The Predictive Value of Pre-treatment Inflammatory Markers in Advanced Non-small-Cell Lung Cancer
Abstract
:1. INTRODUCTION
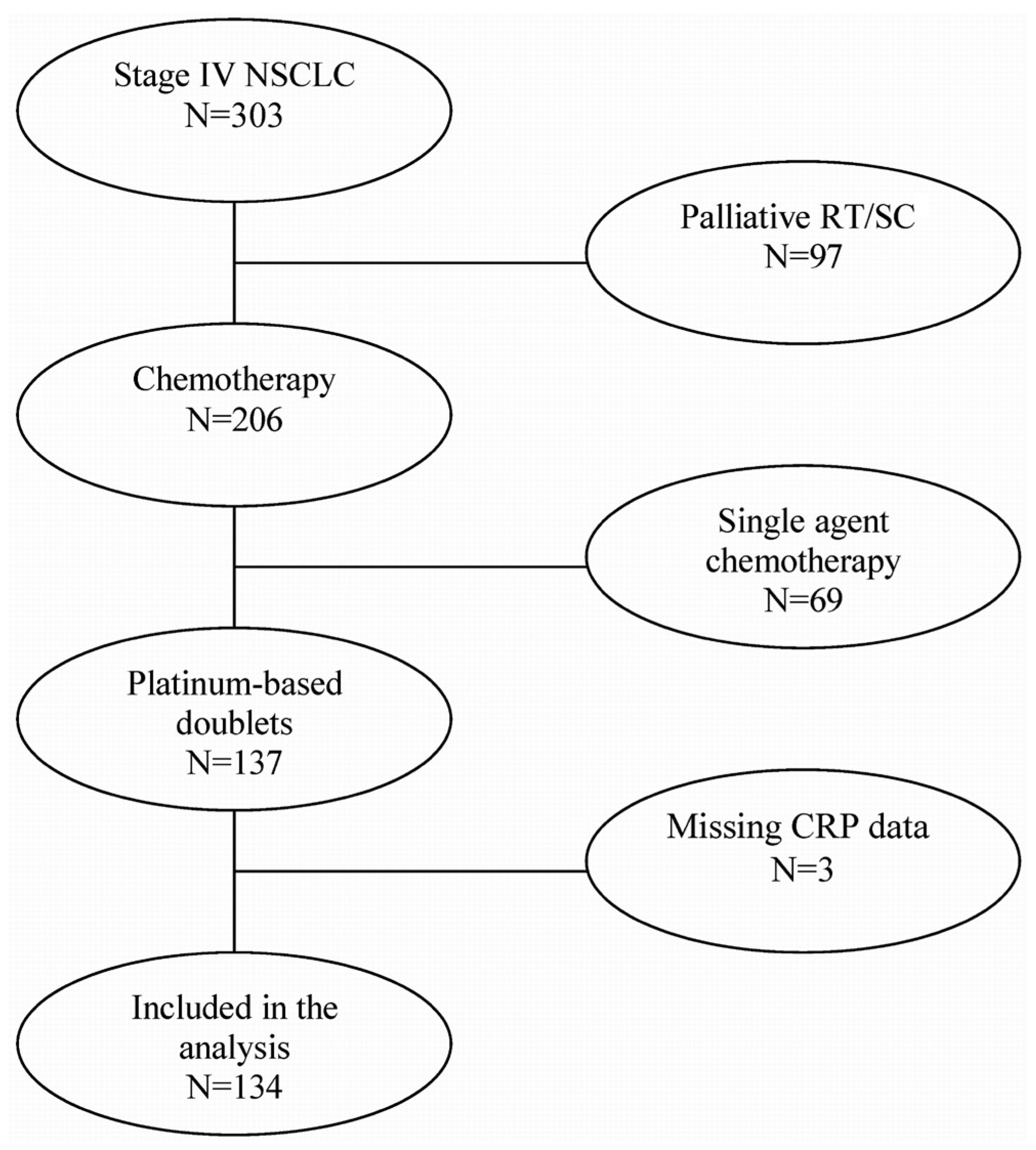
2. PATIENTS AND METHODS
2.1. Participant Identification
2.2. Prognostic Index
- PI 0 for CRP 10 mg/L or less and WBCs 11×109/L or less,
- PI 1 if one of the two markers was elevated, and
- PI 2 if both markers were elevated.
