A Decade of Progress on MAO-Treated Tantalum Surfaces: Advances and Contributions for Biomedical Applications
Abstract
:1. Introduction
2. Understanding the Principles of the MAO Process onto Ta Surfaces
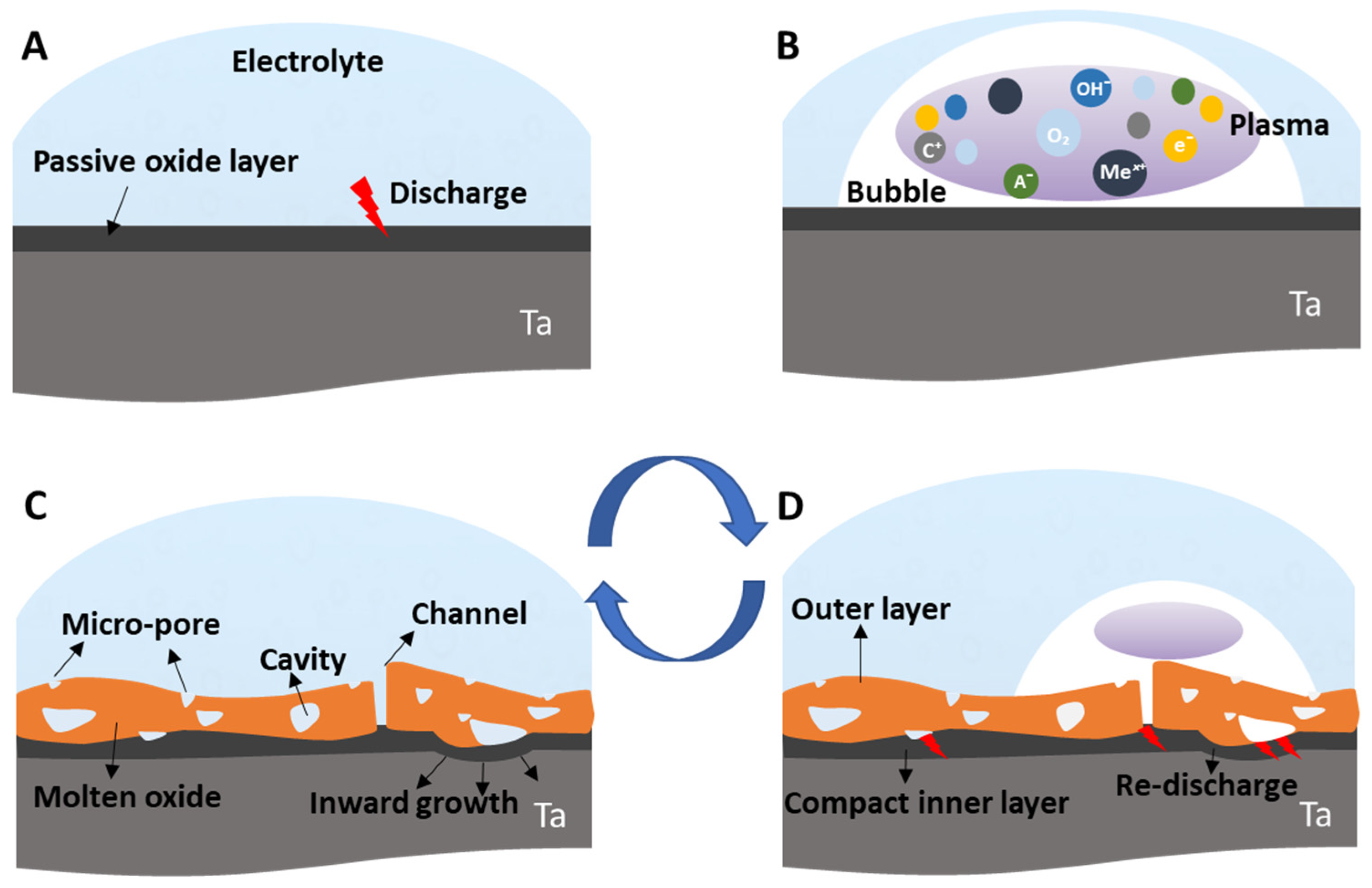
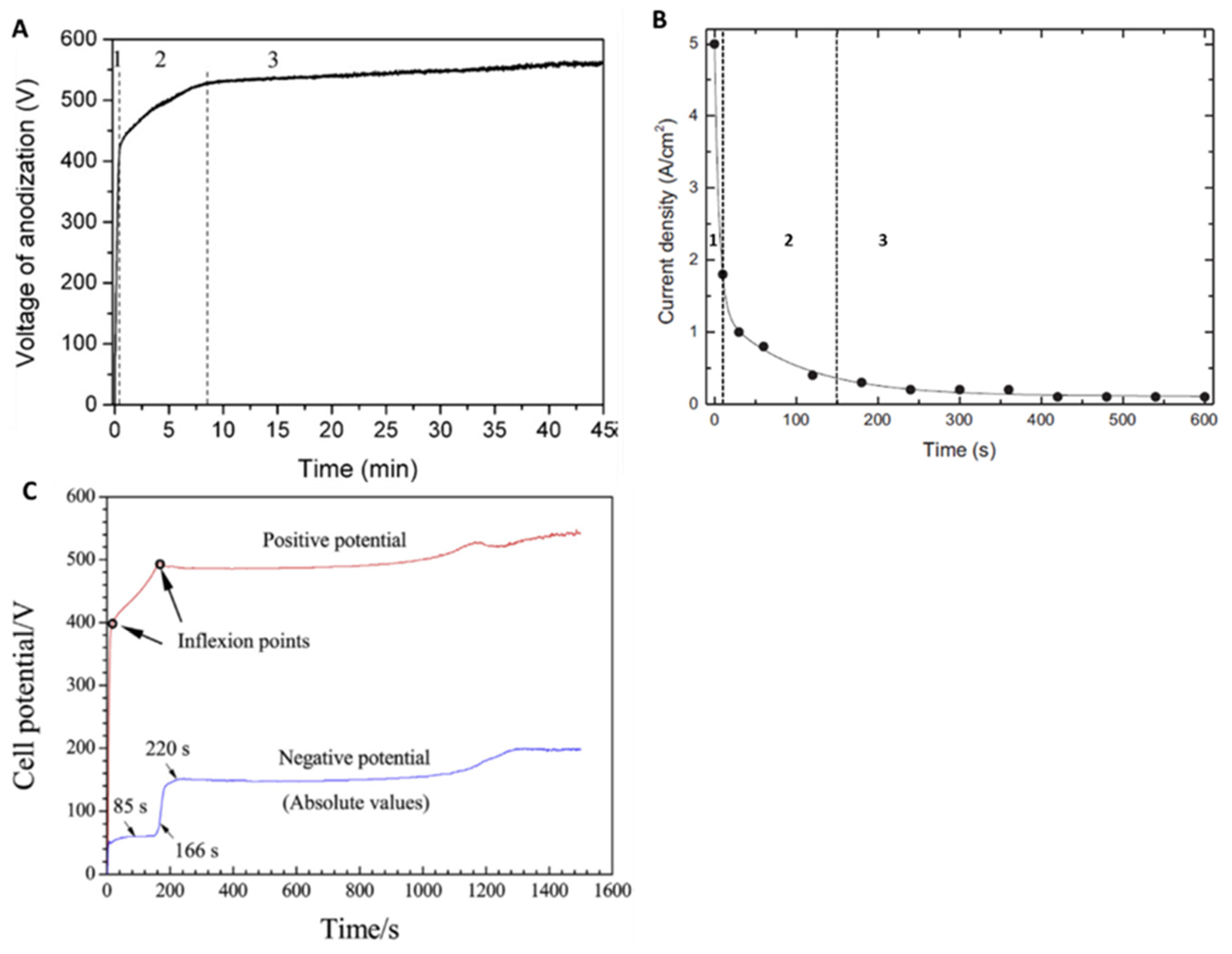
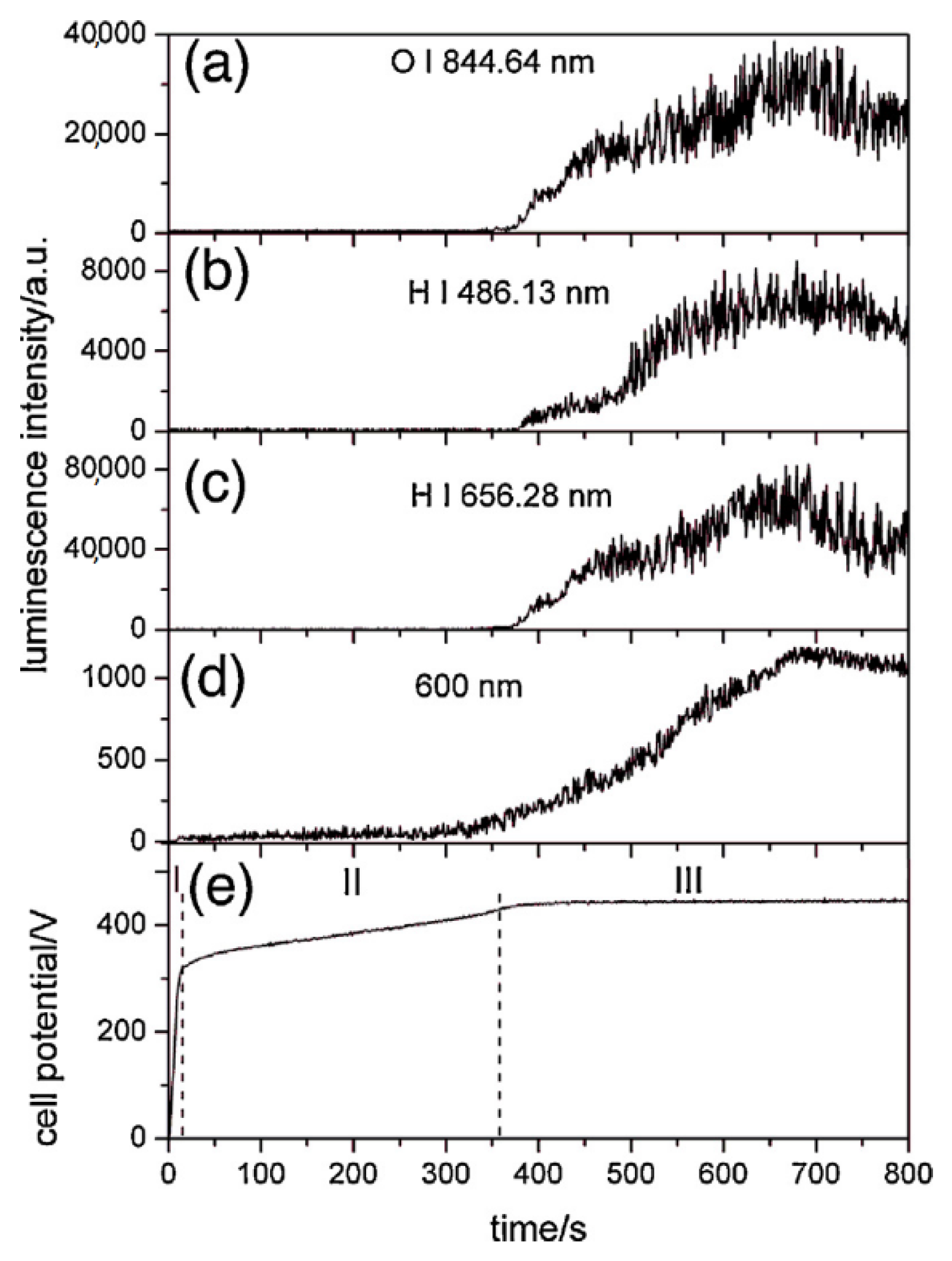
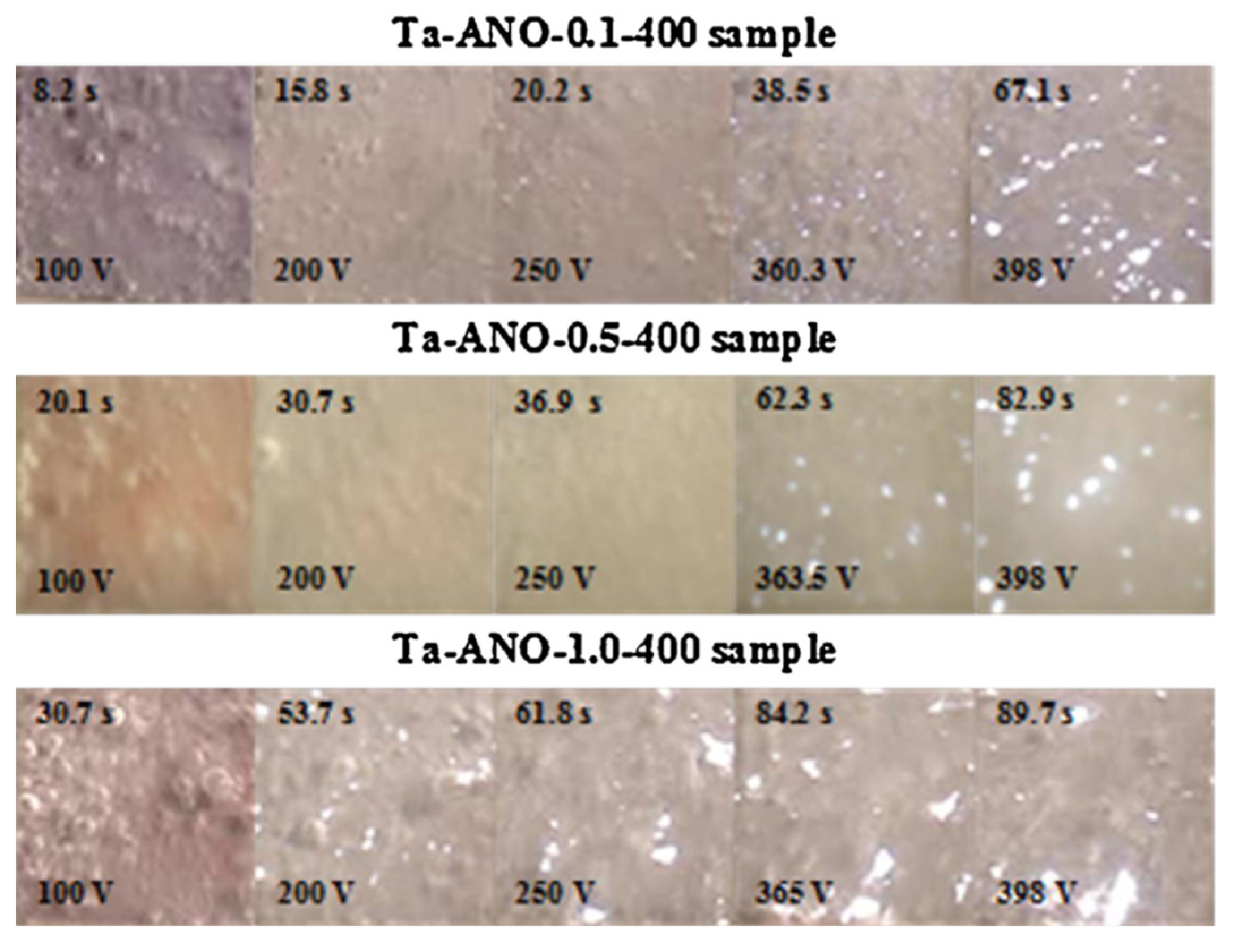
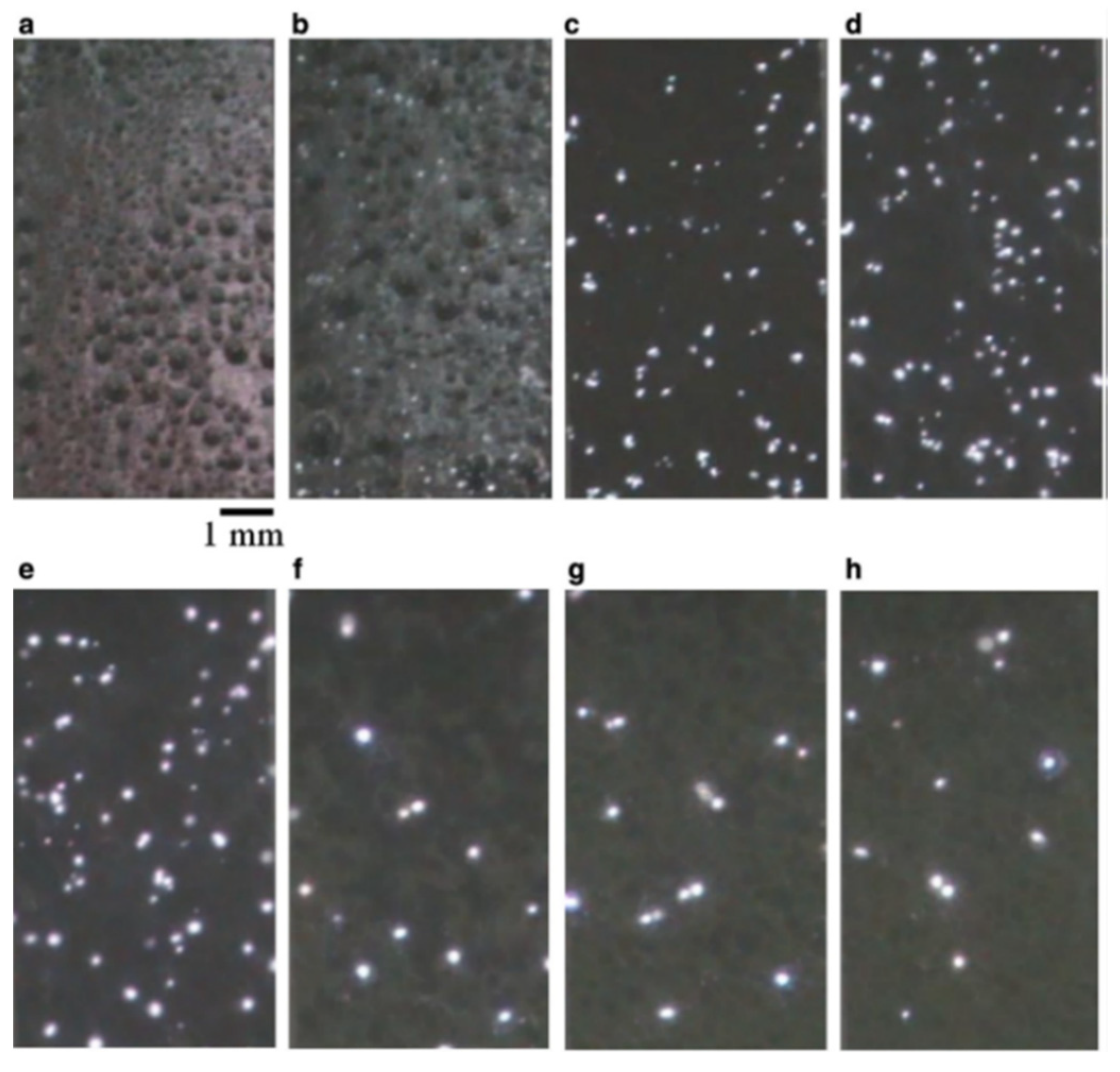
2.1. Transient Plasma Discharge Mechanism
2.2. Gas Evolution Phenomena
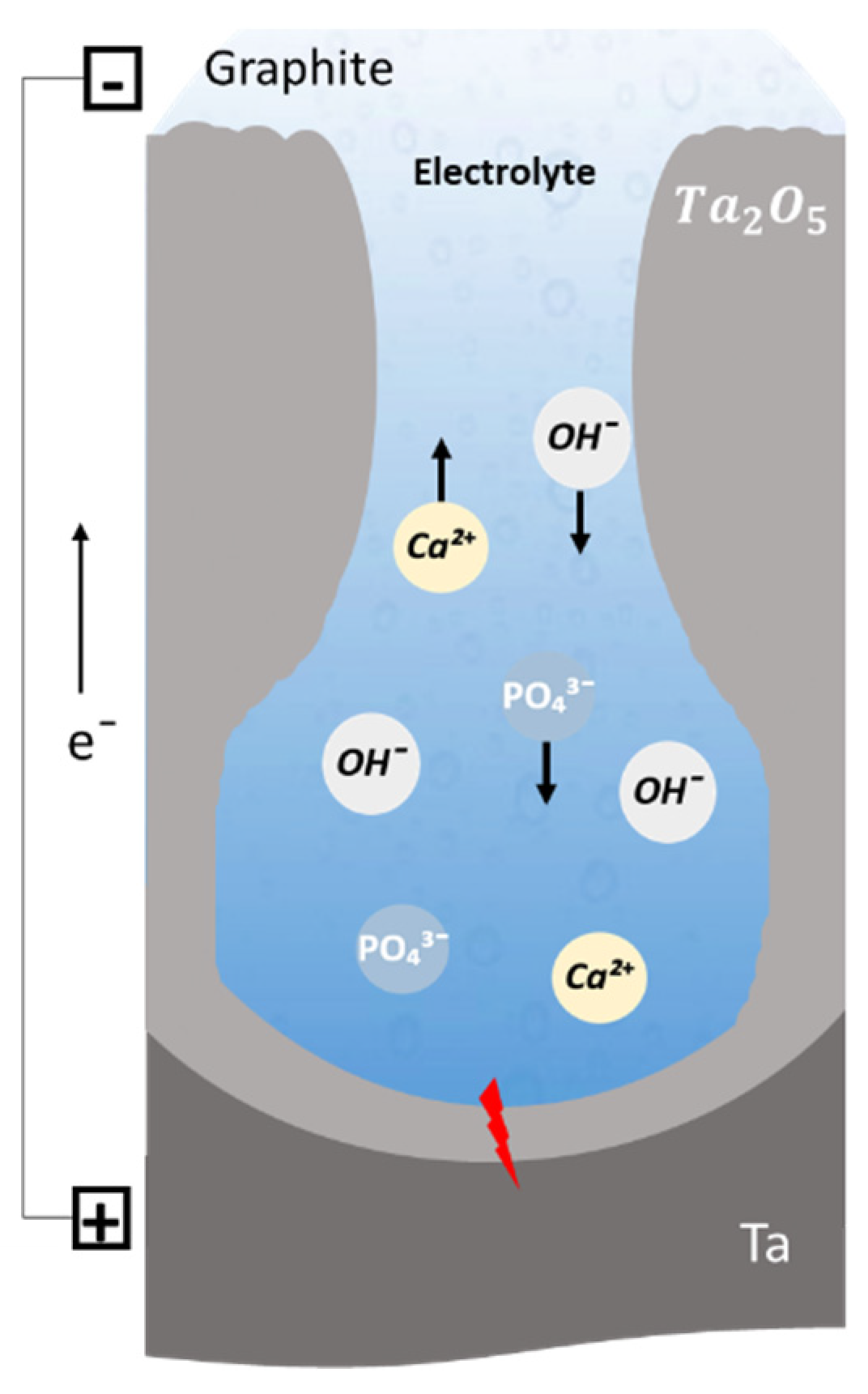
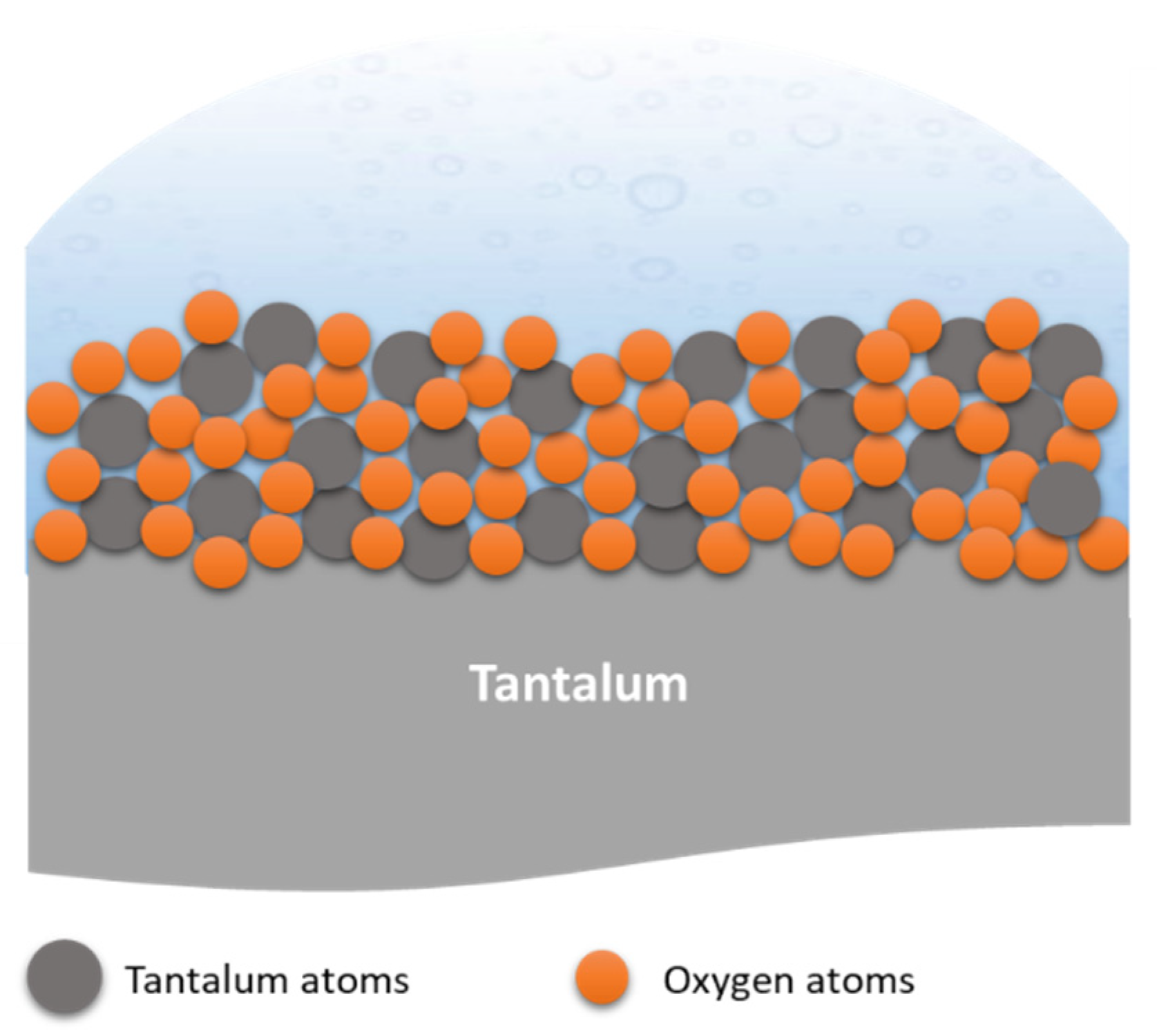
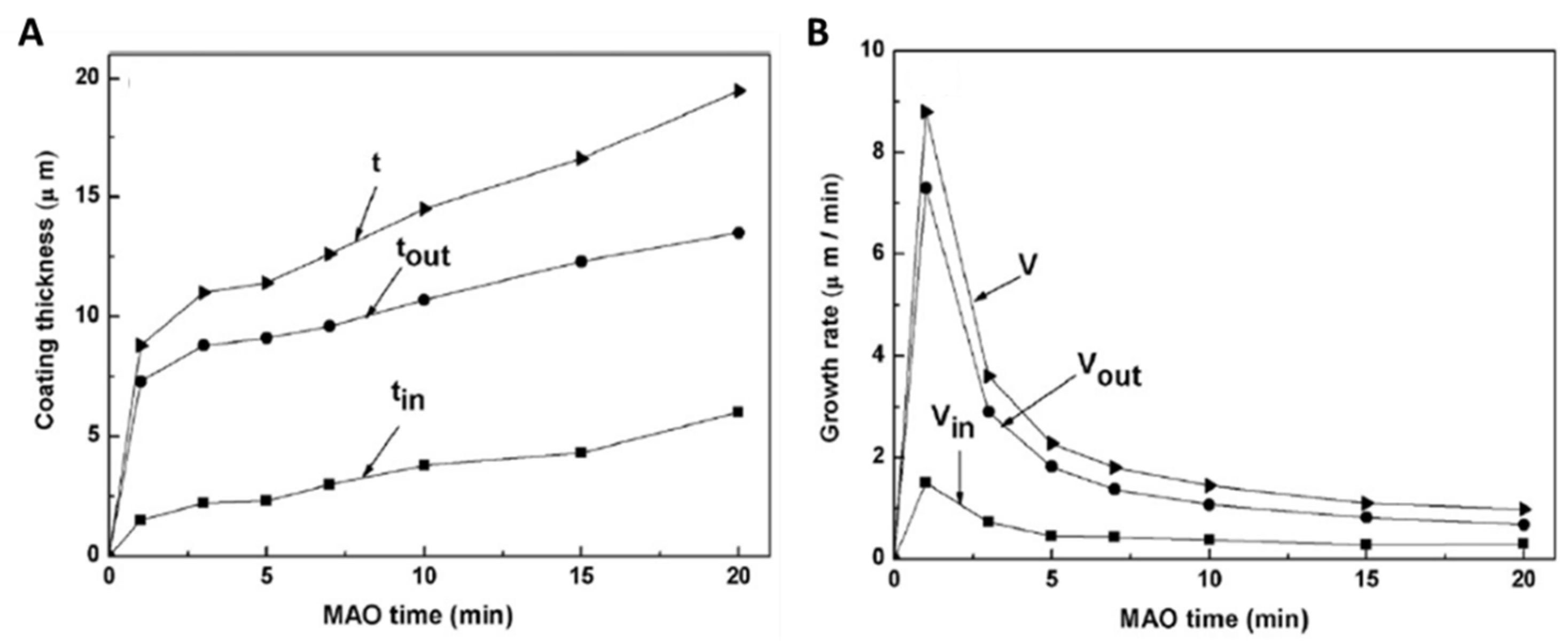
2.3. Growth Mechanism of Ta2O5 Anodic Coating
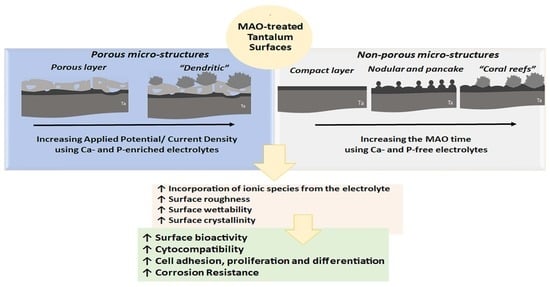
3. Ta Surface Functionalization by MAO
| Type of Microstructures | Electrolyte | Working Conditions | Time (s) | Temperature (°C) | Pre-Treatment | Applications | References |
|---|---|---|---|---|---|---|---|
| Porous | 0.2 M CaA + 0.02 M β-GP | AC: V+ = 350, 400, 450, 480 V, V− = 70 V, 100 Hz, duty ratio of 26% | 600 | 20 | Polished with abrasive papers; ultrasonically cleaned in acetone, ethanol, and DI water | Biomedical | [69] |
| AC: V+ = 350, 450 V, V− = 70 V, 100 Hz, duty ratio of 26% | 60–1200 | 20 | Polished with abrasive papers; ultrasonically cleaned in acetone, ethanol, and DI water | Orthopedic implants | [65] | ||
| AC: V+ = 470 V, V− = 100 V, 100 Hz, duty ratio of 26% | 300 | Biomedical | [70] | ||||
| AC: V = 250–480 V, 100 Hz, duty ratio of 40% | 300, 600, 900 and 1800 | 30 | Polished with abrasive papers; ultrasonically cleaned in acetone, ethanol, and DI water; dried at 60 °C | Orthopedic implants | [71] | ||
| AC: 160–300 V | 60–300 | RT | Washed in DI water; ultrasonic clean in acetone; cleaned in ethanol and air-jet dried | Dental implants | [72] | ||
| AC: 350, 450 and 500 V, 100 Hz, duty cycle 60% | 60–600 | 25–60 | Sonicated in acetone, isopropyl alcohol, and DI water baths | Biomedical | [55] | ||
| A: 0.35–0.7 M CaA B: 0.7 M CaA + 0.04–0.08 M β-GP C: 0.7 M CaA + 0.08 M β-GP + 0.01–0.1 M MgA | DC: 150–200 V | 1800 | RT | Ultrasonically cleaned in benzine and ethanol for 5 min each; rinsed in DI water and dried in air | Dental implants | [73] | |
| 0.35 M CaA + 0.12 M β-GP | DC: 200 V | 1800 | RT | Ultrasonically cleaned in ethanol and DI water for 5 min each | Dental implants | [74] | |
| (0.1, 0.5, 1.0 M) K2SiO3 + 5 g/dm3 KOH | DC: 0.1 A/dm2 up to 100, 200 or 400 V | 120 | Etched in 1 M HF and 4 M H2SO4; cleaned in DI water and ultrasonically cleaned in propanol and DI water | Orthopedic implants | [62] | ||
| A: 0.5 M Ca(H2PO2)2 0.5 M Ca(H2PO2)2 + 1.15 M Ca(HCOO)2 C: 0.5 M Ca(H2PO2)2 + 1.15 M Mg(CH3COO)2 D:0.5 M Ca(H2PO2)2+ 1.5 M Mg(CH3COO)2 | DC: 150 mA/cm2 up to 200, 300, 400 or 500 V | 300 | Polished with abrasive paper; etched in 4 M H2SO4 and 1M HF; ultrasonically cleaned in DI water | Biomedical | [23] | ||
| A: 0.5 M Ca(H2PO2)2 B: 0.5 M Ca(H2PO2)2 + 1.15 M Ca(HCOO)2 C: 0.5 M Ca(H2PO2)2 + 1.15 M Mg(CH3COO)2 | DC: 150 mA/cm2 up to 200, 300 or 400 V | [64] | |||||
| 0.001 M H4SiW12O4 | DC: 70 mA/cm2 | 15–2400 | 21 | Ultrasonically cleaned in acetone, ethanol, and DI water and dried in a warm air stream | Catalysis and semiconductor | [46] | |
