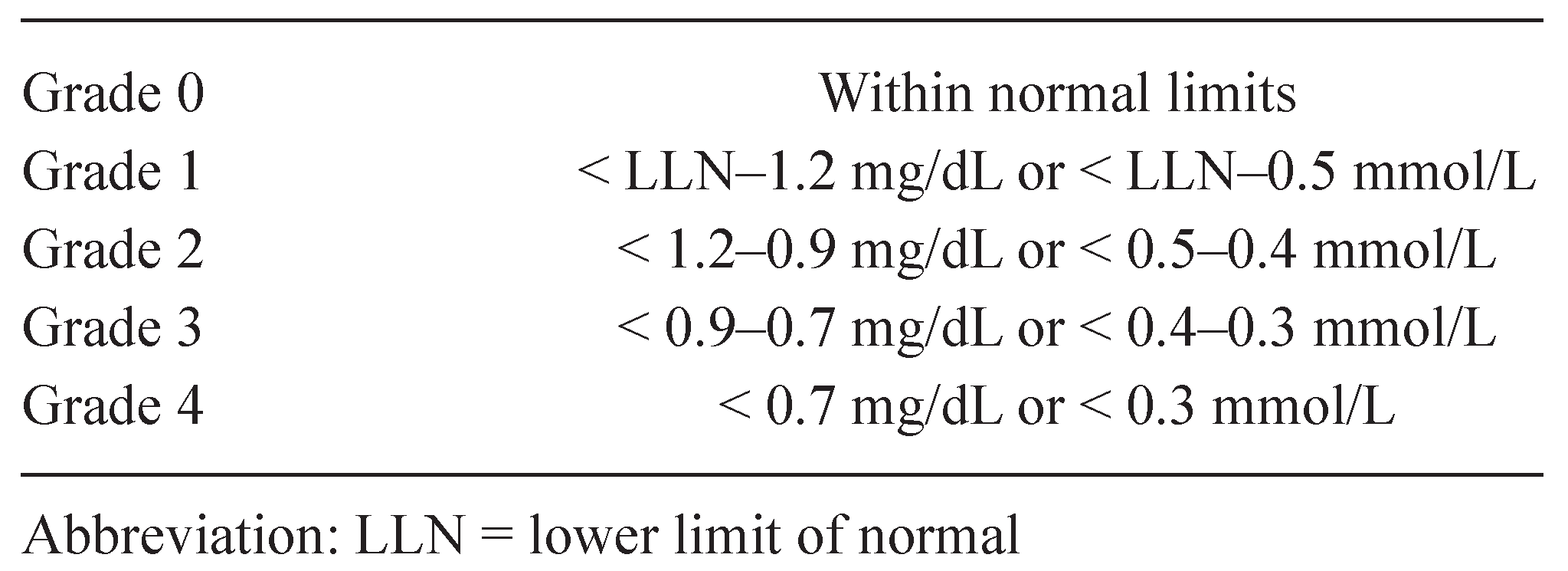
4. HYPOMAGNESEMIA
4.1. Management of hypomagnesemia
5. DIARRHEA
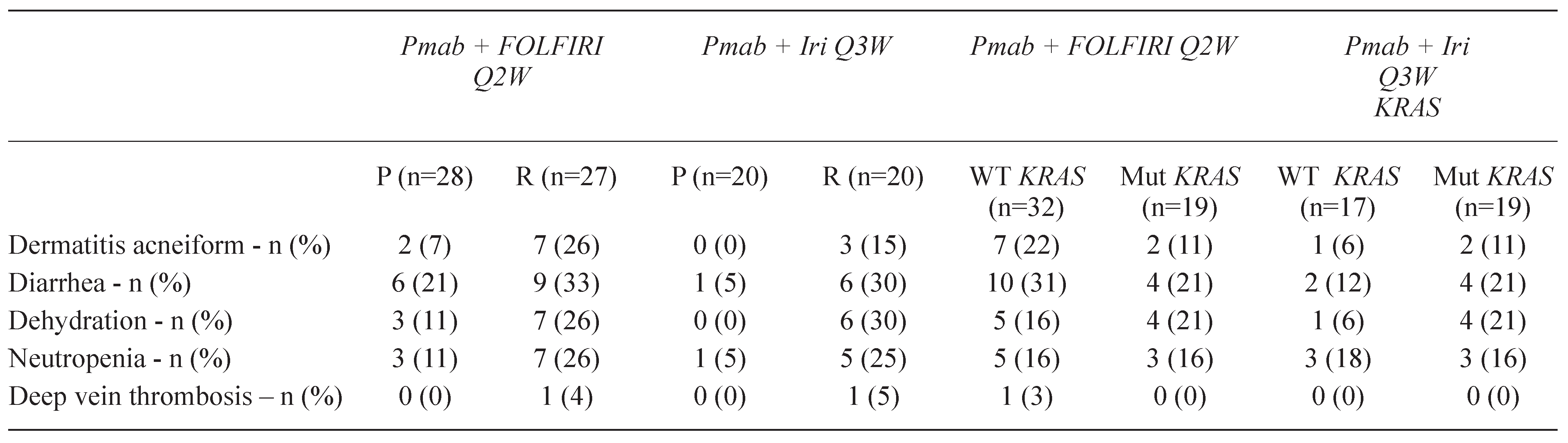
 |
6. INFUSION REACTIONS
7. CONCLUSIONS
Acknowledgments
References
- Di Marco, E.; Albanese, E.; Benso, S.; et al. Expression of epidermal growth factor receptor and transforming growth factor alpha in human larynx carcinoma. Cancer Lett. 1992, 65, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995, 19, 183–232. [Google Scholar] [PubMed]
- Miettinen, P.J.; Berger, J.E.; Meneses, J.; et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995, 376, 337–341. [Google Scholar] [CrossRef]
- Klapper, L.N.; Kirschbaum, M.H.; Sela, M.; et al. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res. 2000, 77, 25–79. [Google Scholar]
- Alroy, I.; Yarden, Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997, 410, 83–86. [Google Scholar] [CrossRef]
- Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell. 2000, 103, 211–225. [Google Scholar] [CrossRef]
- Yarden, Y. The EGFR family and its ligands in human cancer. Signaling mechanisms and therapeutic opportunities. Eur J Cancer. 2001, 37 (Suppl. S4), S3–8. [Google Scholar] [CrossRef]
- Castillo, L.; Etienne-Grimaldi, M.C.; Fischel, J.L.; et al. Pharmacological background of EGFR targeting. Ann Oncol. 2004, 15, 1007–1012. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001, 7, 2958–2970. [Google Scholar]
- Mayer, A.; Takimoto, M.; Fritz, E.; et al. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993, 71, 2454–2460. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, J.M.; Harper, ME. EGFR and cancer prognosis. Eur J Cancer. 2001, 37 (Suppl. S4), S9–S15. [Google Scholar] [CrossRef]
- Ciardiello, F. An update of new targets for cancer treatment: receptor-mediated signals. Ann Oncol. 2002, 13 (Suppl S4), 29–38. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Humblet, Y.; Siena, S.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecanrefractory metastatic colorectal cancer. N Engl J Med. 2004, 351, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Vuong, T.; Hayashi, S.; et al. A simple and reproducible scoring system for EGFR in colorectal cancer: application to prognosis and prediction of response to preoperative brachytherapy. Br J Cancer. 2007, 96, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.E.; Zorbas, M.A.; Danels, Y.J.; et al. Cellular growth response to epidermal growth factor in colon carcinoma cells with an amplified epidermal growth factor receptor derived from a familial adenomatous polyposis patient. Cancer Res. 1991, 51, 1452–1459. [Google Scholar]
