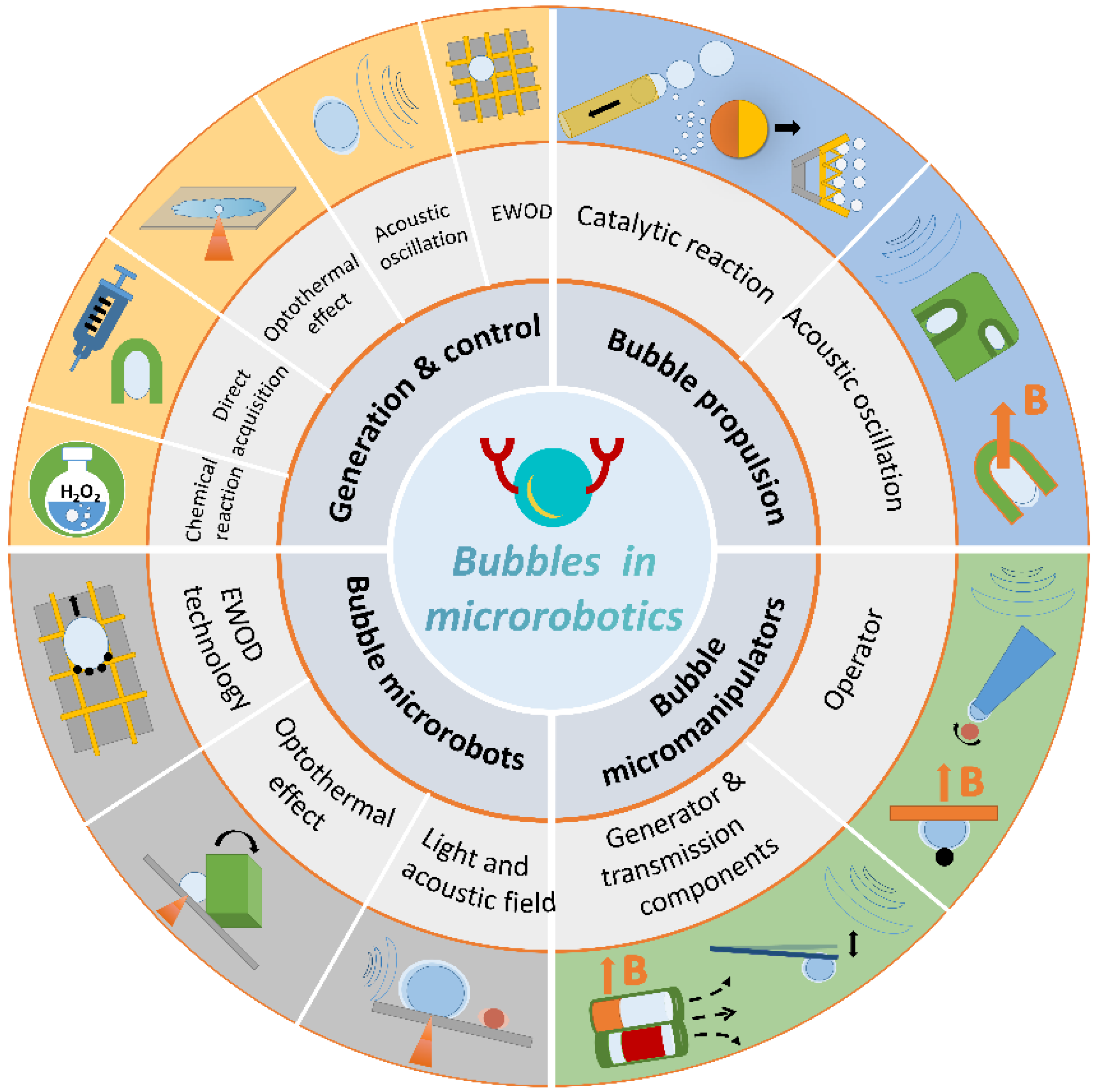
Review of Bubble Applications in Microrobotics: Propulsion, Manipulation, and Assembly
Abstract
1. Introduction
2. Generation and Control of Bubbles
2.1. Chemical Reaction

2.2. Direct Acquisition
2.3. Optothermal Effect
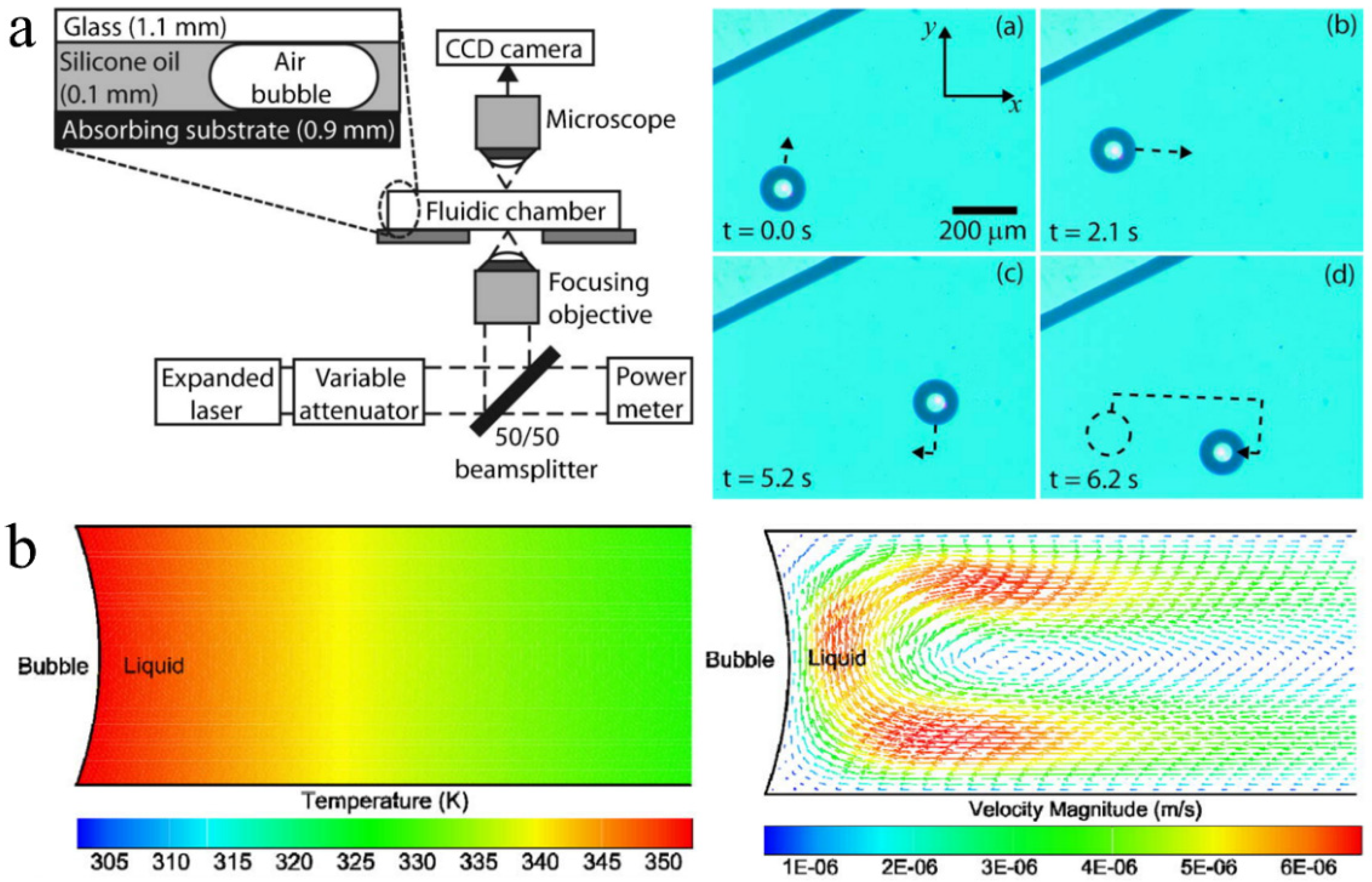
2.4. Acoustic Oscillation
2.5. Electrowetting-On-Dielectric (EWOD)
3. Bubbles Serving as Propulsion System
3.1. Propulsion by Catalytic Reaction Generated Bubbles
3.1.1. Self-Propelled Tubular Micromotors
3.1.2. Self-Propelled Janus Particles
3.1.3. Self-Propelled Micromachines
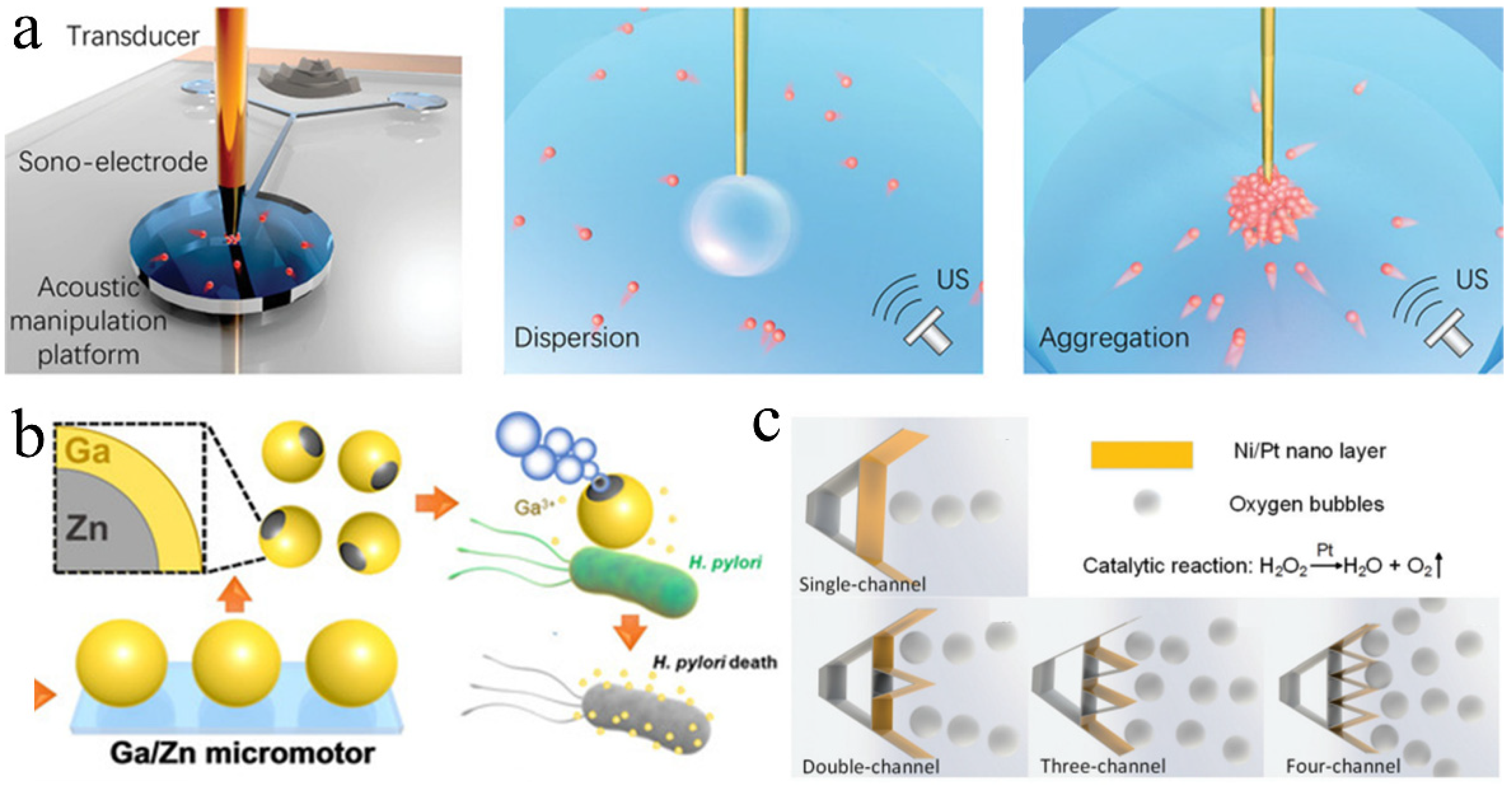
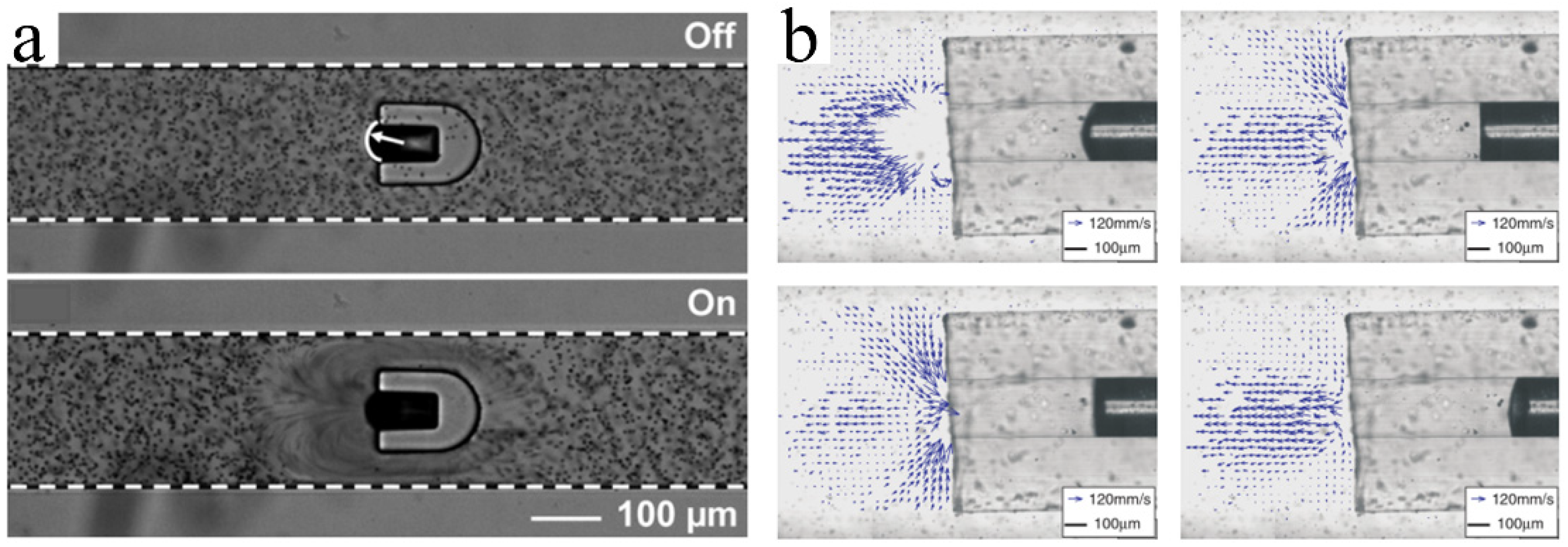
3.2. Propulsion by Acoustic Oscillating Bubbles

4. Bubbles Serving as Micromanipulators
4.1. Bubble Operators
4.2. Bubble Generator and Transmission Components

5. Bubbles Serving as Microrobots
5.1. Bubble Microrobots Used in 2D Manipulation and Assembly
5.1.1. EWOD-Driven Bubble Microrobots
5.1.2. Optothermal Bubble Microrobots
5.1.3. Bubble Microrobots Combining Light and Acoustic Fields
5.2. Bubble Microrobots Used in 3D Manipulation and Assembly

6. Summary and Outlook
- Multi-field combination of bubble microrobots
| Role/Mechanism | Production | Service Life | Advantage | Limit | Application | Ref. |
|---|---|---|---|---|---|---|
| Propulsion/chemical reaction generated bubbles | Chemical reaction | Short, bubbles are generated and quickly separated from the microrobot | Fast driving speed | The generation of bubbles and the movement performance of microrobot is affected by the consumption of chemical fuel; low biocompatibility | Biomedicine, biological detection, environmental purification | [107,136,140] |
| Propulsion/acoustically excited bubbles | Direct acquisition | Related to the shape and hydrophobic properties of the bubble-containing structures and acoustic excitation parameters | Simple and biocompatible equipment | Under long-time acoustic excitation, the change of bubble volume leads to a change of resonance frequency, which affects the motion performance of the microrobots | Targeted drug delivery, microsurgery, manipulation | [34,153,158,163,167,168] |
| Manipulator/manipulator based on acoustic bubble | Direct acquisition | Related to the acoustic excitation parameters | Adsorbable, removable, noninvasive, and flexible | Combined with manipulator, acoustic field, or magnetic field; complex structure | Analysis of living cells and biological samples, manipulation | [172] |
| Manipulator/bubble engine and transmission component | Direct acquisition, optothermal effect | For acoustic bubbles, service life relates to the structural design and acoustic excitation parameters. For optothermal bubbles, it depends on the opening and closing of the laser | Energy conversion, simple structure, and strong controllability | Weak change of flow field caused by bubble generation, oscillation or rupture; limited energy conversion | Energy conversion, cargo transportation, drug delivery | [13,178,181] |
| Microrobot/EWOD technology (2D) | Direct acquisition, chemical reaction | Related to the size of generated bubbles and acoustic excitation parameters | Controllable movement and low energy consumption | Limited movement of bubbles due to the electrode arrangement on the chip | Fluid mixing, micro-object manipulation | [182] |