| The automotive industry, aerospace industry, and gas and oil industries | [58] | ||||||
| 30 mL HF (4%) + 5 mL NH4F + 1 g glycerin + 3g EG + 2 M H3PO4 | DC: 250 V | 900 | Polished with abrasive papers and in Al2O3 suspension | Biomedical | [75] | ||
| 12 g/L Na2SiO3 +10 g/L NaOH + small amount of additives (EDTA) | AC: 450 V, 1000 Hz, duty cycle 20% | 300 | 20 | Polished with abrasive papers to a mirror finish; ultrasonically cleaned in acetone, ethanol, and DI water for 5 min; and dried | Orthopedic implants | [76] | |
| 0.3 M Ca(CH3COO)2.H2O + 0.1 M Na3PO4 | AC: 1.5 A, 500 Hz, duty cycle 10% | 480 | 26 ± 3 | Ultrasonically cleaned in acetone, ethanol, and DI water | Biomedical | [77] | |
| Non-Porous | A: 1 g/L Na2SiO3.5H2O +1 g/L KOH B: 5 g/L Na3PO4 + 1g/L KOH | AC: 0.085 A/cm2 | 1200 | 30 | Biomedical | [67] | |
| 300g Ca(NO3)2 + 300 g Cu(NO3) in 1 L of H3PO4 (85%) | DC: 450 V | 180 | 20 | Polished with abrasive papers | Biomedical and catalysis | [66] | |
| 10 g/L Na2SiO3.9H2O + 1 g/L KOH | AC: j+ = 0.22 A/cm2, j− = 0.11 A/cm2, 1000 Hz, duty cycle 20% | 1500 | Polished with abrasive papers; degreased in ethanol and rinsed in DI water; dried in warm air | Biomedical | [63] | ||
| A: 0.1 M H3PO4 B: 0.1 M oxalic acid | DC: 30–70 mA/cm2 | 10–600 | 21–30 | Ultrasonically cleaned in acetone | Capacitor dielectric, gate insulators in MOS devices | [61] |
4. Ta2O5 Surfaces for Biomedical Applications
4.1. Properties of MAO-Treated Ta Surfaces
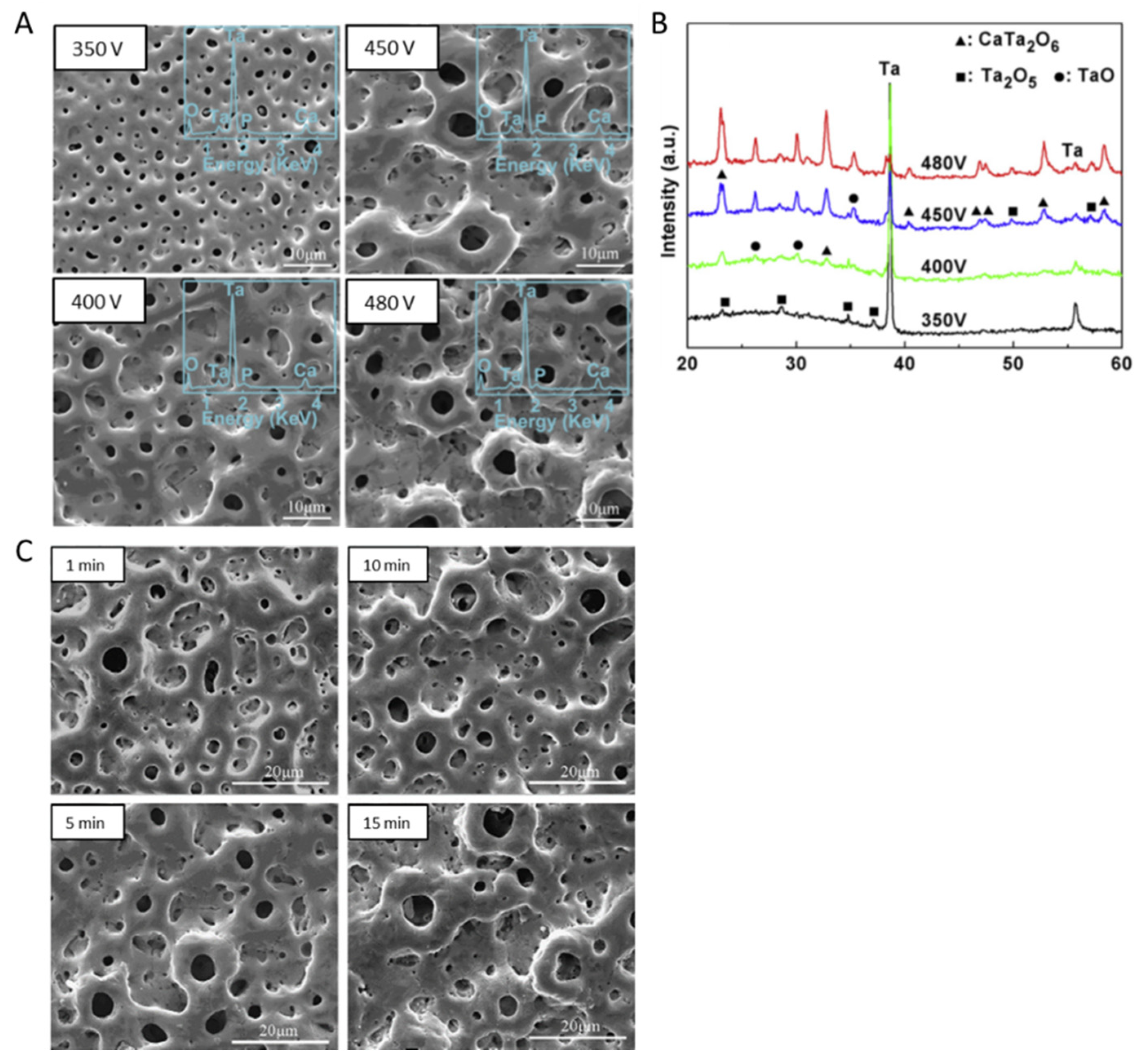
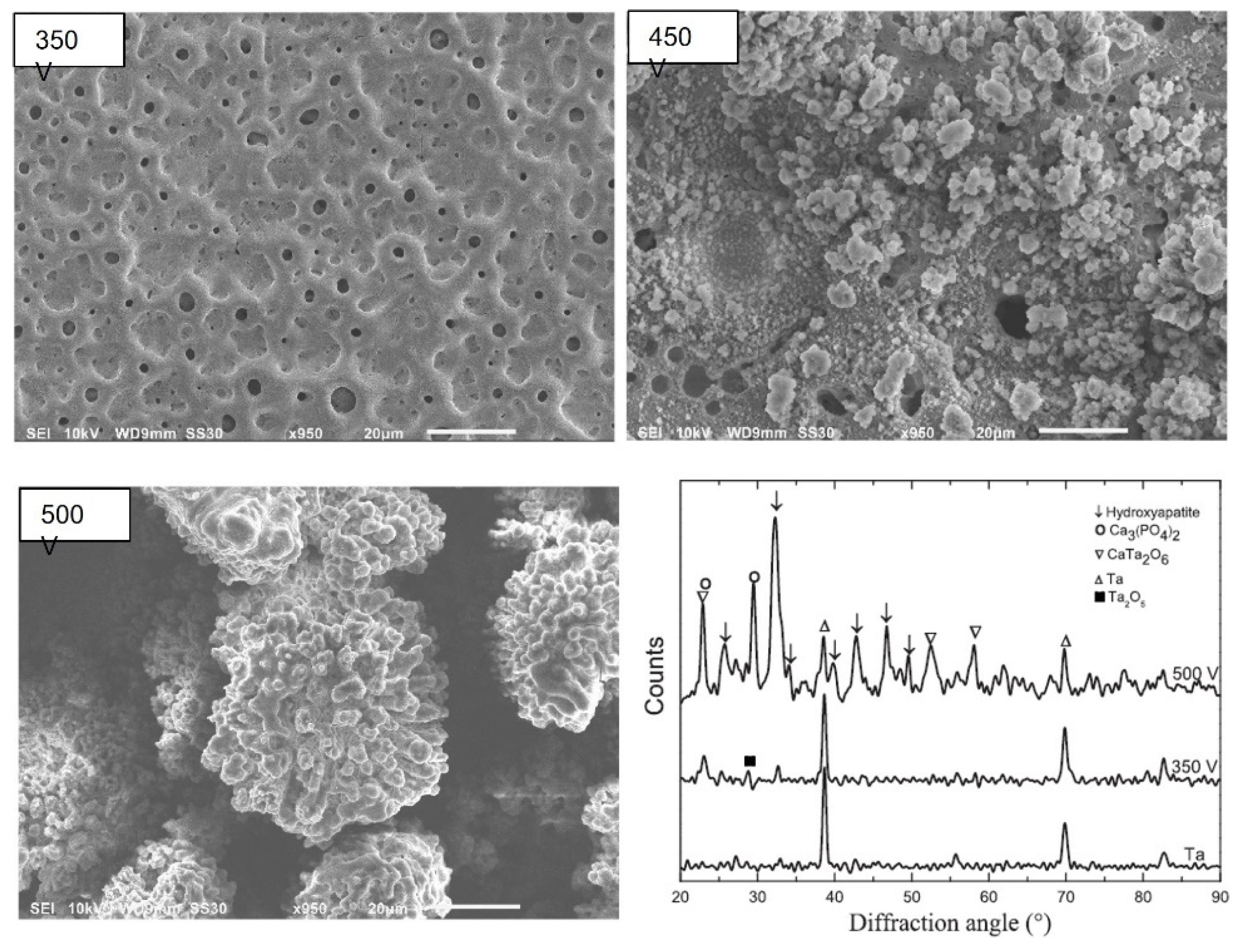
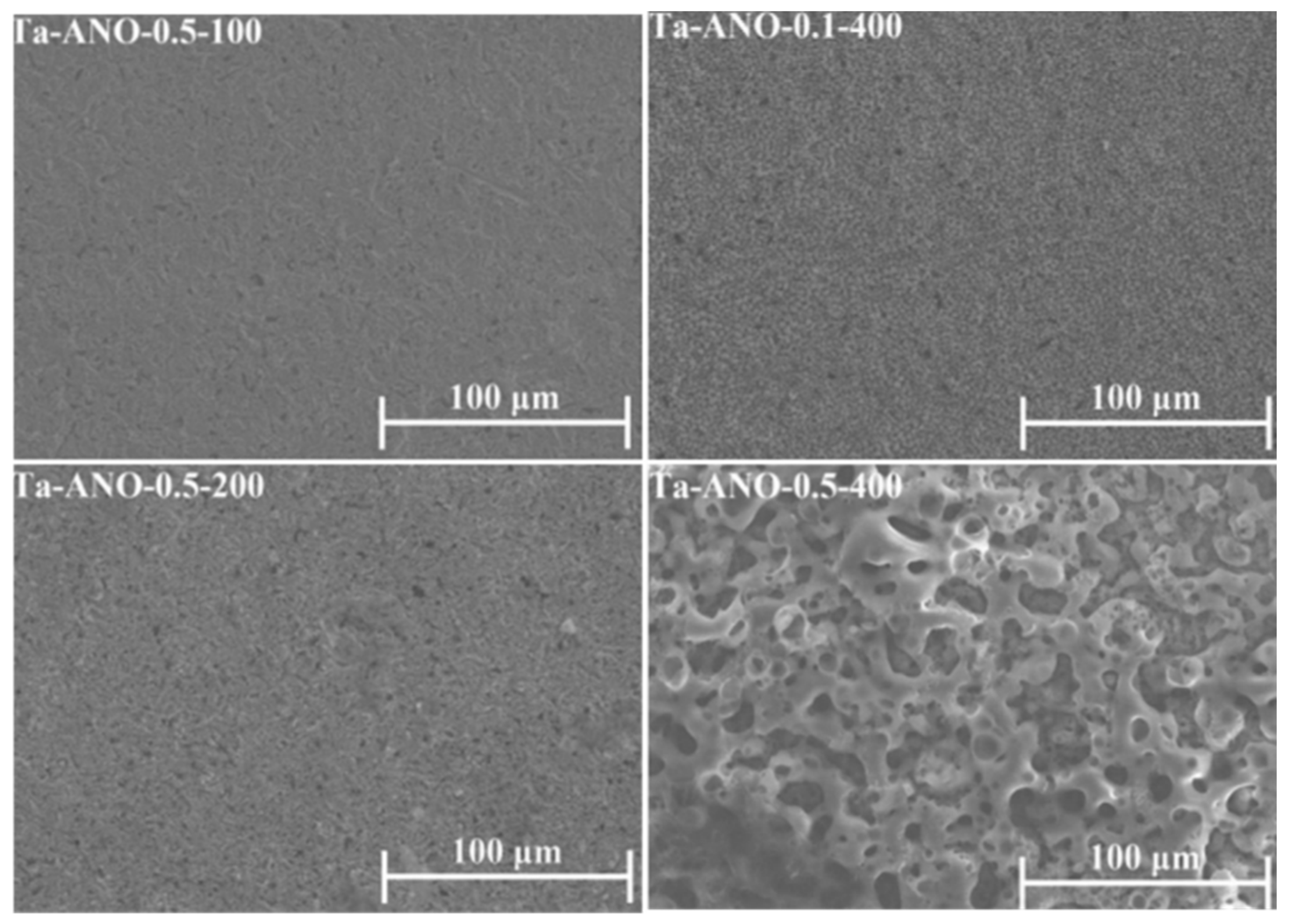
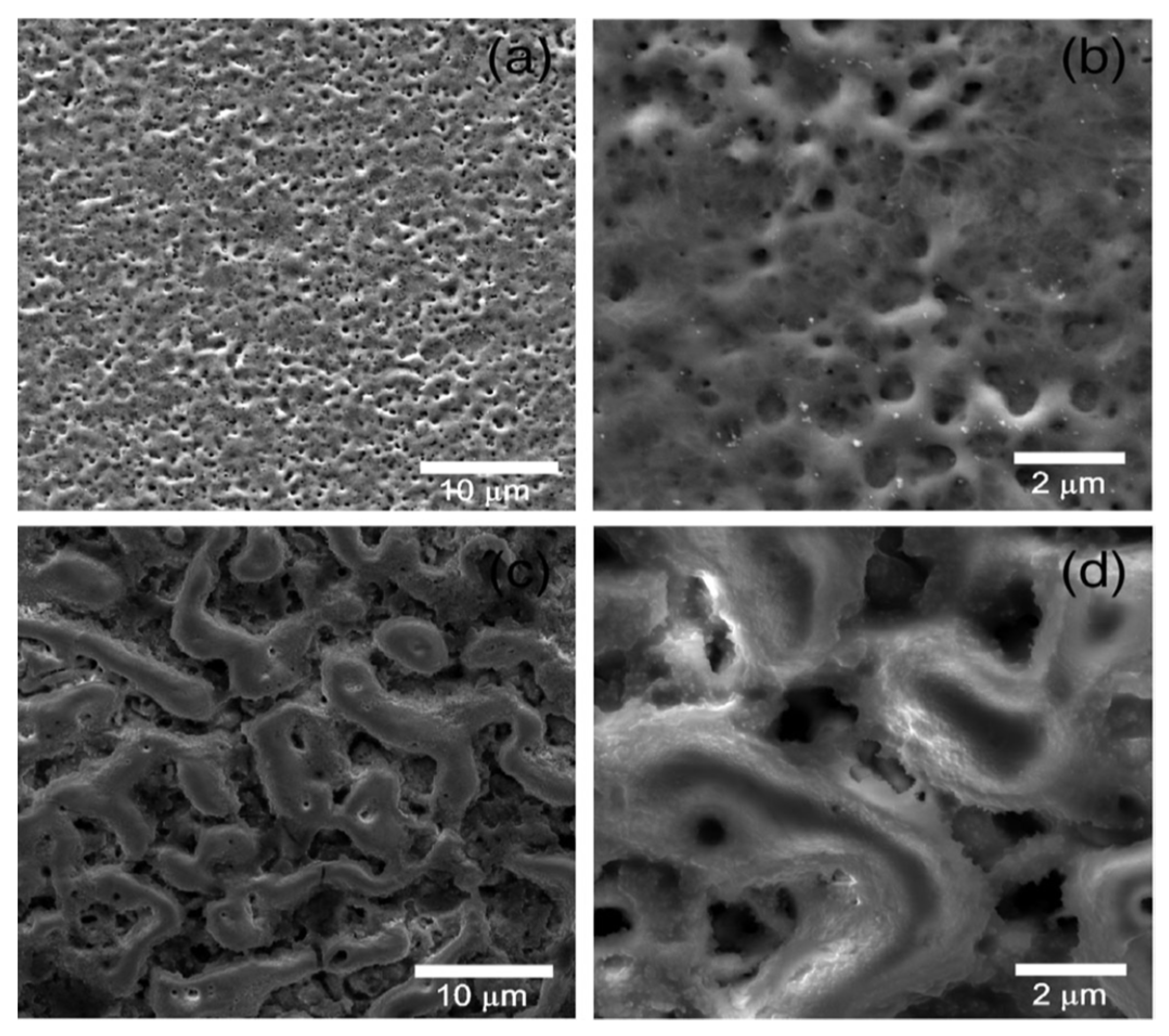
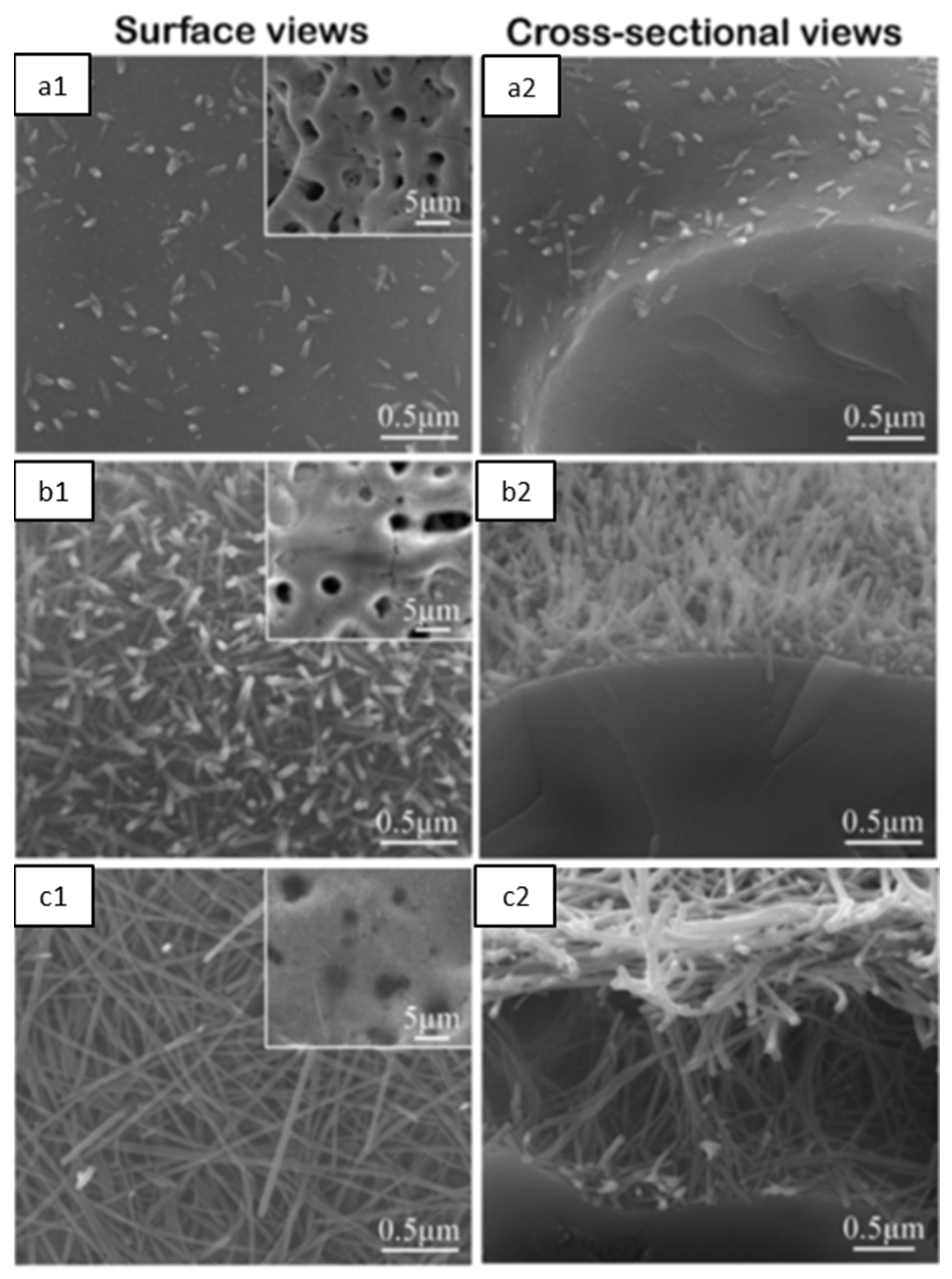
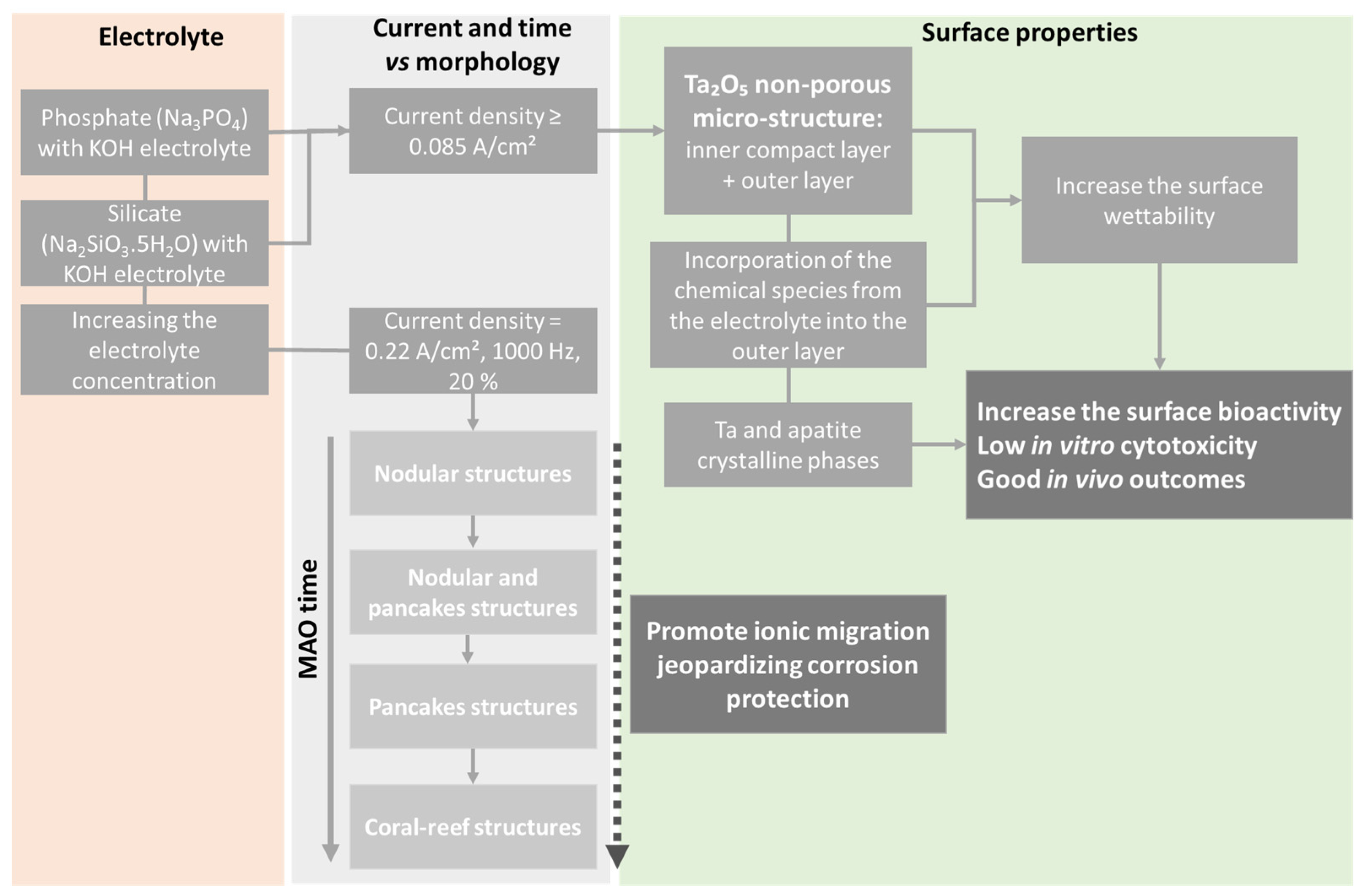
4.1.1. Surface and Cross-Sectional Morphology
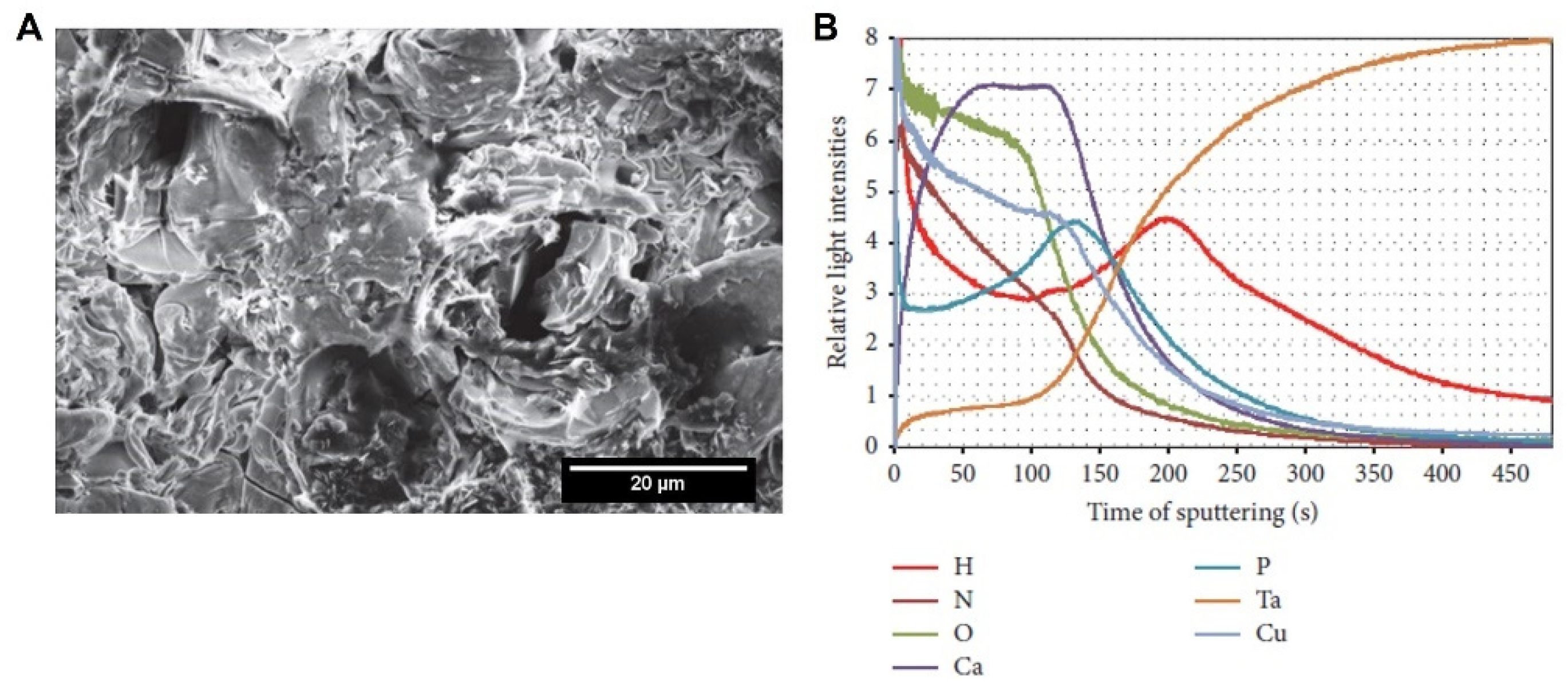
4.1.2. Surface Chemical Composition
4.1.3. Roughness
4.1.4. Surface Wettability
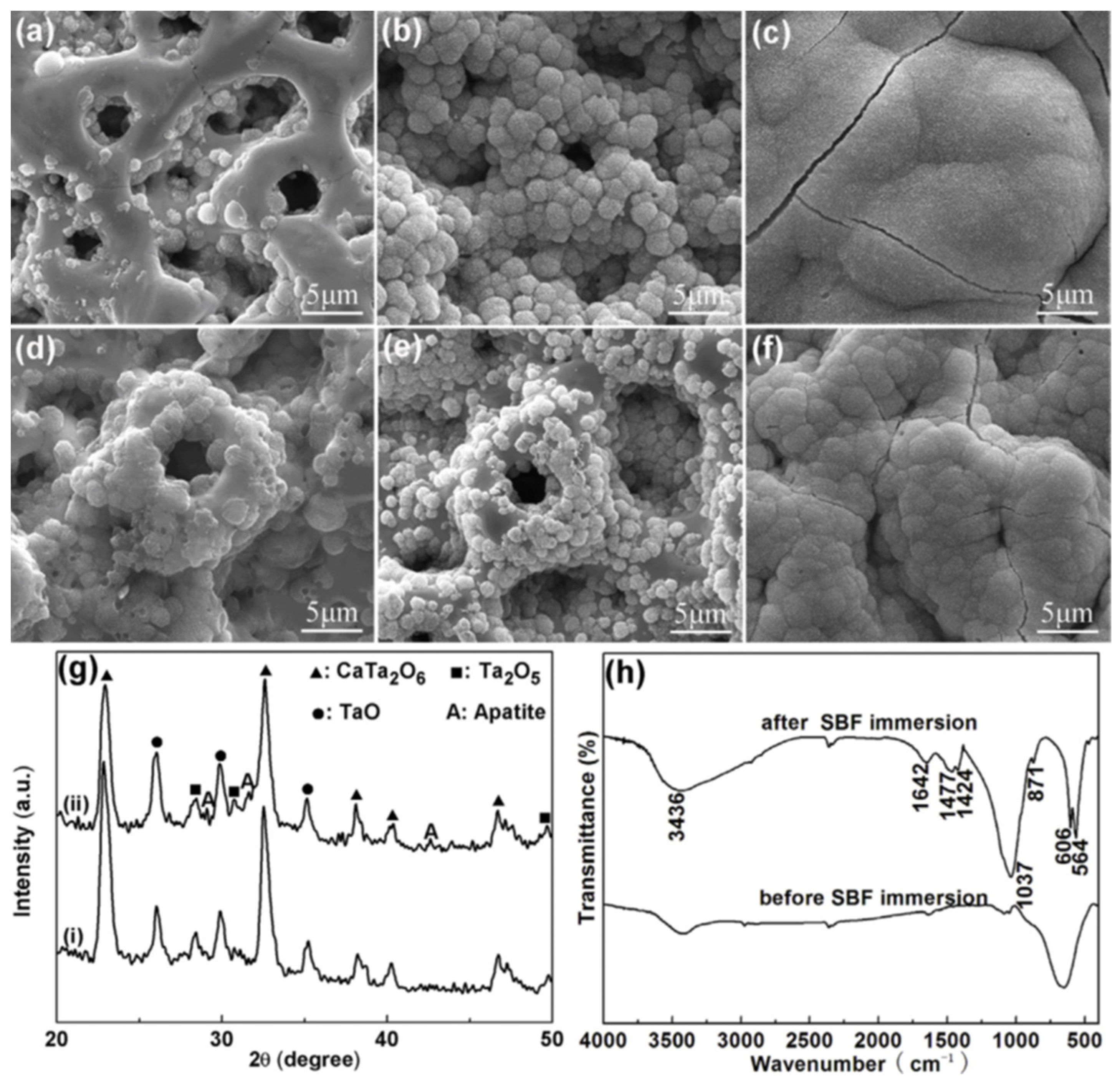
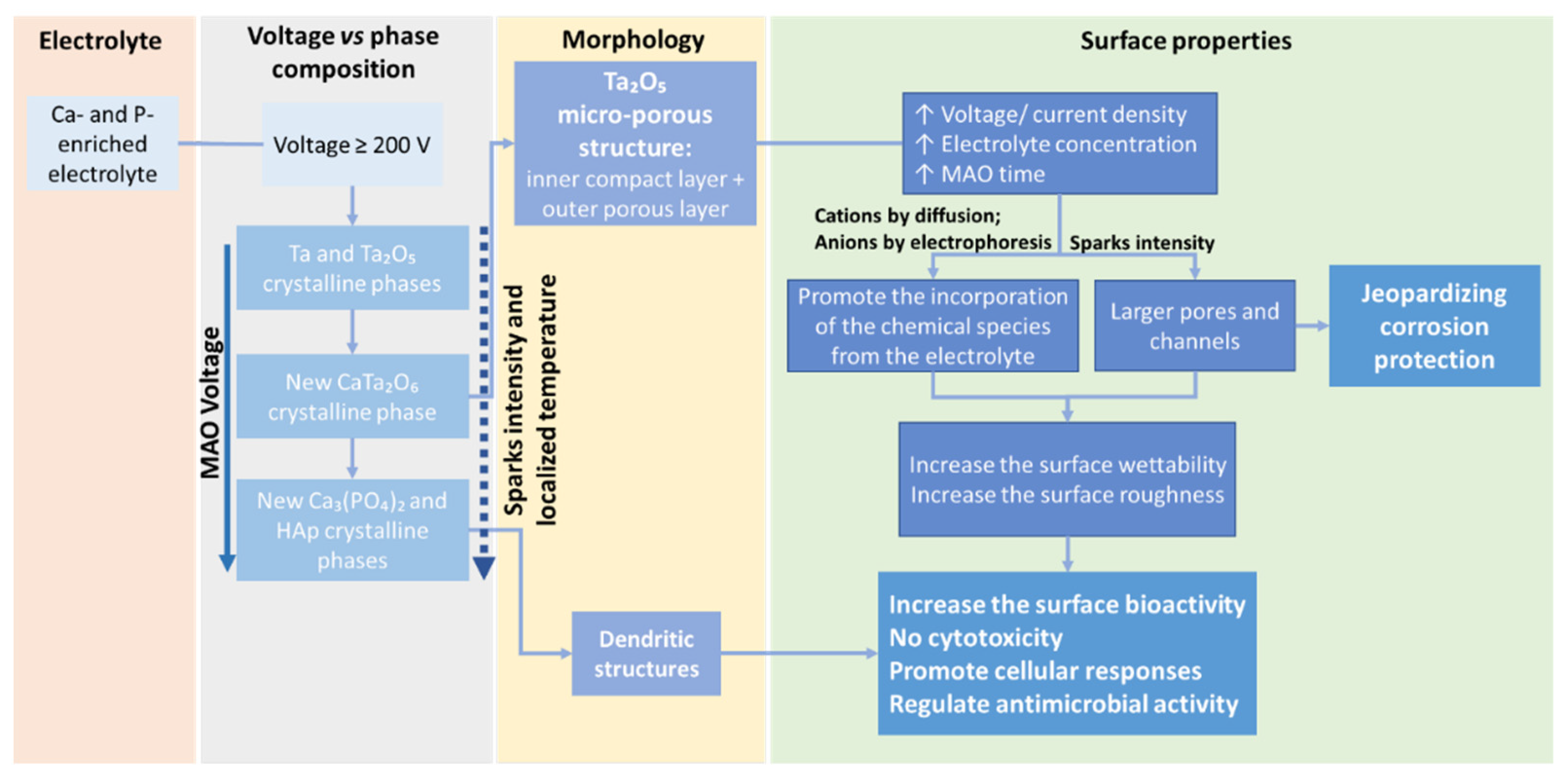
4.1.5. Structural Analysis and Phase Composition
4.2. Functional Properties of MAO-Treated Ta Surface
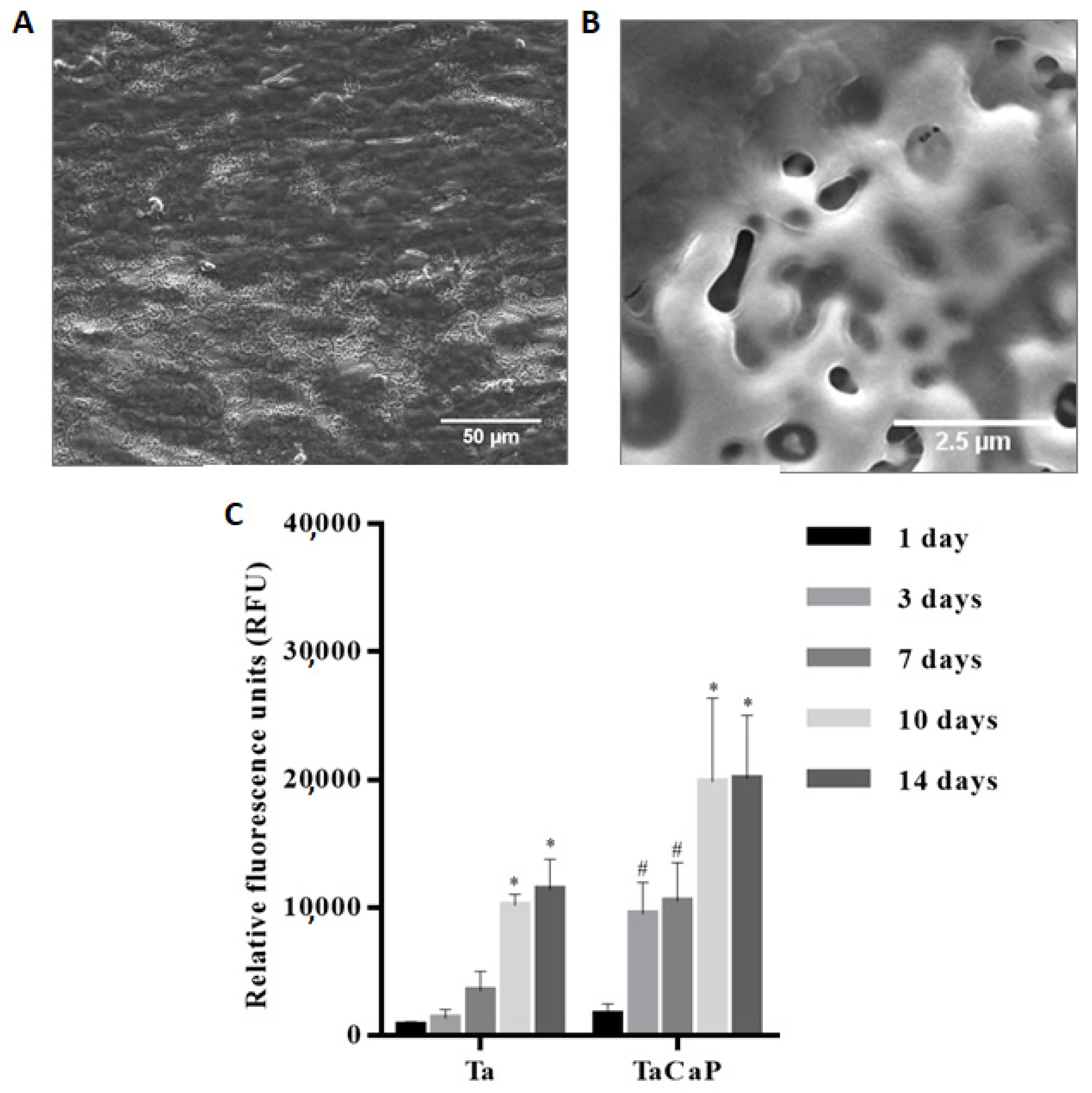
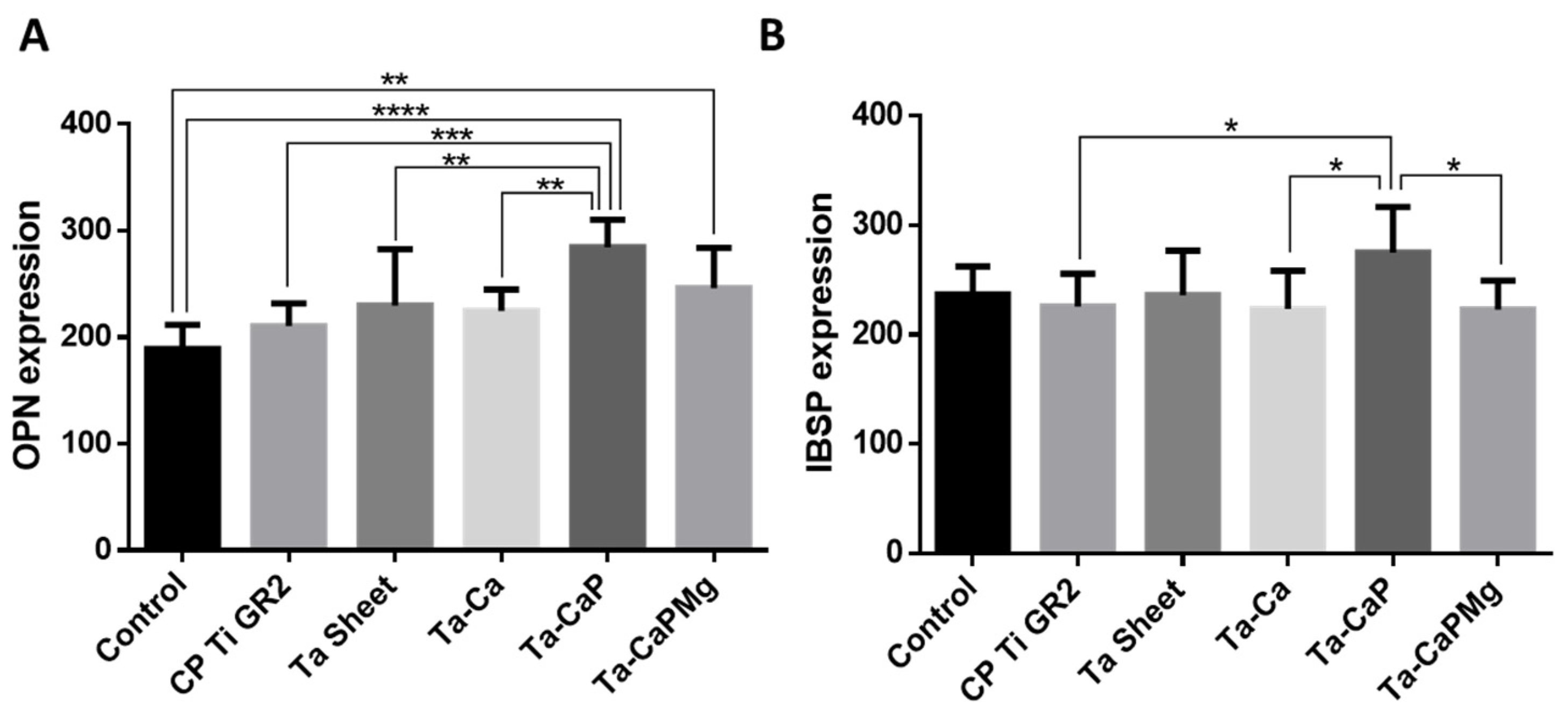
4.2.1. Surface Bioactivity and Biocompatibility
4.2.2. Antimicrobial Activity
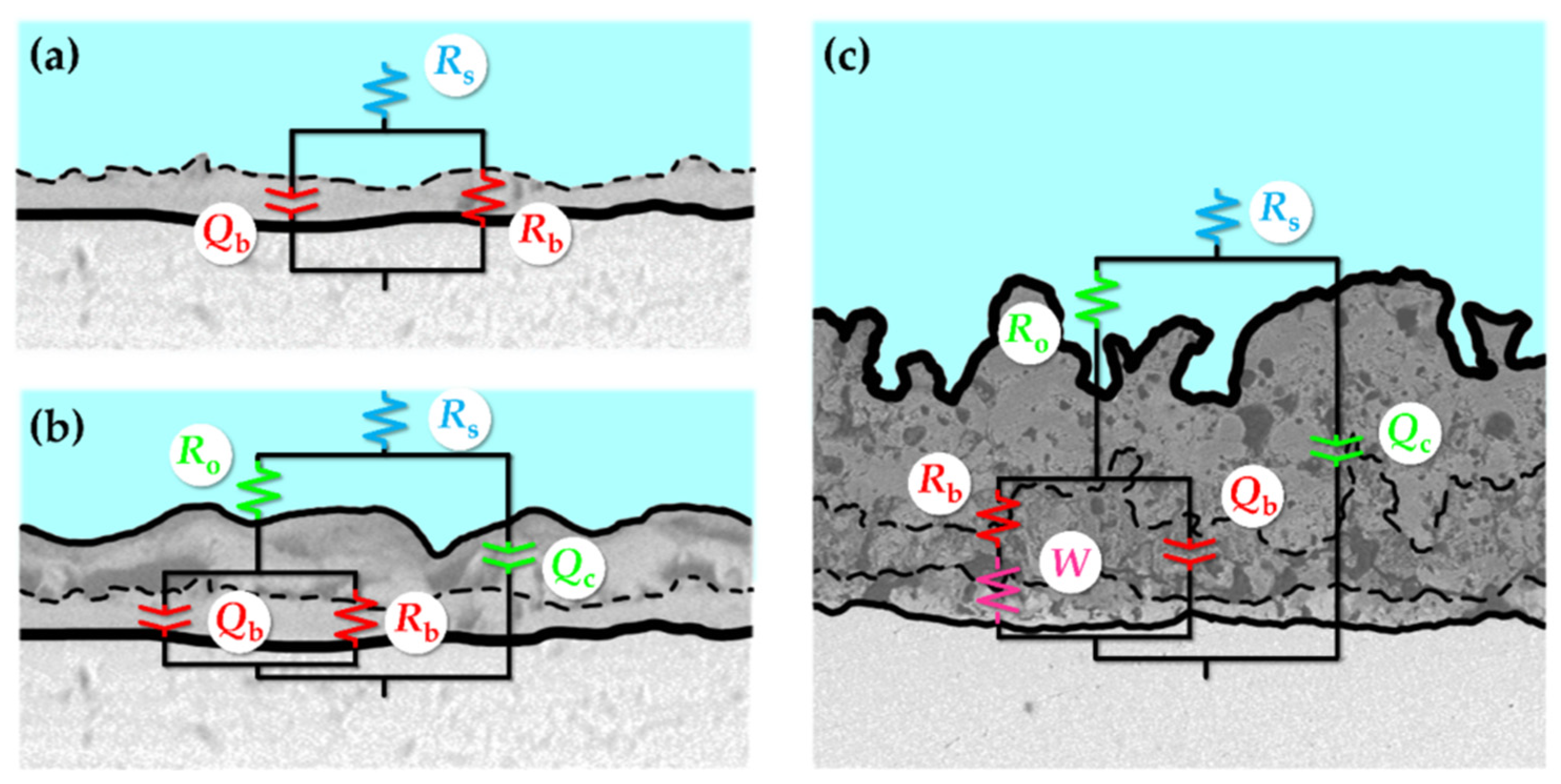
4.2.3. Mechanical Properties and Corrosion Resistance
5. Conclusions and Further Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christensen, C.; De Reus, R.; Bouwstra, S. Tantalum Oxide Thin Films as Protective Coatings for Sensors. J. Micromech. Microeng. 1999, 9, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Mato, S.; Skeldon, P.; Thompson, G.E.; Masheder, D.; Habazaki, H.; Shimizu, K. Anodic Film Growth on Tantalum in Dilute Phosphoric Acid Solution at 20 and 85 °C. Electrochim. Acta 2002, 47, 2761–2767. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Boyd, I.W. Thin Tantalum and Tantalum Oxide Films Grown by Pulsed Laser Deposition. Appl. Surf. Sci. 2000, 168, 234–238. [Google Scholar] [CrossRef]