- Radinsky, R. Modulation of tumour cell gene expression and phenotype by the organ-specific metastatic environment. Cancer Metastasis Rev. 1995, 14, 323–328. [Google Scholar] [CrossRef]
- Radinsky, R.; Risin, S.; Fan, D.; et al. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995, 1, 19–31. [Google Scholar]
- Prewett, M.C.; Hooper, A.T.; Bassi, R.; et al. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002, 8, 994–1003. [Google Scholar]
- Mendelsohn, J.; Baselga, J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003, 21, 2787–2799. [Google Scholar] [CrossRef]
- Mitchell, E.P.; Pérez–Soler, R.; Van Cutsem, E.; et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park). 2007, 21 (Suppl. S5), 4–9. [Google Scholar]
- Lacouture, M.E. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nature Rev Cancer. 2005, 6, 803–812. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Melosky, B.L. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007, 12, 1–5. [Google Scholar]
- Segaert, S.; Tabernero, J.; Chosidow, O.; et al. The management of skin reactions in cancer patients receiving epidermal growth factor receptor targeted therapies. J Dtsch Dermatol Ges. 2005, 3, 599–606. [Google Scholar] [CrossRef]
- Segaert, S.; Van Cutsem, E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005, 16, 1425–1433. [Google Scholar] [CrossRef]
- Folprecht, G.; Lutz, M.P.; Schöffski, P.; et al. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006, 17, 450–456. [Google Scholar] [CrossRef]
- Busam, K.J.; Capodieci, P.; Motzer, R.; et al. Cutaneous sideeffects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001, 144, 1169–1176. [Google Scholar] [CrossRef]
- Molinari, E.; De Quatrebarbes, J.; André, T.; et al. Cetuximabinduced acne. Dermatology. 2005, 211, 330–333. [Google Scholar] [CrossRef]
- Bouché, O.; Brixi-Benmansour, H.; Bertin, A.; et al. Trichomegaly of the eyelashes following treatment with cetuximab. Ann Oncol. 2005, 16, 1711–1712. [Google Scholar] [CrossRef]