| Microrobot/combining light field and acoustic field (2D) | Optothermal effect | Related to the opening and closing of the laser and acoustic excitation parameters | Improves the biocompatibility of optothermal bubbles | Narrow application range | Manipulation, particle classification | [48] |
| Microrobot/optothermal effect (2D/3D) | Optothermal effect | Depends on the opening and closing of the laser | Controllable and flexible bubble position and volume | Limited biomedical applications because of the high temperature around bubbles | Fluid control, cell lysis, manipulation, and assembly | [43,50,190,199,206] |
- Research and application of bionic microrobots based on bubbles
- In vivo microrobots based on bubbles
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Liu, X.; Huang, Q.; Ohta, A.T.; Arai, T. Bubbles in microfluidics: An all-purpose tool for micromanipulation. Lab Chip 2021, 21, 1016–1035. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Trapping and control of bubbles in various microfluidic applications. Lab Chip 2020, 20, 4512–4527. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Yang, D.; Mao, X.; Xie, L.; Gong, L.; Peng, X.; Peng, Q.; Wang, T.; Zhang, H.; Zeng, H. Recent advances in bubble-based technologies: Underlying interaction mechanisms and applications. Appl. Phys. Rev. 2021, 8, 011315. [Google Scholar] [CrossRef]
- Kharaji, Z.G.; Bayareh, M.; Kalantar, V. A review on acoustic field-driven micromixers. Int. J. Chem. React. Eng. 2021, 19, 553–569. [Google Scholar] [CrossRef]
- Bayareh, M.; Ashani, M.N.; Usefian, A. Active and passive micromixers: A comprehensive review. Chem. Eng. Process. Process Intensif. 2020, 147, 107771. [Google Scholar] [CrossRef]
- Liu, B.; Ma, C.; Yang, J.; Li, D.; Liu, H. Study on the Heat Source Insulation of a Thermal Bubble-Driven Micropump with Induction Heating. Micromachines 2021, 12, 1040. [Google Scholar] [CrossRef]
- Mohith, S.; Karanth, P.N.; Kulkarni, S.M. Recent trends in mechanical micropumps and their applications: A review. Mechatronics 2019, 60, 34–55. [Google Scholar] [CrossRef]
- Oskooei, A.; Günther, A. Bubble pump: Scalable strategy for in-plane liquid routing. Lab Chip 2015, 15, 2842–2853. [Google Scholar] [CrossRef]
- Qian, J.Y.; Hou, C.W.; Li, X.J.; Jin, Z.J. Actuation Mechanism of Microvalves: A Review. Micromachines 2020, 11, 172. [Google Scholar] [CrossRef]
- Läubli, N.F.; Shamsudhin, N.; Vogler, H.; Munglani, G.; Grossniklaus, U.; Ahmed, D.; Nelson, B.J. 3D Manipulation and Imaging of Plant Cells using Acoustically Activated Microbubbles. Small Methods 2019, 3, 1800527. [Google Scholar] [CrossRef]
- Tang, Q.; Liang, F.; Huang, L.; Zhao, P.; Wang, W. On-chip simultaneous rotation of large-scale cells by acoustically oscillating bubble array. Biomed. Microdevices 2020, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Giltinan, J.; Diller, E.; Sitti, M. Programmable assembly of heterogeneous microparts by an untethered mobile capillary microgripper. Lab Chip 2016, 16, 4445–4457. [Google Scholar] [CrossRef] [PubMed]
- Villangca, M.J.; Palima, D.; Banas, A.R.; Gluckstad, J. Light-driven micro-tool equipped with a syringe function. Light. Sci. Appl. 2016, 5, e16148. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.T.; Jamshidi, A.; Valley, J.K.; Hsu, H.Y.; Wu, M.C. Optically actuated thermocapillary movement of gas bubbles on an absorbing substrate. Appl. Phys. Lett. 2007, 91, 074103. [Google Scholar] [CrossRef]