- Chaneliere, C.; Autran, J.L.; Devine, R.A.B.; Balland, B. Tantalum Pentoxide (Ta2O5) Thin Films for Advanced Dielectric Applications. Mater. Sci. Eng. R. Rep. 1998, 22, 269–322. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Birss, V.I. Controlled Interconversion of Nanoarray of Ta Dimples and High Aspect Ratio Ta Oxide Nanotubes. Nano Lett. 2009, 9, 1350–1355. [Google Scholar] [CrossRef]
- Xu, G.; Shen, X.; Hu, Y.; Ma, P.; Cai, K. Fabrication of Tantalum Oxide Layers onto Titanium Substrates for Improved Corrosion Resistance and Cytocompatibility. Surf. Coat. Technol. 2015, 272, 58–65. [Google Scholar] [CrossRef]
- Sarraf, M.; Razak, B.A.; Nasiri-Tabrizi, B.; Dabbagh, A.; Kasim, N.H.A.; Basirun, W.J.; Sulaiman, E. Bin Nanomechanical Properties, Wear Resistance and in-Vitro Characterization of Ta2O5Coating on Biomedical Grade Ti–6Al–4V. J. Mech. Behav. Biomed. Mater. 2017, 66, 159–171. [Google Scholar] [CrossRef]
- Hirpara, J.; Chawla, V.; Chandra, R. Investigation of Tantalum Oxynitride for Hard and Anti-Corrosive Coating Application in Diluted Hydrochloric Acid Solutions. Mater. Today Commun. 2020, 23, 101113. [Google Scholar] [CrossRef]
- Almeida Alves, C.F.; Calderón, S.V.; Dias, D.; Carvalho, S. Influence of Oxygen Content on the Electrochemical Behavior of Ta1-XOx Coatings. Electrochim. Acta 2016, 211, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Tu, Q.; Wu, H.; Zhao, C.; Yao, X.; Fan, W.; Zhang, S.; Ni, J.; Zhang, X. Study on Morphology Evolution of Anodic Tantalum Oxide Films in Different Using Stages of H2SO4/HF Electrolyte. Electrochim. Acta 2017, 236, 140–153. [Google Scholar] [CrossRef]
- Namur, R.S.; Reys, K.M.; Marino, C.E.B. Growth and Electrochemical Stability of Compact Tantalum Oxides Obtained in Different Electrolytes for Biomedical Applications. Mater. Res. 2015, 18, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Macak, J.M.; Shrestha, N.K.; Schmuki, P. Thick Self-Ordered Nanoporous Ta2O5Films with Long-Range Lateral Order. J. Electrochem. Soc. 2009, 156, K104–K109. [Google Scholar] [CrossRef]
- Gao, H.; Yang, J.; Jin, X.; Qu, X.; Zhang, F.; Zhang, D.; Chen, H.; Wei, H.; Zhang, S.; Jia, W.; et al. Porous Tantalum Scaffolds: Fabrication, Structure, Properties, and Orthopedic Applications. Mater. Des. 2021, 210, 110095. [Google Scholar] [CrossRef]
- Rupérez, E.; Manero, J.M.; Riccardi, K.; Li, Y.; Aparicio, C.; Gil, F.J. Development of Tantalum Scaffold for Orthopedic Applications Produced by Space-Holder Method. Mater. Des. 2015, 83, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Sieber, I.V.; Schmuki, P. Porous Tantalum Oxide Prepared by Electrochemical Anodic Oxidation. J. Electrochem. Soc. 2005, 152, C639–C644. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Birss, V.I. Controlled Growth and Monitoring of Tantalum Oxide Nanostructures. Nanoscale 2010, 2, 793–798. [Google Scholar] [CrossRef]
- Ruckh, T.; Porter, J.R.; Allam, N.K.; Feng, X.; Grimes, C.A.; Popat, K.C. Nanostructured Tantala as a Template for Enhanced Osseointegration. Nanotechnology 2009, 20, 045102. [Google Scholar] [CrossRef]