- Boucher, K.W.; Davidson, K.; Mirakhur, B.; et al. Paronychia induced by cetuximab, an antiepidermal growth factor receptor antibody. J Am Acad Dermatol. 2002, 47, 632–633. [Google Scholar] [CrossRef]
- Grenader, T.; Gipps, M.; Goldberg, A. Staphylococcus aureus bacteremia secondary to severe erlotinib skin toxicity. Clin Lung Cancer. 2008, 9, 59–60. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.; Van Laethern, JL. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: where are we today? Oncologist. 2009, 14, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Meropol, N.J.; Loehrer, P.J., Sr; et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004, 22, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.; Koopman, M.; Rodenburg, C.J.; et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol. 2008, 19, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Mitchell, E.; Chidiac, T.; et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009, 27, 672–680. [Google Scholar] [CrossRef]
- Saltz, L.B.; Lenz, H.-J.; Kindler, H. L.; et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared With cetuximab and bevacizumab alone in irinotecanrefractory colorectal cancer: the BOND-2 study. Clin Oncol. 2007, 25, 4557–4561. [Google Scholar]
- Jacot, W.; Bessis, D.; Jorda, E.; et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol. 2004, 151, 238–241. [Google Scholar] [CrossRef]
- Bianchini, D.; Jayanth, A.; Chua, Y.J.; et al. Epidermal growth factor receptor inhibitor-related skin toxicity: mechanisms, treatment, and its potential role as a predictive marker. Clin Colorectal Cancer. 2008, 7, 33–43. [Google Scholar] [CrossRef]
- Mitchell, E.P.; LaCouture, M.; Shearer, H.; et al. Updated results of STEPP, a phase 2, open-label study of pre-emptive versus reactive skin toxicity treatment in metastatic colorectal cancer (mCRC) patients receiving panitumumab+FOLFIRI or irinotecan-only chemotherapy as second-line treatment. European Society for Medical Oncology (ESMO) International Symposium. 10th World Congress on Gastrointestinal Cancer; June 25–28, 2008; Barcelona, Spain. (Abstract O-021) Available online at: www.worldgicancer.com/WCGI/WGIC08/WGIC08_press- info_combined.pdf; cited December 15, 2008.