- Guix, M.; Mayorga-Martinez, C.C.; Merkoçi, A. Nano/micromotors in (bio)chemical science applications. Chem. Rev. 2014, 114, 6285–6322. [Google Scholar] [CrossRef]
- Tasoglu, S.; Gurkan, U.A.; Wang, S.Q.; Demirci, U. Manipulating biological agents and cells in micro-scale volumes for applications in medicine. Chem. Soc. Rev. 2013, 42, 5788–5808. [Google Scholar] [CrossRef]
- Lin, Y.; Geng, X.; Chi, Q.; Wang, C.; Wang, Z. Driving Forces of the Bubble-Driven Tubular Micromotor Based on the Full Life-Cycle of the Bubble. Micromachines 2019, 10, 415. [Google Scholar] [CrossRef]
- Solovev, A.A.; Mei, Y.; Urena, E.B.; Huang, G.; Schmidt, O.G. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 2009, 5, 1688–1692. [Google Scholar] [CrossRef]
- Surdo, S.; Diaspro, A.; Duocastella, M. Micromixing with spark-generated cavitation bubbles. Microfluid. Nanofluidics 2017, 21, 82. [Google Scholar] [CrossRef]
- Ma, Z.; Melde, K.; Athanassiadis, A.G.; Schau, M.; Richter, H.; Qiu, T.; Fischer, P. Spatial ultrasound modulation by digitally controlling microbubble arrays. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Ye, Y.C.; Luan, J.B.; Wang, M.; Chen, Y.M.; Wilson, D.A.; Peng, F.; Tu, Y.F. Fabrication of Self-Propelled Micro- and Nanomotors Based on Janus Structures. Chem. A Eur. J. 2019, 25, 8663–8680. [Google Scholar] [CrossRef]
- Chen, C.; Karshalev, E.; Guan, J.; Wang, J. Magnesium-Based Micromotors: Water-Powered Propulsion, Multifunctionality, and Biomedical and Environmental Applications. Small 2018, 14, 1704252. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Karshalev, E.; Li, J.; Soto, F.; Castillo, R.; Campos, I.; Mou, F.; Guan, J.; Wang, J. Transient Micromotors That Disappear When No Longer Needed. ACS Nano 2016, 10, 10389–10396. [Google Scholar] [CrossRef] [PubMed]
- Battino, R.; Rettich, T.R.; Tominaga, T. The Solubility of Nitrogen and Air in Liquids. J. Phys. Chem. Ref. Data 1984, 13, 563–600. [Google Scholar] [CrossRef]
- Orbay, S.; Ozcelik, A.; Lata, J.; Kaynak, M.; Wu, M.; Huang, T.J. Mixing high-viscosity fluids via acoustically driven bubbles. J. Micromech. Microeng. 2017, 27, 015008. [Google Scholar] [CrossRef]
- Pereiro, I.; Khartchenko, A.F.; Petrini, L.; Kaigala, G.V. Nip the bubble in the bud: A guide to avoid gas nucleation in microfluidics. Lab Chip 2019, 19, 2296–2314. [Google Scholar] [CrossRef]
- Peng, T.; Zhou, M.; Yuan, S.; Jiang, B. Trapping stable bubbles in hydrophobic microchannel for continuous ultrasonic microparticle manipulation. Sens. Actuators A Phys. 2021, 331, 113045. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, X.; Cao, M.; Yu, C.; Li, K.; Jiang, L. Superhydrophobic helix: Controllable and directional bubble transport in an aqueous environment. J. Mater. Chem. A 2016, 4, 16865–16870. [Google Scholar] [CrossRef]
- Yu, C.; Cao, M.; Dong, Z.; Wang, J.; Li, K.; Jiang, L. Spontaneous and Directional Transportation of Gas Bubbles on Superhydrophobic Cones. Adv. Funct. Mater. 2016, 26, 3236–3243. [Google Scholar] [CrossRef]