- Uslu, E.; Öztatlı, H.; Garipcan, B.; Ercan, B. Fabrication and Cellular Interactions of Nanoporous Tantalum Oxide. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2020, 108, 2743–2753. [Google Scholar] [CrossRef]
- Zeng, Y.-J.; Twan, S.-C.; Wang, K.-W.; Huang, H.-H.; Hsu, Y.-B.; Wang, C.-Y.; Lan, M.-Y.; Lee, S.-W. Enhanced Biocompatibility in Anodic TaOx Nanotube Arrays. Nanoscale Res. Lett. 2017, 12, 557. [Google Scholar] [CrossRef] [Green Version]
- Cristea, D.; Ghiuță, I.; Munteanu, D. Tantalum Based Materials for Implants and Prosthesis Applications. Bull. Transilv. Univ. Braşov 2015, 8, 151–158. [Google Scholar]
- Alves, C.F.A.; Cavaleiro, A.; Carvalho, S. Bioactivity Response of Ta1-XOx Coatings Deposited by Reactive DC Magnetron Sputtering. Mater. Sci. Eng. C 2016, 58, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Bao, C.; Wismeijer, D.; Wu, G. The Physicochemical/Biological Properties of Porous Tantalum and the Potential Surface Modification Techniques to Improve Its Clinical Application in Dental Implantology. Mater. Sci. Eng. C 2015, 49, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sowa, M.; Woszczak, M.; Kazek-Kęsik, A.; Dercz, G.; Korotin, D.M.; Zhidkov, I.S.; Kurmaev, E.Z.; Cholakh, S.O.; Basiaga, M.; Simka, W. Influence of Process Parameters on Plasma Electrolytic Surface Treatment of Tantalum for Biomedical Applications. Appl. Surf. Sci. 2017, 407, 52–63. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.; Wang, J.; Chen, S.; Ma, Y.; Zhang, Z. Study on the Anticorrosion, Biocompatibility, and Osteoinductivity of Tantalum Decorated with Tantalum Oxide Nanotube Array Films. ACS Appl. Mater. Interfaces 2012, 4, 4516–4523. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.V.; Migowski, P.; Wender, H.; Eberhardt, D.; Weibel, D.E.; Sonaglio, F.C.; Zapata, M.J.M.; Dupont, J.; Feil, A.F.; Teixeira, S.R. Ta2O5Nanotubes Obtained by Anodization: Effect of Thermal Treatment on the Photocatalytic Activity for Hydrogen Production. J. Phys. Chem. C 2012, 116, 14022–14030. [Google Scholar] [CrossRef]
- Kado, Y.; Hahn, R.; Lee, C.-Y.; Schmuki, P. Strongly Enhanced Photocurrent Response for Na Doped Ta3N5-Nano Porous Structure. Electrochem. Commun. 2012, 17, 67–70. [Google Scholar] [CrossRef]
- Su, Z.; Grigorescu, S.; Wang, L.; Lee, K.; Schmuki, P. Fast Fabrication of Ta2O5Nanotube Arrays and Their Conversion to Ta3N5 for Efficient Solar Driven Water Splitting. Electrochem. Commun. 2015, 50, 15–19. [Google Scholar] [CrossRef]
- Cristea, D.; Cunha, L.; Gabor, C.; Ghiuta, I.; Croitoru, C.; Marin, A.; Velicu, L.; Besleaga, A.; Vasile, B. Tantalum Oxynitride Thin Films: Assessment of the Photocatalytic Efficiency and Antimicrobial Capacity. Nanomaterials 2019, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Schmuki, P. Highly Ordered Nanoporous Ta2O5Formed by Anodization of Ta at High Temperatures in a Glycerol/Phosphate Electrolyte. Electrochem. Commun. 2011, 13, 542–545. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wen, C. Fabrication and Characterization of Nanoporous Niobia, and Nanotubular Tantala, Titania and Zirconia via Anodization. J. Funct. Biomater. 2015, 6, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Zaffora, A.; Cho, D.-Y.; Lee, K.-S.; Di Quarto, F.; Waser, R.; Santamaria, M.; Valov, I. Electrochemical Tantalum Oxide for Resistive Switching Memories. Adv. Mater. 2017, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Greiner, M.T.; Kruse, P. Robust Inorganic Membranes from Detachable Ultrathin Tantalum Oxide Films. Nano Lett. 2007, 7, 2676–2683. [Google Scholar] [CrossRef]