- Lacouture, M.E. Insights into the pathophysiology and management of dermatologic toxicities to EGFR-targeted therapies in colorectal cancer. Cancer Nurs. 2007, 30 (Suppl. S1), S17–S26. [Google Scholar] [CrossRef]
- Hidalgo, M.; Siu, L.L.; Nemunaitis, J.; et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001, 19, 3267–3279. [Google Scholar] [CrossRef]
- Foon, K.A.; Yang, X.D.; Weiner, L.M.; et al. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys. 2004, 58, 984–990. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Schwartz, G.H.; Gollob, J.A.; et al. Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol. 2004, 22, 3003–3015. [Google Scholar] [CrossRef]
- Kimyai-Asadi, A.; Jih, M.H. Follicular toxic effects of chimeric anti-epidermal growth factor receptor antibody cetuximab used to treat human solid tumors. Arch Dermatol. 2002, 138, 129–131. [Google Scholar] [CrossRef]
- Walon, L.; Gilbeau, C.; Lachapelle, JM. [Acneiform eruptions induced by cetuximab]. Ann Dermatol Venereol. 2003, 130, 443–446. [Google Scholar] [PubMed]
- Lenz, H.J.; Van Cutsem, E.; Khambata-Ford, S.; et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006, 24, 4914–4921. [Google Scholar] [CrossRef]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.S.; et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Siena, S.; Humblet, Y.; et al. An open-label, singlearm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol. 2008, 19, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nowacki, M.; Lang, I.; et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial. J Clin Oncol. 2007, 25, 4000. [Google Scholar] [CrossRef]
- Hecht, J.R.; Patnaik, A.; Berlin, J.; et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer. 2007, 110, 980–988. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Peeters, M.; Siena, S.; et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007, 25, 1658–1664. [Google Scholar] [CrossRef]
- Tejpar, S.; Peeters, M.; Humblet, Y.; et al. Phase I/II study of cetuximab dose-escalation in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST): Pharmacokinetic (PK), Pharmacodynamic (PD) and efficacy data. J Clin Oncol. 2007, 25, 4037. [Google Scholar] [CrossRef]
- Melosky, B.; Burkes, R.; Rayson, D.; et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009, 16, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Scope, A.; Agero, A.L.; Dusza, S.W.; et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007, 25, 5390–5396. [Google Scholar] [CrossRef]
- Mitchell, E.P.; LaCouture, M.E.; Shearer, H.; et al. A phase II, open-label trial of skin toxicity (ST) evaluation (STEPP) in metastatic colorectal cancer (mCRC) patients (pts) receiving panitumumab (pmab) + FOLFIRI or irinotecan-only chemotherapy (CT) as 2nd-line treatment (tx): Interim analysis. J Clin Oncol. 2008, 26, 15007. [Google Scholar] [CrossRef]
- Grothey, A. Recognizing and managing toxicities of molecular targeted therapies for colorectal cancer. Oncology (Williston Park). 2006, 20 (Suppl. S10), 21–28. [Google Scholar] [PubMed]
- Agero, A.L.; Dusza, S.W.; Benvenuto-Andrade, C.; et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006, 55, 657–670. [Google Scholar] [CrossRef]
- Perez-Soler, R. Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer 2006, 8 (Suppl. S1), S7–S14. [Google Scholar] [CrossRef] [PubMed]
- Porzio, G.; Aielli, F.; Verna, L.; et al. Efficacy of pregabalin in the management of cetuximab-related itch. J Pain Symptom Manage. 2006, 32, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J., Jr; Kim, E.S.; Eaby, B.; et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007, 12, 610–621. [Google Scholar] [CrossRef]
- Dick, F.E.; Crawford, G.H. Managing cutaneous side effects of epidermal growth factor receptor (HER1/EGFR) inhibitors. Commun Oncol. 2005, 2, 492–496. [Google Scholar] [CrossRef]
- Roé, E.; García Muret, M.P.; Marcuello, E.; et al. Description and management of cutaneous side effects during cetuximab or erlotinib treatments: a prospective study of 30 patients. J Am Acad Dermatol. 2006, 55, 429–437. [Google Scholar] [CrossRef]