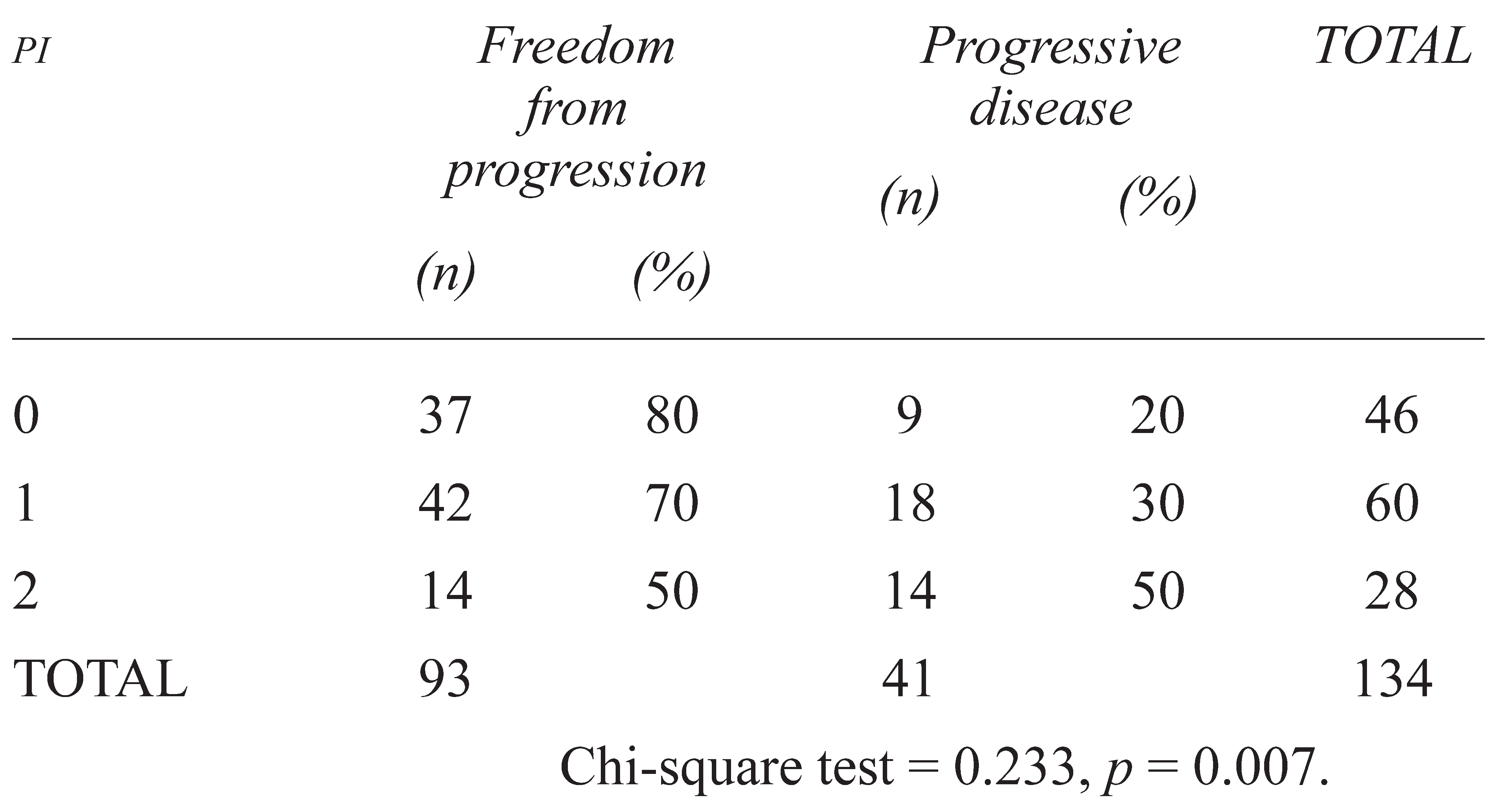
2.3. Response to Chemotherapy
2.4. Statistical Analysis
3. RESULTS
4. DISCUSSION
5. CONCLUSIONS
Acknowledgments
Conflicts of Interest
References
- Brundage, M.D.; Davies, D.; Mackillop, W.J. Prognostic factors in non-small cell lung cancer: A decade of progress. Chest 2002, 122, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Tesniere, A.; Ghiringhelli, F.; Kroemer, G.; Zitvogel, L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 2008, 68, 4026–4030. [Google Scholar] [CrossRef]
- Deans, C.; Rose-Zerilli, M.; Wigmore, S.; et al. Host cytokine genotype is related to adverse prognosis and systemic inflammation in gastro-oesophageal cancer. Ann Surg Oncol 2007, 14, 329–339. [Google Scholar] [CrossRef]
- Hilmy, M.; Campbell, R.; Bartlett, J.M.; McNicol, A.M.; Underwood, M.A.; McMillan, D.C. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer 2006, 95, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Watine, J. Comments on: Haemostatic abnormalities in lung cancer: Prognostic implications, Buccheri et al., Eur J Cancer, 33, pp. 50–55, 1997. Eur J Cancer 1998, 34, 430. [Google Scholar]