- Cheon, K.-H.; Park, C.; Kang, M.-H.; Kang, I.-G.; Lee, M.-K.; Lee, H.; Kim, H.-E.; Jung, H.-D.; Jang, T.-S. Construction of Tantalum/Poly(Ether Imide) Coatings on Magnesium Implants with Both Corrosion Protection and Osseointegration Properties. Bioact. Mater. 2021, 6, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; He, Q.; Ding, Z.; Liao, C.; Chen, D.; Ou, L. Fabrication and Performance of ZnO Doped Tantalum Oxide Multilayer Composite Coatings on TI6AL4V for Orthopedic Application. Nanomaterials 2019, 9, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, C.F.A.; Serra, R.; Bayat, R.; Ferreira, F.; Cavaleiro, A.; Carvalho, S. Synergetic Effect of Thickness and Oxygen Addition on the Electrochemical Behaviour of Tantalum Oxide Coatings Deposited by HiPIMS in DOMS Mode. Electrochim. Acta 2022, 423, 140497. [Google Scholar] [CrossRef]
- Wei, W.; Macak, J.M.; Schmuki, P. High Aspect Ratio Ordered Nanoporous Ta2O5Films by Anodization of Ta. Electrochem. Commun. 2008, 10, 428–432. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, S.; Yang, X.; Wang, X.; Sun, H.; Huo, M. Synthesis of Coral-Like Tantalum Oxide Films via Anodization in Mixed Organic-Inorganic Electrolytes. PLoS ONE 2013, 8, e66447. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, H.; Singh, S.; Kruse, P. Formation of Dimpled Tantalum Surfaces from Electropolishing. J. Electrochem. Soc. 2007, 154, C728. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Horwood, C.A.; Abhayawardhana, A.D.; Birss, V.I. New Insights into the Initial Stages of Ta Oxide Nanotube Formation on Polycrystalline Ta Electrodes. Nanoscale 2013, 5, 1494–1498. [Google Scholar] [CrossRef]
- El-Sayed, H.; Singh, S.; Greiner, M.T.; Kruse, P. Formation of Highly Ordered Arrays of Dimples on Tantalum at the Nanoscale. Nano Lett. 2006, 6, 2995–2999. [Google Scholar] [CrossRef]
- Allam, N.K.; Feng, X.J.; Grimes, C.A. Self-Assembled Fabrication of Vertically Oriented Ta2O5 Nanotube Arrays, and Membranes Thereof, by One-Step Tantalum Anodization. Chem. Mater. 2008, 20, 6477–6481. [Google Scholar] [CrossRef]
- Momeni, M.M.; Mirhosseini, M.; Chavoshi, M. Fabrication of Ta2O5Nanostructure Films via Electrochemical Anodisation of Tantalum. Surf. Eng. 2017, 33, 83–89. [Google Scholar] [CrossRef]
- Fialho, L.; Almeida Alves, C.F.; Marques, L.S.; Carvalho, S. Development of Stacked Porous Tantalum Oxide Layers by Anodization. Appl. Surf. Sci. 2020, 511, 145542. [Google Scholar] [CrossRef]
- Alves, C.F.A.; Calderon, C.V.; Ferreira, P.J.; Marques, L.; Carvalho, S. Passivation and Dissolution Mechanisms in Ordered Anodic Tantalum Oxide Nanostructures. Appl. Surf. Sci. 2020, 513, 145575. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A Review of Recent Work on Discharge Characteristics during Plasma Electrolytic Oxidation of Various Metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef] [Green Version]