- Chang, G.C.; Yang, T.Y.; Chen, K.C.; et al. Complications of therapy in cancer patients: Case 1. Paronychia and skin hyperpigmentation induced by gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004, 22, 4646–4648. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.T.; Kris, M.G.; Pao, W.; et al. Practical management of patients with non-small-cell lung cancer treated with gefitinib. J Clin Oncol. 2005, 23, 165–174. [Google Scholar] [CrossRef]
- Dainichi, T.; Tanaka, M.; Tsuruta, N.; et al. Development of multiple paronychia and periungual granulation in patients treated with gefitinib, an inhibitor of epidermal growth factor receptor. Dermatology. 2003, 207, 324–325. [Google Scholar] [CrossRef]
- Nakano, J.; Nakamura, M. Paronychia induced by gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Dermatol. 2003, 30, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Sauer, T.; Lund-Johansen, F.; et al. Epidermal growth factor receptor inhibition induces trichomegaly. Acta Oncol. 2003, 42, 345–346. [Google Scholar] [CrossRef]
- Pascual, J.C.; Bañuls, J.; Belinchon, I.; et al. Trichomegaly following treatment with gefitinib (ZD1839). Br J Dermatol. 2004, 151, 1111–1112. [Google Scholar] [CrossRef] [PubMed]
- Drelich, D.A.; Rose, L.; Ramirez, M.; et al. Dermatological toxicities of panitumumab in the treatment of patients with metastatic colorectal cancer (mCRC) from three clinical studies. J Clin Oncol 2007, 25 (Suppl. S18), 14551. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Basti, S.; Patel, J.; et al. The SERIES clinic: an interdisciplinary approach to the management of toxicities of EGFR inhibitors. J Support Oncol. 2006, 4, 236–238. [Google Scholar]
- Berger, B.; Belka, C. Severe skin reaction secondary to concomitant radiotherapy plus cetuximab. Radiat Oncol. 2008, 3, 5. [Google Scholar] [CrossRef]
- Bölke, E.; Gerber, P.A.; Lammering, G.; et al. Development and management of severe cutaneous side effects in head-and-neck cancer patients during concurrent radiotherapy and cetuximab. Strahlenther Onkol. 2008, 184, 105–110. [Google Scholar] [CrossRef]
- Azad, A. Severe cutaneous toxicity following treatment with radiotherapy and cetuximab: a case report. Cases J. 2009, 2, 25. [Google Scholar] [CrossRef]
- Giro, C.; Berger, B.; Bölke, E.; et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol. 2009, 90, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Pryor, D.I.; Porceddu, S.V.; Burmeister, B.H.; et al. Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother Oncol. 2009, 90, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Tonini, G.; Vincenzi, B.; Santini, D.; et al. Ocular toxicity related to cetuximab monotherapy in an advanced colorectal cancer patient. J Natl Cancer Inst. 2005, 97, 606–607. [Google Scholar] [CrossRef]
- Dranko, S.; Kinney, C.; Ramanathan, R.K. Ocular toxicity related to cetuximab monotherapy in patients with colorectal cancer. Clin Colorectal Cancer. 2006, 6, 224–225. [Google Scholar] [CrossRef]
- Erbitux (cetuximab) Product Monograph. Montreal, Quebec: Bristol-Myers Squibb Canada; 2009.
- Tejpar, S.; Piessevaux, H.; Claes, K.; et al. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol. 2007, 8, 387–394. [Google Scholar] [CrossRef]
- Sobrero, A.F.; Maurel, J.; Fehrenbacher, L.; et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008, 26, 2311–2319. [Google Scholar] [CrossRef]
- Jonker, D.J.; Karapetis, C.S.; Moore, M.; et al. Randomized phase III trial of cetuximab monotherapy plus best supportive care (BSC) versus BSC alone in patients with pretreated metastatic epidermal growth factor receptor (EGFR)-positive colorectal carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) and the Australasian Gastro-Intestinal Trials Group (AGITG) [abstract]. Presented at the American Association for Cancer Research Annual Meeting; April 14–18, 2007, Los Angeles, CA. In: Program and proceedings of the 2007 annual meeting of the American Association for Cancer Research. Philadelphia, PA: American Association for Cancer Research, 2007.
