Biochemotherapy for the Treatment of Metastatic Malignant Melanoma: A Clinical Practice Guideline
Abstract
:1. QUESTIONS
- What is the role of biochemotherapy in the treatment of metastatic malignant melanoma?
- What are the adverse effects and effects on quality of life of biochemotherapy as a treatment option?
2. CHOICE OF TOPIC AND RATIONALE
3. METHODS
3.1. Guideline Development
3.2. Literature Search Strategy
4. RESULTS
5. DSG CONSENSUS PROCESS
6. REPORT APPROVAL PANEL
6.1. Results
- Was there consistency in the biochemotherapy regimens tested in the phase II trials that led to the reported phase III trials?
- Might variation in the response rates across the reported trials be related to different tumour response evaluation criteria?
- Was it appropriate to use 12-month mortality data for the meta-analysis, when the report Introduction indicates that median survival for this patient group is 6–8 months?
6.2. Modifications/Actions
- acknowledged that an optimum regimen had not been identified for biochemotherapy, and that the regimens used in the phase II and phase III trials had varied, generally corresponding with institutional or organizational preferences.
- indicated that the criteria used to define tumour response in most trials were those of the World Health Organization (or a similar definition) and added a brief statement to the Trial Descriptions section of the systematic review to summarize those data.
- agreed that 11–12 months was a reasonable time point for data pooling in meta-analysis, because the median survival for most of the reported trials fell close to that range. In addition, the pooled 6-month survival data showed a result similar to that obtained at 12 months, and that finding was indicated in the Results section of the report.
- Finally, although the PEBC guidelines are considered in policy determination, the authors considered that their main purpose was to provide guidance for clinicians, and they therefore did not wish to comment on policy-determining outcomes. In developing the recommendations, all relevant outcomes were considered, and the DSG felt that the current wording of the recommendation accurately reflected that fact. The recommendation was therefore not revised.
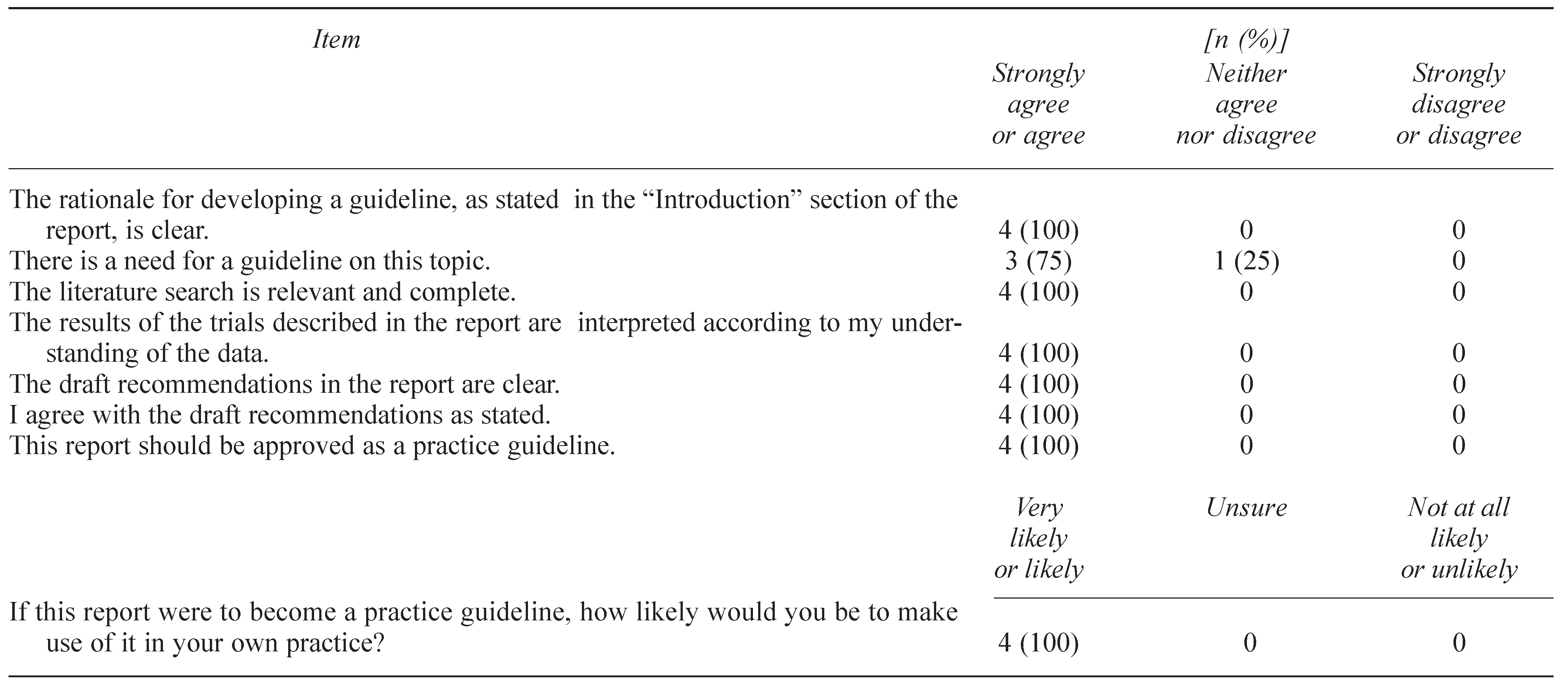
7. PRACTITIONER FEEDBACK
7.1. Methods
7.2. Results
7.3. Summary of Written Comments
8. PRACTICE GUIDELINE
8.1. Target Population
8.2. Recommendations
9. PRACTICE GUIDELINE DATE
References
- Canadian Cancer Society and the National Cancer Institute of Canada. Canadian Cancer Statistics 2006; Canadian Cancer Society: Toronto, ON, Canada, 2006. [Google Scholar]
- Browman, G.P.; Levine, M.N.; Mohide, E.A.; et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol 1995, 13, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Petrella, T.; Quirt, I.; Verma, S.; Haynes, A.E.; Charette, M.; Bak, K. on behalf of the Melanoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev 2007, 33, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lee, S.; Flaherty, L.E.; Sosman, J.A.; Sondak, V.K.; Kirkwood, J.M. A prospective randomized phase III trial of concurrent biochemotherapy (BCT) with cisplatin, vinblastine, dacarbazine (CVD), IL-2 and interferon alpha-2b (IFN) versus CVD alone in patients with metastatic melanoma (E3695): an ECOGcoordinated Intergroup trial [abstract 2847]. Proc Am Soc Clin Oncol 2003, 21. [Available online at: www.asco.org/ASCO/ Abstracts+%26+Virtual+Meeting/Abstracts?&vmview= abst_detail_view&confID=23&abstractID=101244;cited March 4, 2008].