- Petković, M.; Stojadinović, S.; Vasilić, R.; Zeković, L. Characterization of Oxide Coatings Formed on Tantalum by Plasma Electrolytic Oxidation in 12-Tungstosilicic Acid. Appl. Surf. Sci. 2011, 257, 10590–10594. [Google Scholar] [CrossRef]
- Rizwan, M.; Alias, R.; Zaidi, U.Z.; Mahmoodian, R.; Hamdi, M. Surface Modification of Valve Metals Using Plasma Electrolytic Oxidation for Antibacterial Applications: A Review. J. Biomed. Mater. Res.-Part A 2018, 106, 590–605. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation-an Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (Peo) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Dunleavy, C.S.; Golosnoy, I.O.; Curran, J.A.; Clyne, T.W. Characterisation of Discharge Events during Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2009, 203, 3410–3419. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-D.; Zhang, H.; Dong, X.-D.; Ning, C.-Y.; Fok, A.S.L.; Wang, Y. Physicochemical Properties and in Vitro Cytocompatibility of Modified Titanium Surfaces Prepared via Micro-Arc Oxidation with Different Calcium Concentrations. Appl. Surf. Sci. 2015, 329, 347–355. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Chen, C.; Zhao, Z. Review of the Biocompatibility of Micro-Arc Oxidation Coated Titanium Alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Chen, D.; Wang, R.; Li, D.; Guo, C.; Jiang, G.; Shen, D.; Yu, S.; Nash, P. Micro-Structures and Growth Mechanisms of Plasma Electrolytic Oxidation Coatings on Aluminium at Different Current Densities. Surf. Coat. Technol. 2017, 321, 236–246. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, T.; Li, S.; Cheng, Y.; Cao, J.; Xie, H. The Effects of Anion Deposition and Negative Pulse on the Behaviours of Plasma Electrolytic Oxidation (PEO)—A Systematic Study of the PEO of a Zirlo Alloy in Aluminate Electrolytes. Electrochim. Acta 2017, 225, 47–68. [Google Scholar] [CrossRef]
- Antonio, R.F.; Rangel, E.C.; Mas, B.A.; Duek, E.A.R.; Cruz, N.C. Growth of Hydroxyapatite Coatings on Tantalum by Plasma Electrolytic Oxidation in a Single Step. Surf. Coat. Technol. 2019, 357, 698–705. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of Plasma Electrolytic Oxidation of Titanium Substrates: Mechanism, Properties, Applications and Limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Tu, W.; Cheng, Y.; Wang, X.; Zhan, T.; Han, J.; Cheng, Y. Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy in Aluminate-Tungstate Electrolytes and the Coating Formation Mechanism. J. Alloys Compd. 2017, 725, 199–216. [Google Scholar] [CrossRef]
- Stojadinović, S.; Jovović, J.; Petković, M.; Vasilić, R.; Konjević, N. Spectroscopic and Real-Time Imaging Investigation of Tantalum Plasma Electrolytic Oxidation (PEO). Surf. Coat. Technol. 2011, 205, 5406–5413. [Google Scholar] [CrossRef]