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin Cancer Res 2009, 15, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, M.; Ayzac, L.; Ingenbleek, Y.; Kostka, T.; Boisson, R.C.; Bienvenu, J. Usefulness of the prognostic inflammatory and nutritional index (PINI) in hospitalized elderly patients. Int J Vitam Nutr Res 1998, 68, 189–195. [Google Scholar]
- Brown, D.J.; Milroy, R.; Preston, T.; McMillan, D.C. The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol 2007, 60, 705–708. [Google Scholar] [CrossRef]
- Casamassima, A.; Picciariello, M.; Quaranta, M.; et al. C-Reactive protein: A biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 base immunotherapy. J Urol 2005, 173, 52–55. [Google Scholar] [CrossRef]
- Clinchy, B.; Fransson, A.; Druvefors, B.; et al. Preoperative interleukin-6 production by mononuclear blood cells predicts survival after radical surgery for colorectal carcinoma. Cancer 2007, 109, 1742–1749. [Google Scholar] [CrossRef]
- Kelly, L.; White, S.; Stone, P.C. The B12/CRP index as a simple prognostic indicator in patients with advanced cancer: A confirmatory study. Ann Oncol 2007, 18, 1395–1399. [Google Scholar] [CrossRef]
- MacDonald, N.; Kasymjanova, G.; Dobson, S.; et al. Prognostic value of baseline inflammatory markers in inoperable non-small cell lung cancer (NSCLC) [abstract 17035]. Proc Am Soc Clin Oncol 2006, 24. Available online: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=31987 (accessed on 10 June 2010).