- Ahmed, D.; Ozcelik, A.; Bojanala, N.; Nama, N.; Upadhyay, A.; Chen, Y.; Hanna-Rose, W.; Huang, T.J. Rotational manipulation of single cells and organisms using acoustic waves. Nat. Commun. 2016, 7, 11085. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, C.; Gao, Y.; Wu, M.; Yazdi, A.A.; Xu, J. Acoustofluidic micromixer on lab-on-a-foil devices. Sens. Actuators B Chem. 2019, 287, 312–319. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Mitra, D. Multiphysical phenomenon of air bubble growth in polydimethylsiloxane channel corners under microfluidic negative pressure-driven flow. Int. J. Heat Mass Transf. 2015, 91, 611–618. [Google Scholar] [CrossRef]
- Ren, L.; Nama, N.; McNeill, J.M.; Soto, F.; Yan, Z.; Liu, W.; Wang, W.; Wang, J.; Mallouk, T.E. 3D steerable, acoustically powered microswimmers for single-particle manipulation. Sci. Adv. 2019, 5, eaax3084. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wu, M. Biologically inspired micro-robotic swimmers remotely controlled by ultrasound waves. Lab Chip 2021, 21, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, H.; Wang, Y.; Zhu, C.; Wang, S.; Cao, J.; Zhu, S. Accumulating microparticles and direct-writing micropatterns using a continuous-wave laser-induced vapor bubble. Lab Chip 2011, 11, 3816–3820. [Google Scholar] [CrossRef]
- Ghosh, S.; Ranjan, A.D.; Das, S.; Sen, R.; Roy, B.; Roy, S.; Banerjee, A. Directed Self-Assembly Driven Mesoscale Lithography Using Laser-Induced and Manipulated Microbubbles: Complex Architectures and Diverse Applications. Nano Lett. 2021, 21, 10–25. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamamoto, Y.; Tamura, M.; Tokonami, S.; Iida, T. Damage-free light-induced assembly of intestinal bacteria with a bubble-mimetic substrate. Commun. Biol. 2021, 4, 385. [Google Scholar] [CrossRef]
- Zhang, Q.; Pang, Y.; Schiffbauer, J.; Jemcov, A.; Chang, H.-C.; Lee, E.; Luo, T. Light-Guided Surface Plasmonic Bubble Movement via Contact Line De-Pinning by In-Situ Deposited Plasmonic Nanoparticle Heating. ACS Appl. Mater. Interfaces 2019, 11, 48525–48532. [Google Scholar] [CrossRef]
- Lin, L.; Peng, X.; Mao, Z.; Li, W.; Yogeesh, M.N.; Rajeeva, B.B.; Perillo, E.P.; Dunn, A.K.; Akinwande, D.; Zheng, Y. Bubble-Pen Lithography. Nano Lett. 2016, 16, 701–708. [Google Scholar] [CrossRef]
- Baffou, G.; Polleux, J.; Rigneault, H.; Monneret, S. Super-Heating and Micro-Bubble Generation around Plasmonic Nanoparticles under cw Illumination. J. Phys. Chem. C 2014, 118, 4890–4898. [Google Scholar] [CrossRef]
- Ghosh, S.; Biswas, A.; Roy, B.; Banerjee, A. Self-assembly and complex manipulation of colloidal mesoscopic particles by active thermocapillary stress. Soft Matter 2019, 15, 4703–4713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zaytsev, M.E.; Lajoinie, G.; The, H.L.; Eijkel, J.C.T.; van den Berg, A.; Versluis, M.; Weckhuysen, B.M.; Zhang, X.; Zandvliet, H.J.W.; et al. Giant and explosive plasmonic bubbles by delayed nucleation. Proc. Natl. Acad. Sci. USA 2018, 115, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Ge, Z.; Jiao, N.; Liu, L. 2D to 3D Manipulation and Assembly of Microstructures Using Optothermally Generated Surface Bubble Microrobots. Small 2019, 15, 1902815. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.J.; Yang, S.T.; Shen, N.; Elhadj, S.; Raman, R.N.; Guss, G.; Bass, I.L.; Nostrand, M.C.; Wegner, P.J. Micro-Shaping, Polishing, and Damage Repair of Fused Silica Surfaces Using Focused Infrared Laser Beams. Adv. Eng. Mater. 2015, 17, 247–252. [Google Scholar] [CrossRef]
- Song, B.-S.; Noda, S.; Asano, T.; Akahane, Y. Ultra-high-Q photonic double-heterostructure nanocavity. Nat. Mater. 2005, 4, 207–210. [Google Scholar] [CrossRef]