- Oliveira, F.G.; Ribeiro, A.R.; Perez, G.; Archanjo, B.S.; Gouvea, C.P.; Araújo, J.R.; Campos, A.P.C.; Kuznetsov, A.; Almeida, C.M.; Maru, M.M.; et al. Understanding Growth Mechanisms and Tribocorrosion Behaviour of Porous TiO2 Anodic Films Containing Calcium, Phosphorous and Magnesium. Appl. Surf. Sci. 2015, 341, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.R.; Ko, Y.G.; Shin, D.H. Effect of Electrolyte on Surface Properties of Pure Titanium Coated by Plasma Electrolytic Oxidation. J. Alloys Compd. 2011, 509, S478–S481. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Vasilić, R. Luminescence of Oxide Films during the Electrolytic Oxidation of Tantalum. Electrochim. Acta 2015, 152, 323–329. [Google Scholar] [CrossRef]
- Sowa, M.; Kazek-k, A.; Socha, R.P.; Dercz, G.; Michalska, J.; Simka, W. Modification of Tantalum Surface via Plasma Electrolytic Oxidation in Silicate Solutions. Electrochim. Acta 2013, 114, 627–636. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Q.; Zhu, Z.; Tu, W.; Cheng, Y.; Skeldon, P. Potential and Morphological Transitions during Bipolar Plasma Electrolytic Oxidation of Tantalum in Silicate Electrolyte. Ceram. Int. 2020, 46, 13385–13396. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials 2018, 11, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, F.; Han, Y. Structural Characteristics and Outward-Inward Growth Behavior of Tantalum Oxide Coatings on Tantalum by Micro-Arc Oxidation. Surf. Coat. Technol. 2013, 214, 110–116. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Chapon, P.; Raaen, S.; Sandim, H.R.Z. XPS and GDOES Characterization of Porous Coating Enriched with Copper and Calcium Obtained on Tantalum via Plasma Electrolytic Oxidation. J. Spectrosc. 2016, 2016, 7093071. [Google Scholar] [CrossRef]
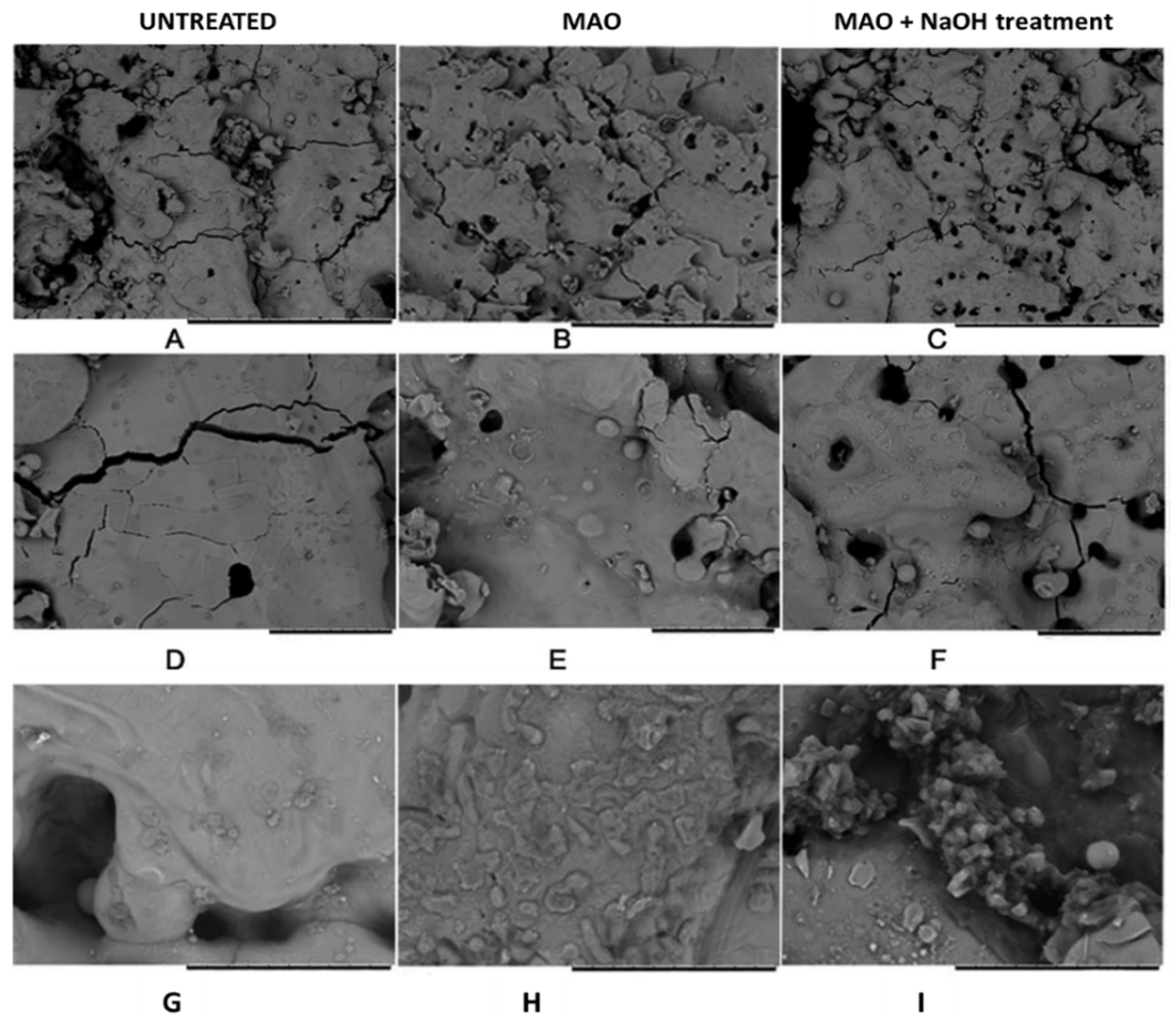
- Gao, H.; Jie, Y.F.; Wang, Z.Q.; Wan, H.; Gong, L.; Lu, R.C.; Xue, Y.K.; Li, D.; Wang, H.Y.; Hao, L.N.; et al. Bioactive Tantalum Metal Prepared by Micro-Arc Oxidation and NaOH Treatment. J. Mater. Chem. B 2014, 2, 1216–1224. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Molaei, M.; Babaei, K. Influence of Electrolyte Composition and Voltage on the Microstructure and Growth Mechanism of Plasma Electrolytic Oxidation (PEO) Coatings on Tantalum: A Review. Anal. Bioanal. Electrochem. 2020, 12, 517–535. [Google Scholar]
- Wang, C.; Wang, F.; Han, Y. The Structure, Bond Strength and Apatite-Inducing Ability of Micro-Arc Oxidized Tantalum and Their Response to Annealing. Appl. Surf. Sci. 2016, 361, 190–198. [Google Scholar] [CrossRef]
- Wang, C.; Fan, Z.; Han, Y. Formation and Osteoblast Behavior of HA Nano-Rod/Fiber Patterned Coatings on Tantalum in Porous and Compact Forms. J. Mater. Chem. B 2015, 3, 5442–5454. [Google Scholar] [CrossRef]
- Zhao, Q.-M.; Li, G.-Z.; Yang, H.-L.; Gu, X.-F. Surface Modification of Biomedical Tantalum by Micro-Arc Oxidation. Mater. Technol. 2017, 32, 90–95. [Google Scholar] [CrossRef]
- Goularte, M.A.P.C.; Barbosa, G.F.; Cruz, N.C.; Hirakata, L.M. Achieving Surface Chemical and Morphologic Alterations on Tantalum by Plasma Electrolytic Oxidation. Int. J. Implant Dent. 2016, 2, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
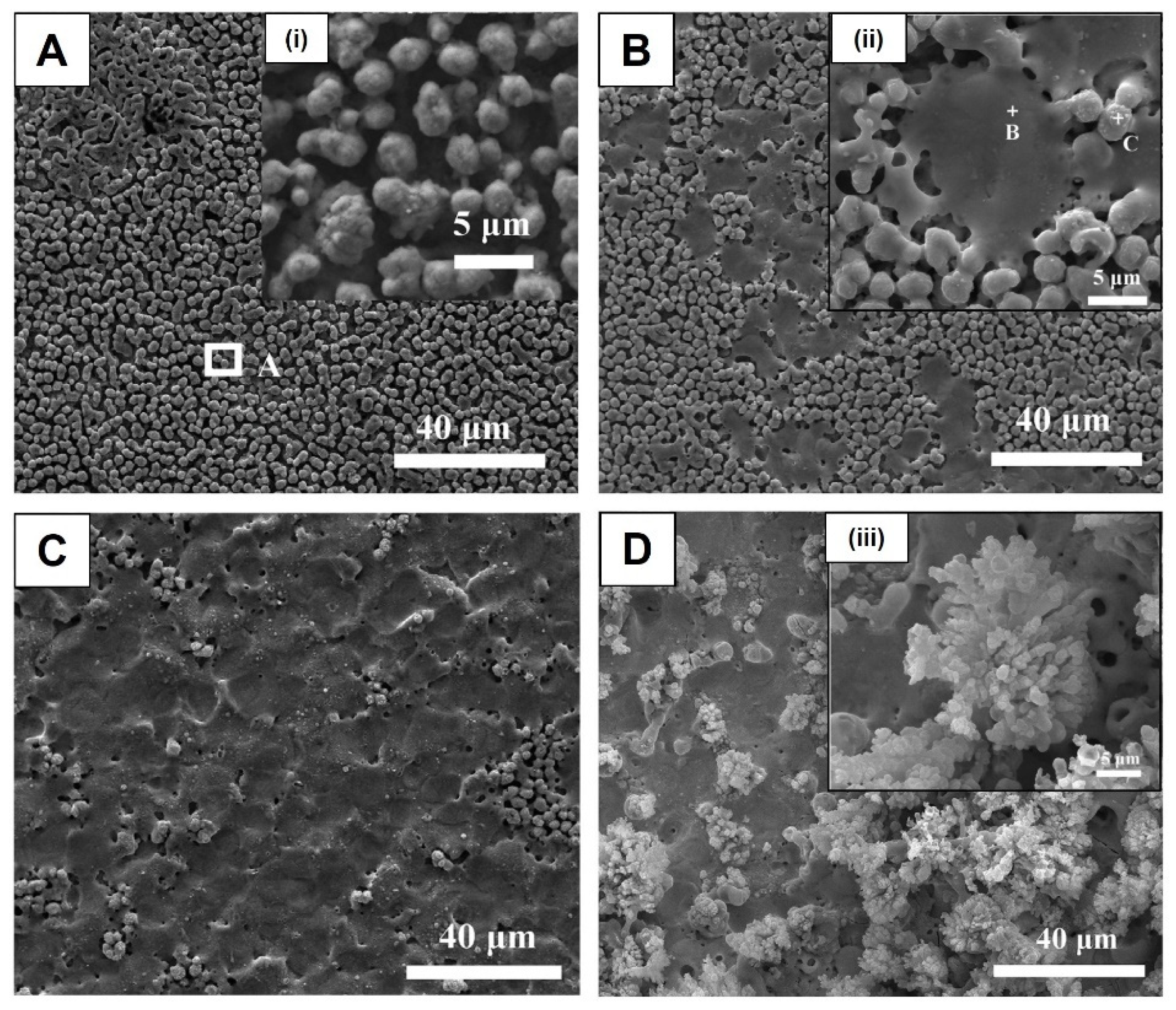