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; André, F.; Tesniere, A.; Kroemer, G. The anticancer immune response: Indispensable for therapeutic success? J Clin Invest 2008, 118, 1991–2001. [Google Scholar] [CrossRef]
- McKeown, D.J.; Brown, D.J.; Kelly, A.; Wallace, A.M.; McMillan, D.C. The relationship between circulating concentrations of C-reactive protein, inflammatory cytokines and cytokine receptors in patients with non-small-cell lung cancer. Br J Cancer 2004, 91, 1993–1995. [Google Scholar] [CrossRef]
- Heikkila, K.; Harris, R.; Lowe, G.; et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: Findings from two prospective cohorts and a meta-analysis. Cancer Causes Control 2009, 20, 15–26. [Google Scholar] [CrossRef]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef]
- Koch, A.; Fohlin, H.; Sorenson, S. Prognostic significance of C-reactive protein and smoking in patients with advanced non-small cell lung cancer treated with first-line palliative chemotherapy. J Thorac Oncol 2009, 4, 326–332. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Schifreen, R.S.; Gorman, E.; Tuhy, P.M.; Bienvenu, J.; Warkentin, D.L. Development and performance of a fully automated method for assay of C-reactive protein in the aca discrete clinical analyzer. Clin Chem 1988, 34, 1646–1649. [Google Scholar] [CrossRef]
- Jewish General Hospital, Department of Diagnostic Medicine. Normal Reference Range Table; Jewish General Hospital: Montreal, QC, 2008. [Google Scholar]
- Julka, P.K.; Doval, D.C.; Gupta, S.; Rath, G.K. Response assessment in solid tumours: A comparison of WHO, SWOG and RECIST guidelines. Br J Radiol 2008, 81, 444–449. [Google Scholar] [CrossRef]
- Armaiz–Pena, G.N.; Lutgendorf, S.K.; Cole, S.W.; Sood, A.K. Neuroendocrine modulation of cancer progression. Brain Behav Immun 2009, 23, 10–15. [Google Scholar] [CrossRef]
- Apetoh, L.; Obeid, M.; Tesniere, A.; et al. Immunogenic chemotherapy: Discovery of a critical protein through proteomic analyses of tumor cells. Cancer Genomics Proteomics 2007, 4, 65–70. [Google Scholar]
- Ullrich, E.; Ménard, C.; Flament, C.; et al. Dendritic cells and innate defense against tumor cells. Cytokine Growth Factor Rev 2008, 19, 79–92. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 7, 1073–1081. [Google Scholar] [CrossRef]
- Geissbühler, P.; Mermillod, B.; Rapin, C.H. Elevated serum vitamin B12 levels associated with CRP as a predictive factor of mortality in palliative care cancer patients: A prospective study over five years. J Pain Symptom Manage 2000, 20, 93–103. [Google Scholar] [CrossRef]
- Guillem, P.; Triboulet, J.P. Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus 2005, 18, 146–150. [Google Scholar] [CrossRef]
- Karakiewicz, P.I.; Hutterer, G.C.; Trinh, Q.D.; et al. C-Reactive protein is an informative predictor of renal cell carcinoma– specific mortality. Cancer 2007, 110, 1241–1247. [Google Scholar] [CrossRef]
- Graff, J.; Lalani, A.S.; Lee, S.; et al. C-Reactive protein as a prognostic marker for men with androgen-independent prostate cancer (AIPC): Results from the ASCENT trial [abstract 5074]. Proc Am Soc Clin Oncol 2007, 25. Available online: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=47&abstractID=31092 (accessed on 10 June 2010).