- Wong, B.T.; Francoeur, M.; Mengüç, M.P. A Monte Carlo simulation for phonon transport within silicon structures at nanoscales with heat generation. Int. J. Heat Mass Transf. 2011, 54, 1825–1838. [Google Scholar] [CrossRef]
- Garrido, P.L.; Hurtado, P.I.; Nadrowski, B. Simple one-dimensional model of heat conduction which obeys Fourier’s law. Phys. Rev. Lett. 2001, 86, 5486–5489. [Google Scholar] [CrossRef]
- Xie, Y.; Nama, N.; Li, P.; Mao, Z.; Huang, P.H.; Zhao, C.; Costanzo, F.; Huang, T.J. Probing Cell Deformability via Acoustically Actuated Bubbles. Small 2016, 12, 902–910. [Google Scholar] [CrossRef]
- Holdeman, J.T.; Kim, J.W. Computation of incompressible thermal flows using Hermite finite elements. Comput. Methods Appl. Mech. Eng. 2010, 199, 3297–3304. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, Y.; Mao, Z.; Zhao, Y.; Rufo, J.; Yang, S.; Guo, F.; Mai, J.D.; Huang, T.J. Theory and experiment on particle trapping and manipulation via optothermally generated bubbles. Lab Chip 2014, 14, 384–391. [Google Scholar] [CrossRef]
- Hu, W.; Ishii, K.S.; Ohta, A.T. Micro-assembly using optically controlled bubble microrobots. Appl. Phys. Lett. 2011, 99, 094103. [Google Scholar] [CrossRef]
- Karbalaei, A.; Kumar, R.; Cho, H.J. Thermocapillarity in Microfluidics-A Review. Micromachines 2016, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Higuera, F.J. Steady thermocapillary-buoyant flow in an unbounded liquid layer heated nonuniformly from above. Phys. Fluids 2000, 12, 2186–2197. [Google Scholar] [CrossRef]
- Hu, W.; Ishii, K.S.; Fan, Q.; Ohta, A.T. Hydrogel microrobots actuated by optically generated vapour bubbles. Lab Chip 2012, 12, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Fan, Q.; Ohta, A.T. An opto-thermocapillary cell micromanipulator. Lab Chip 2013, 13, 2285–2291. [Google Scholar] [CrossRef]
- Fan, Q.; Hu, W.; Ohta, A.T. Laser-induced microbubble poration of localized single cells. Lab Chip 2014, 14, 1572–1578. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, T.H.; Kung, Y.C.; Teitell, M.A.; Chiou, P.Y. 3D pulsed laser-triggered high-speed microfluidic fluorescence-activated cell sorter. Analyst 2013, 138, 7308–7315. [Google Scholar] [CrossRef]
- Hashmi, A.; Yu, G.; Reilly-Collette, M.; Heiman, G.; Xu, J. Oscillating bubbles: A versatile tool for lab on a chip applications. Lab Chip 2012, 12, 4216–4227. [Google Scholar] [CrossRef]
- Wu, J.; Nyborg, W.L. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Deliv. Rev. 2008, 60, 1103–1116. [Google Scholar] [CrossRef]
- Yang, D.X.; Zhang, Q.; Zhang, Z.Z.; Yuan, Z.Y.; Xu, G.Y.; Wu, J.; Zhang, M.S.; Guo, X.S.; Tu, J.; Zhang, D. The influence of ultrasound-induced microbubble cavitation on the viability, migration and cell cycle distribution of melanoma cells. Appl. Acoust. 2021, 179, 108056. [Google Scholar] [CrossRef]
- Longuet-Higgins, M.S. Viscous streaming from an oscillating spherical bubble. Proc. R. Soc. A-Math. Phys. Eng. Sci. 1998, 454, 725–742. [Google Scholar] [CrossRef]