- Bajetta, E.; Del, V.M.; Nova, P.; et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-α2b in metastatic melanoma. Ann Oncol 2006, 17, 571–577. [Google Scholar] [PubMed]
- Atzpodien, J.; Neuber, K.; Kamanabrou, D.; et al. IL-2 and IFN-α: results of a prospectively randomized trial of the Cooperative Advanced Malignant Melanoma Chemoimmunotherapy Group (ACIMM). Br J Cancer 2002, 86, 179–184. [Google Scholar] [PubMed]
- Ridolfi, R.; Chiarion–Sileni, V.; Guida, M.; et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol 2002, 20, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Eton, O.; Legha, S.S.; Bedikian, A.Y.; et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol 2002, 20, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Schwartzentruber, D.J.; Hwu, P.; Marincola, F.M.; Topalian, S.L. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol 1999, 17, 968–975. [Google Scholar] [PubMed]
- Keilholz, U.; Punt, C.J.; Gore, M.; et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol 2005, 23, 6747–6755. [Google Scholar] [PubMed]
- Hauschild, A.; Garbe, C.; Stolz, W.; et al. Dacarbazine and interferon alpha with or without interleukin 2 in metastatic melanoma: a randomized phase III multicentre trial of the Dermatologic Cooperative Oncology Group (DeCOG). Br J Cancer 2001, 84, 1036–1042. [Google Scholar] [PubMed]
- Keilholz, U.; Goey, S.H.; Punt, C.J.A.; Proebstle, T.M.; Salzmann, R.; Scheibenbogen, C. Interferon alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: a randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol 1997, 15, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lee, S.; Flaherty, L.E.; Sosman, J.A.; Sondak, V.K.; Kirkwood, J.M. A prospective randomized phase III trial of concurrent biochemotherapy (BCT) with cisplatin, vinblastine, dacarbazine (CVD), IL-2 and interferon alpha-2b (IFN) versus CVD alone in patients with metastatic melanoma (E3695): an ECOGcoordinated Intergroup trial [slides]. 2003. [Available online (use the “Slides” control)at: www.asco.org/portal/site/ASCO/menuitem.34d60f5624ba07fd506fe310ee37a01d/?vgnextoid= 76f8201eb61a7010VgnVCM100000ed730ad1RCRD&vm view=abst_detail_view&confID=23&abstractID=101244; cited January 9, 2007].
- Sasse, A.; Sasse, E.; Clark, L.; Ulloa, L.; Clark, O. Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database Syst Rev 2007, CD005413. [Google Scholar] [CrossRef] [PubMed]
- Chiarion–Sileni, V.; Del Bianco, P.; De Salvo, G.L.; et al. Quality of life evaluation in a randomised trial of chemotherapy versus bio-chemotherapy in advanced melanoma patients. Eur J Cancer 2003, 39, 1577–1585. [Google Scholar] [PubMed]
© 2008 by the author. Multimed Inc.
Share and Cite
Verma, S.; Petrella, T.; Hamm, C.; Bak, K.; Charette, M.; the members of the Melanoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Biochemotherapy for the Treatment of Metastatic Malignant Melanoma: A Clinical Practice Guideline. Curr. Oncol. 2008, 15, 85-89. https://doi.org/10.3747/co.v15i2.173
Verma S, Petrella T, Hamm C, Bak K, Charette M, the members of the Melanoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Biochemotherapy for the Treatment of Metastatic Malignant Melanoma: A Clinical Practice Guideline. Current Oncology. 2008; 15(2):85-89. https://doi.org/10.3747/co.v15i2.173
Chicago/Turabian StyleVerma, S., T. Petrella, C. Hamm, K. Bak, M. Charette, and the members of the Melanoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. 2008. "Biochemotherapy for the Treatment of Metastatic Malignant Melanoma: A Clinical Practice Guideline" Current Oncology 15, no. 2: 85-89. https://doi.org/10.3747/co.v15i2.173
APA StyleVerma, S., Petrella, T., Hamm, C., Bak, K., Charette, M., & the members of the Melanoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. (2008). Biochemotherapy for the Treatment of Metastatic Malignant Melanoma: A Clinical Practice Guideline. Current Oncology, 15(2), 85-89. https://doi.org/10.3747/co.v15i2.173