- Suh, S.Y.; Ahn, H.Y. A prospective study on C-reactive protein as a prognostic factor for survival time of terminally ill cancer patients. Support Care Cancer 2007, 15, 613–620. [Google Scholar] [CrossRef]
- Hara, M.; Matsuzaki, Y.; Shimuzu, T.; et al. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer Res 2007, 27, 3001–3004. [Google Scholar]
- Siemes, C.; Visser, L.E.; Coebergh, J.W.; et al. C-Reactive protein levels, variation in the C-reactive protein gene, and cancer risk: The Rotterdam Study. J Clin Oncol 2006, 24, 5216–5222. [Google Scholar] [CrossRef]
- Du Clos, T.W.; Mold, C. C-Reactive protein: An activator of innate immunity and a modulator of adaptive immunity. Immunol Res 2004, 30, 261–277. [Google Scholar] [CrossRef]
- Peisajovich, A.; Marnell, L.; Mold, C.; Du Clos, T.W. C-Reactive protein at the interface between innate immunity and inflammation. Expert Rev Clin Immunol 2008, 4, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Panek, B.; Chyczewska, E.; Ossolińska, M.; Naumnik, W.; Izycki–Herman, T.; Korniluk, M. Evaluation of some functions of polymorphonuclear granulocytes in lung cancer patients during chemotherapy [Polish]. Pneumonol Alergol Pol 2005, 73, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Teramukai, S.; Kitano, T.; Kishida, Y.; et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: An analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 2009, 5, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, C.; Vasile, E.; Bernardini, I.; Orlandini, C.; Andreuccetti, M.; Falcone, A. Baseline elevated leukocyte count in peripheral blood is associated with poor survival in patients with advanced non-small cell lung cancer: A prognostic model. J Cancer Res Clin Oncol 2008, 134, 1143–1149. [Google Scholar] [CrossRef]
- Kato, K.; Hitsuda, Y.; Kawasaki, Y.; et al. The value of serum C-reactive protein as a survival determinant in patients with advanced non-small-cell lung cancer [Japanese]. Nihon Kokyuki Gakkai Zasshi 2000, 38, 575–580. [Google Scholar]
- Mignot, G.; Ullrich, E.; Bonmort, M.; et al. The critical role of IL-15 in the antitumour effects mediated by the combination therapy imatinib and IL-2. J Immunol 2008, 180, 6477–6483. [Google Scholar] [CrossRef]
- MacDonald, N. Cancer cachexia and targeting chronic inflammation: A unified approach to cancer treatment and palliative/supportive care. J Support Oncol 2007, 5, 157–162. [Google Scholar]
- Wilop, S.; Crysandt, M.; Bendel, M.; Mahnken, A.H.; Osieka, R.; Jost, E. Correlation of C-reactive protein with survival and radiographic response to first-line platinum-based chemotherapy in advanced non-small cell lung cancer. Onkologie 2008, 31, 665–670. [Google Scholar] [CrossRef]
- Beer, T.M.; Ryan, C.W.; Venner, P.M.; et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: A report from the ASCENT investigators. J Clin Oncol 2007, 25, 669–674. [Google Scholar] [CrossRef]
- Qin, T.J.; An, G.L.; Zhao, X.H.; et al. Combined treatment of oxaliplatin and capecitabine in patients with metastatic esophageal squamous cell cancer. World J Gastroenterol 2009, 15, 871–876. [Google Scholar] [CrossRef]