- Alves, C.F.A.; Fialho, L.; Marques, S.M.; Pires, S.; Rico, P.; Palacio, C.; Carvalho, S. MC3T3-E1 Cell Response to Microporous Tantalum Oxide Surfaces Enriched with Ca, P and Mg. Mater. Sci. Eng. C 2021, 124, 112008. [Google Scholar] [CrossRef] [PubMed]
- Fialho, L.; Grenho, L.; Fernandes, M.H.; Carvalho, S. Porous Tantalum Oxide with Osteoconductive Elements and Antibacterial Core-Shell Nanoparticles: A New Generation of Materials for Dental Implants. Mater. Sci. Eng. C 2021, 120, 111761. [Google Scholar] [CrossRef]
- Sopata, M.; Karpiński, T.M.; Jakubowicz, J.; Sopata, M. Development of Tantalum with Highly Hydrophilic Surface and Antimicrobial Properties Obtained by Micro-Arc Oxidation Process. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2021, 109, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-M.; Ni, X.-H.; Zhu, X.-Y.; Liu, X.-D.; Zhang, L.; Zhang, Z.-Y.; Ren, L.-B.; Wang, J.-Y.; Yi, L. Preparation, Characterisation and Biocompatibility of Microarc Oxidation Coating on Tantalum. Surf. Innov. 2020, 9, 174–181. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Gu, W.; Zhang, Z.; Chen, Y.; Wu, T.; Wang, Q.; Tang, H. Surface Functionalization of Selective Electron Beam Melting Pure Tantalum by Micro-Arc Oxidation. Surf. Coat. Technol. 2021, 428, 127880. [Google Scholar] [CrossRef]
- Fialho, L.; Carvalho, S. Surface Engineering of Nanostructured Ta Surface with Incorporation of Osteoconductive Elements by Anodization. Appl. Surf. Sci. 2019, 495, 143573. [Google Scholar] [CrossRef]
- Benčina, M.; Iglič, A.; Mozetič, M.; Junkar, I. Crystallized TiO2 Nanosurfaces in Biomedical Applications. Nanomaterials 2020, 10, 1121. [Google Scholar] [CrossRef]
- Saeed, E.M.; Dawood, N.M.; Hasan, S.F. Improvement Corrosion Resistance of Ni-Ti Alloy by TiO2 Coating and Hydroxyapatite/TiO2 Composite Coating Using Micro Arc Oxidation Process. Mater. Today Proc. 2021, 42, 2789–2796. [Google Scholar] [CrossRef]
- Pesode, P.A.; Barve, S.B. Recent Advances on the Antibacterial Coating on Titanium Implant by Micro-Arc Oxidation Process. Mater. Today Proc. 2021, 47, 5652–5662. [Google Scholar] [CrossRef]
- Li, C.-Y.; Yu, C.; Zeng, R.-C.; Zhang, B.-C.; Cui, L.-Y.; Wan, J.; Xia, Y. In Vitro Corrosion Resistance of a Ta2O5Nanofilm on MAO Coated Magnesium Alloy AZ31 by Atomic Layer Deposition. Bioact. Mater. 2020, 5, 34–43. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fialho, L.; Alves, C.F.A.; Carvalho, S. A Decade of Progress on MAO-Treated Tantalum Surfaces: Advances and Contributions for Biomedical Applications. Nanomaterials 2022, 12, 2319. https://doi.org/10.3390/nano12142319
Fialho L, Alves CFA, Carvalho S. A Decade of Progress on MAO-Treated Tantalum Surfaces: Advances and Contributions for Biomedical Applications. Nanomaterials. 2022; 12(14):2319. https://doi.org/10.3390/nano12142319
Chicago/Turabian StyleFialho, Luísa, Cristiana F. Almeida Alves, and Sandra Carvalho. 2022. "A Decade of Progress on MAO-Treated Tantalum Surfaces: Advances and Contributions for Biomedical Applications" Nanomaterials 12, no. 14: 2319. https://doi.org/10.3390/nano12142319