- Wu, J.R.; Du, G.H. Streaming generated by a bubble in an ultrasound field. J. Acoust. Soc. Am. 1997, 101, 1899–1907. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, S. Manipulation of Biological Objects Using Acoustic Bubbles: A Review. Integr. Comp. Biol. 2014, 54, 959–968. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Z.C.; Merritt, B.; Strack, D.; Xu, J.; Lee, S. Onset of particle trapping and release via acoustic bubbles. Lab Chip 2016, 16, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.; Neild, A. Selective particle trapping using an oscillating microbubble. Lab Chip 2011, 11, 3710–3715. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Mao, X.; Shi, J.; Juluri, B.K.; Huang, T.J. A millisecond micromixer via single-bubble-based acoustic streaming. Lab Chip 2009, 9, 2738–2741. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. A bubble-driven microfluidic transport element for bioengineering. Proc. Natl. Acad. Sci. USA 2004, 101, 9523–9527. [Google Scholar] [CrossRef]
- van der Wijngaart, W.; Chugh, D.; Man, E.; Melin, J.; Stemme, G. A Low-Temperature Thermopneumatic Actuation Principle for Gas Bubble Microvalves. J. Micromech. Syst. 2007, 16, 765–774. [Google Scholar] [CrossRef]
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef]
- Dijkink, R.J.; van der Dennen, J.P.; Ohl, C.D.; Prosperetti, A. The ‘acoustic scallop’: A bubble-powered actuator. J. Micromech. Microeng. 2006, 16, 1653–1659. [Google Scholar] [CrossRef]
- Fair, R.B. Digital microfluidics: Is a true lab-on-a-chip possible? Microfluid. Nanofluidics 2007, 3, 245–281. [Google Scholar] [CrossRef]
- Chae, J.B.; Lee, S.J.; Yang, J.; Chung, S.K. 3D electrowetting-on-dielectric actuation. Sens. Actuators A Phys. 2015, 234, 331–338. [Google Scholar] [CrossRef]
- Zhao, Y.; Cho, S.K. Micro air bubble manipulation by electrowetting on dielectric (EWOD): Transporting, splitting, merging and eliminating of bubbles. Lab Chip 2007, 7, 273–280. [Google Scholar] [CrossRef]
- Chung, S.K.; Cho, S.K. On-chip manipulation of objects using mobile oscillating bubbles. J. Micromech. Microeng. 2008, 18, 125024. [Google Scholar] [CrossRef]
- Chung, S.K.; Kwon, J.O.; Cho, S.K. Manipulation of Micro/Mini-objects by AC-Electrowetting-Actuated Oscillating Bubbles: Capturing, Carrying and Releasing. J. Adhes. Sci. Technol. 2012, 26, 1965–1983. [Google Scholar] [CrossRef]
- Cheng, M.; Xiao, M.; Shi, F. Self-propelling mini-motor and its applications in supramolecular self-assembly and energy conversion. Sci. Sin. Chim. 2017, 47, 40–61. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Zhang, G.; Zhou, D.; Chang, X. Propulsion mechanisms and applications of multiphysics- driven micro- and nanomotors. Chin. Sci. Bull. 2016, 62, 122–135. [Google Scholar] [CrossRef]
- Ren, B.; Cai, Y.; Dong, R. Light-driven micro/namomotors: Mechanisms and performances. Chin. Sci. Bull. 2016, 62, 152–166. [Google Scholar] [CrossRef]
- Xu, T.; Song, Y.; Zhang, X.; Huang, J.; Liu, C. Controlling the micro/nanomotors motion and their application in precision medicine. Sci. Sin. Chim. 2017, 47, 29–38. [Google Scholar] [CrossRef]
- Huang, T.; Yu, J.; Zhang, L.; Jin, D.; Duan, H. Magnetic micro-/nanoscale swimmers: Current status and potential applications. Chin. Sci. Bull. 2016, 62, 136–151. [Google Scholar] [CrossRef][Green Version]
- Si, T.; Lin, X.; Wu, Z.; He, Q. Controlled molecular assembly of self-propelled colloid motors and their biomedical applications. Sci. Sin. Chim. 2016, 47, 3–13. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, J.; Cui, H.; Wang, L. Research progress about the applications of self-propellant Janus particles in water environment. Sci. Sin. Chim. 2016, 47, 70–81. [Google Scholar] [CrossRef]