- Al Murri, A.M.; Bartlett, J.M.; Canney, P.A.; Doughty, J.C.; Wilson, C.; McMillan, D.C. C. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer 2006, 94, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dagg, K.; Scott, H.R. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer 2005, 92, 1834–1836. [Google Scholar] [CrossRef]
- Ackerman, W.E., 3rd; Zhang, J.M. Serum hs-CRP as a useful marker for predicting the efficacy of lumbar epidural steroid injections on pain relief in patients with lumbar disc herniations. J Ky Med Assoc 2006, 104, 295–299. [Google Scholar] [PubMed]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer 2004, 90, 1704–1706. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Chaput, N.; Ménard, C.; et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med 2006, 12, 214–219. [Google Scholar] [CrossRef]
- Bisoendial, R.J.; Birjmohun, R.S.; Akdim, F.; et al. C-Reactive protein elicits white blood cell activation in humans. Am J Med 2009, 122, 582.e1–582.e9. [Google Scholar] [CrossRef]
- Assenat, E.; Gerbal–Chaloin, S.; Maurel, P.; Vilarem, M.J.; Pascussi, J.M. Is nuclear factor kappa-Β the missing link between inflammation, cancer and alteration in hepatic drug metabolism in patients with cancer? Eur J Cancer 2006, 42, 785–792. [Google Scholar] [CrossRef]
- Robertson, G.R.; Liddle, C.; Clarke, S.J. Inflammation and altered drug clearance in cancer: Transcriptional repression of a human CYP3A4 transgene in tumor-bearing mice. Clin Pharmacol Ther 2008, 83, 894–897. [Google Scholar] [CrossRef]
- Kacevska, M.; Robertson, G.R.; Clarke, S.J.; Liddle, C. Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: Impact and implications for chemotherapeutic drug dosing. Expert Opin Drug Metab Toxicol 2008, 4, 137–149. [Google Scholar] [CrossRef]
- Sharma, R.; Kacevska, M.; London, R.; Clarke, S.J.; Liddle, C.; Robertson, G. Downregulation of drug transport and metabolism in mice bearing extra-hepatic malignancies. Br J Cancer 2008, 98, 91–97. [Google Scholar] [CrossRef]

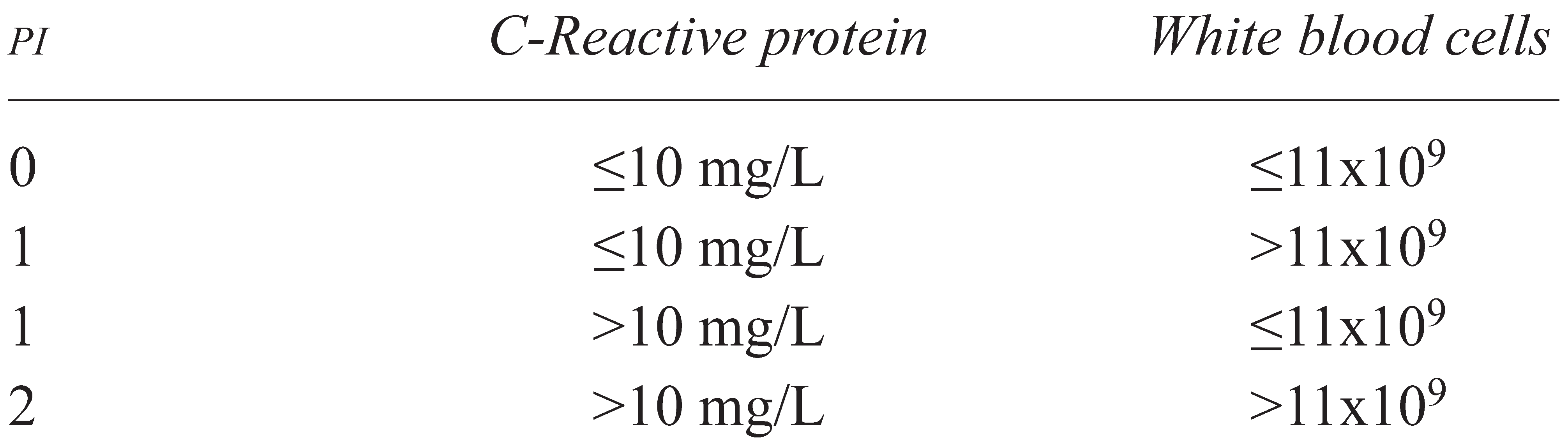 |
 |
 |
 |
 |
© 2008 by the author. Multimed Inc.
Share and Cite
Kasymjanova, G.; MacDonald, N.; Agulnik, J.S.; Cohen, V.; Pepe, C.; Kreisman, H.; Sharma, R.; Small, D. The Predictive Value of Pre-treatment Inflammatory Markers in Advanced Non-small-Cell Lung Cancer. Curr. Oncol. 2010, 17, 52-58. https://doi.org/10.3747/co.v17i4.567
Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, Kreisman H, Sharma R, Small D. The Predictive Value of Pre-treatment Inflammatory Markers in Advanced Non-small-Cell Lung Cancer. Current Oncology. 2010; 17(4):52-58. https://doi.org/10.3747/co.v17i4.567
Chicago/Turabian StyleKasymjanova, G., N. MacDonald, J. S. Agulnik, V. Cohen, C. Pepe, H. Kreisman, R. Sharma, and D. Small. 2010. "The Predictive Value of Pre-treatment Inflammatory Markers in Advanced Non-small-Cell Lung Cancer" Current Oncology 17, no. 4: 52-58. https://doi.org/10.3747/co.v17i4.567
APA StyleKasymjanova, G., MacDonald, N., Agulnik, J. S., Cohen, V., Pepe, C., Kreisman, H., Sharma, R., & Small, D. (2010). The Predictive Value of Pre-treatment Inflammatory Markers in Advanced Non-small-Cell Lung Cancer. Current Oncology, 17(4), 52-58. https://doi.org/10.3747/co.v17i4.567