- Giusti, R.M.; Shastri, K.A.; Cohen, M.H.; et al. FDA drug approval summary: panitumumab (Vectibix). Oncologist. 2007, 12, 577–583. [Google Scholar] [CrossRef]
- Saif, M.W.; Cohenuram, M. Role of panitumumab in the management of metastatic colorectal cancer. Clin Colorectal Cancer. 2006, 6, 118–124. [Google Scholar] [CrossRef]
- Saif, M.W. Management of hypomagnesemia in cancer patients receiving chemotherapy. J Support Oncol. 2008, 6, 243–248. [Google Scholar]
- Schrag, D.; Chung, K.Y.; Flombaum, C.; et al. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. 2005, 97, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Peeters, M.; Siena, S.; et al. Panitumumab significantly improves progression-free survival in patients with metastatic colorectal cancer. Ann Oncol 2006, 17, vi19–vi27. [Google Scholar]
- Fakih, M.G.; Wilding, G.; Lombardo, J. Cetuximab-induced hypomagnesemia in patients with colorectal cancer. Clin Colorectal Cancer. 2006, 6, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Cho, C.D.; Halsey, J.; et al. Phase II study of gefitinib, fluorouracil, leucovorin, and oxaliplatin therapy in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2005, 23, 5613–5619. [Google Scholar] [CrossRef]
- Iannello, S.; Belfiore, F. Hypomagnesemia. A review of pathophysiological, clinical and therapeutical aspects. Panminerva Med. 2001, 43, 177–209. [Google Scholar]
- Fakih, M. Management of anti-EGFR-targeting monoclonal antibody-induced hypomagnesemia. Oncology (Williston Park). 2008, 22, 74–76. [Google Scholar]
- Berlin, J.; Posey, J.; Tchekmedyian, S.; et al. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin Colorectal Cancer. 2007, 6, 427–432. [Google Scholar] [CrossRef]
- Held-Warmkessel, J. Camp-Sorrell, D., Hawkins, R.A., Eds.; Diarrhea. In Clinical manual for the oncology advanced practice nurse, 2nd ed.; Oncology Nursing Society: Pittsburgh, PA, USA, 2006; pp. 425–433. [Google Scholar]
- Maroun, J.A.; Anthony, L.B.; Blais, N.; et al. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Curr Oncol. 2007, 14, 13–20. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Allen, R.; Spigel, D.R.; et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007, 25, 3644–3648. [Google Scholar] [CrossRef]
- Needle, M.N. Safety experience with IMC-C225, an anti-epidermal growth factor receptor antibody. Semin Oncol. 2002, 29 (Suppl S14), 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Mirakhur, B.; Chan, E.; et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008, 358, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.L.; Pfeiffer, P.; Jensen, B.V. Re-treatment with cetuximab in patients with severe hypersensitivity reactions to cetuximab. Two case reports. Acta Oncol. 2006, 45, 1137–1138. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Glynne-Jones, R.; Thaler, J.; et al. Infusion-related reactions (IRR) associated with cetuximab plus irinotecan treatment in patients with irinotecan-resistant metastatic colorectal cancer (mCRC): Findings from the MABEL study. J Clin Oncol. 2007, 25, 4137. [Google Scholar] [CrossRef]
- Saif, M.W.; Syrigos, K.I.; Hotchkiss, S.; et al. Successful desensitization with cetuximab after an infusion reaction to panitumumab in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2009, 65, 107–112. [Google Scholar] [CrossRef]
- Saif, M.W.; Peccerillo, J.; Potter, V. Successful re-challenge with panitumumab in patients who developed hypersensitivity reactions to cetuximab: report of three cases and review of literature. Cancer Chemother Pharmacol. 2009, 63, 1017–1022. [Google Scholar] [CrossRef]

 |
 |
 |
 |
 |
© 2008 by the author. Multimed Inc.
Share and Cite
Fakih, M.; Vincent, M. Adverse Events Associated with Anti-EGFR Therapies for the Treatment of Metastatic Colorectal Cancer. Curr. Oncol. 2010, 17, 18-30. https://doi.org/10.3747/co.v17is1.615
Fakih M, Vincent M. Adverse Events Associated with Anti-EGFR Therapies for the Treatment of Metastatic Colorectal Cancer. Current Oncology. 2010; 17(s1):18-30. https://doi.org/10.3747/co.v17is1.615
Chicago/Turabian StyleFakih, M., and M. Vincent. 2010. "Adverse Events Associated with Anti-EGFR Therapies for the Treatment of Metastatic Colorectal Cancer" Current Oncology 17, no. s1: 18-30. https://doi.org/10.3747/co.v17is1.615
APA StyleFakih, M., & Vincent, M. (2010). Adverse Events Associated with Anti-EGFR Therapies for the Treatment of Metastatic Colorectal Cancer. Current Oncology, 17(s1), 18-30. https://doi.org/10.3747/co.v17is1.615