- Zha, F.; Wang, T.; Luo, M.; Guan, J. Tubular Micro/Nanomotors: Propulsion Mechanisms, Fabrication Techniques and Applications. Micromachines 2018, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.G.; Eberl, K. Nanotechnology—Thin solid films roll up into nanotubes. Nature 2001, 410, 168. [Google Scholar] [CrossRef] [PubMed]
- Manesh, K.M.; Cardona, M.; Yuan, R.; Clark, M.; Kagan, D.; Balasubramanian, S.; Wang, J. Template-Assisted Fabrication of Salt-Independent Catalytic Tubular Microengines. ACS Nano 2010, 4, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ambrosi, A.; Pumera, M. Self-propelled nanojets via template electrodeposition. Nanoscale 2013, 5, 1319–1324. [Google Scholar] [CrossRef]
- Mei, Y.; Huang, G.; Solovev, A.A.; Ureña, E.B.; Mönch, I.; Ding, F.; Reindl, T.; Fu, R.K.Y.; Chu, P.K.; Schmidt, O.G. Versatile Approach for Integrative and Functionalized Tubes by Strain Engineering of Nanomembranes on Polymers. Adv. Mater. 2008, 20, 4085–4090. [Google Scholar] [CrossRef]
- Solovev, A.A.; Xi, W.; Gracias, D.H.; Harazim, S.M.; Deneke, C.; Sanchez, S.; Schmidt, O.G. Self-Propelled Nanotools. Acs Nano 2012, 6, 1751–1756. [Google Scholar] [CrossRef]
- Wrede, P.; Medina-Sánchez, M.; Fomin, V.M.; Schmidt, O.G. Switching Propulsion Mechanisms of Tubular Catalytic Micromotors. Small 2021, 17, 2006449. [Google Scholar] [CrossRef]
- Manjare, M.; Yang, B.; Zhao, Y.P. Bubble-Propelled Microjets: Model and Experiment. J. Phys. Chem. C 2013, 117, 4657–4665. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Li, T.; Song, W.; Zhang, G. Hydrodynamics and propulsion mechanism of self-propelled catalytic micromotors: Model and experiment. Soft Matter 2014, 10, 7511–7518. [Google Scholar] [CrossRef] [PubMed]
- Klingner, A.; Khalil, I.S.M.; Magdanz, V.; Fomin, V.M.; Schmidt, O.G.; Misra, S. Modeling of Unidirectional-Overloaded Transition in Catalytic Tubular Microjets. J. Phys. Chem. C 2017, 121, 14854–14863. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.; Ye, M.; Li, M.; Liu, R.; Mei, Y. Dynamics of catalytic tubular microjet engines: Dependence on geometry and chemical environment. Nanoscale 2011, 3, 5083–5089. [Google Scholar] [CrossRef]
- Mei, Y.; Solovev, A.A.; Sanchez, S.; Schmidt, O.G. Rolled-up nanotech on polymers: From basic perception to self-propelled catalytic microengines. Chem. Soc. Rev. 2011, 40, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Wang, H.; Pumera, M. Beyond platinum: Silver-catalyst based bubble-propelled tubular micromotors. Chem. Commun. 2016, 52, 4333–4336. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Zou, X.; Sun, J.; He, Q. Biodegradable protein-based rockets for drug transportation and light-triggered release. ACS Appl. Mater. Interfaces 2015, 7, 250–255. [Google Scholar] [CrossRef]
- Solovev, A.A.; Smith, E.J.; Bof’ Bufon, C.C.; Sanchez, S.; Schmidt, O.G. Light-controlled propulsion of catalytic microengines. Angew. Chem. Int. Ed. Engl. 2011, 50, 10875–10878. [Google Scholar] [CrossRef]
- Xu, T.; Soto, F.; Gao, W.; Garcia-Gradilla, V.; Li, J.; Zhang, X.; Wang, J. Ultrasound-modulated bubble propulsion of chemically powered microengines. J. Am. Chem. Soc. 2014, 136, 8552–8555. [Google Scholar] [CrossRef]
- Zhao, G.; Viehrig, M.; Pumera, M. Challenges of the movement of catalytic micromotors in blood. Lab Chip 2013, 13, 1930–1936. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, G.; Mei, Y.; Men, C. Manipulation and applications of rolled-up microtubular engine. Sci. Sin. Chim. 2016, 47, 14–28. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Wu, J.; Ju, H. Bubble-Propelled Jellyfish-like Micromotors for DNA Sensing. ACS Appl. Mater. Interfaces 2019, 11, 13581–13588. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Jiang, Y.; Su, J.; Deng, Z.; Mou, F.; Xu, L.; Guan, J. Surface Charge-Reversible Tubular Micromotors for Extraction of Nucleic Acids in Microsystems. Chem. Asian J. 2019, 14, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, B.E.F.; Lopez-Ramirez, M.A.; Báez, D.F.; Jodra, A.; Singh, V.V.; Kaufmann, K.; Wang, J. Aptamer-Modified Graphene-Based Catalytic Micromotors: Off–On Fluorescent Detection of Ricin. ACS Sens. 2016, 1, 217–221. [Google Scholar] [CrossRef]
- Liang, C.; Zhan, C.; Zeng, F.; Xu, D.; Wang, Y.; Zhao, W.; Zhang, J.; Guo, J.; Feng, H.; Ma, X. Bilayer Tubular Micromotors for Simultaneous Environmental Monitoring and Remediation. ACS Appl. Mater. Interfaces 2018, 10, 35099–35107. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Zhang, H. Individual behaviors and dynamic self-assembly of active colloids. Chin. Sci. Bull. 2016, 62, 194–208. [Google Scholar] [CrossRef]
- Lu, X.; Shen, H.; Wei, Y.; Ge, H.; Wang, J.; Peng, H.; Liu, W. Ultrafast Growth and Locomotion of Dandelion-Like Microswarms with Tubular Micromotors. Small 2020, 16, e2003678. [Google Scholar] [CrossRef]
- Lu, X.; Wei, Y.; Ou, H.; Zhao, C.; Shi, L.; Liu, W. Universal Control for Micromotor Swarms with a Hybrid Sonoelectrode. Small 2021, 17, e2104516. [Google Scholar] [CrossRef]
- Walther, A.; Muller, A.H.E. Janus particles. Soft Matter 2008, 4, 663–668. [Google Scholar] [CrossRef]
- Yang, S.K.; Guo, F.; Kiraly, B.; Mao, X.L.; Lu, M.Q.; Leong, K.W.; Huang, T.J. Microfluidic synthesis of multifunctional Janus particles for biomedical applications. Lab Chip 2012, 12, 2097–2102. [Google Scholar] [CrossRef]
- Kong, L.; Mou, F.; Jiang, Y.; Li, X.; Guan, J. Design strategies and structure simplification methods of self-propelled micro-/nanomotors. Chin. Sci. Bull. 2016, 62, 107–121. [Google Scholar] [CrossRef][Green Version]
- Paxton, W.F.; Kistler, K.C.; Olmeda, C.C.; Sen, A.; Angelo, S.K.S.; Cao, Y.; Mallouk, T.E.; Lammert, P.E.; Crespi, V.H. Catalytic nanomotors: Autonomous movement of striped nanorods. J. Am. Chem. Soc. 2004, 126, 13424–13431. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.F.; Sen, A.; Mallouk, T.E. Motility of catalytic nanoparticles through self-generated forces. Chemistry 2005, 11, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Fournier-Bidoz, S.; Arsenault, A.C.; Manners, I.; Ozin, G.A. Synthetic self-propelled nanorotors. Chem. Commun. 2005, 4, 441–443. [Google Scholar] [CrossRef]
- Howse, J.R.; Jones, R.A.L.; Ryan, A.J.; Gough, T.; Vafabakhsh, R.; Golestanian, R. Self-motile colloidal particles: From directed propulsion to random walk. Phys. Rev. Lett. 2007, 99, 048102. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.G.; Zhao, Y.P. Autonomously motile catalytic nanomotors by bubble propulsion. Appl. Phys. Lett. 2009, 94, 163104. [Google Scholar] [CrossRef]
- Walther, A.; Muller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, Z.; Zheng, X. The mechanisms of the self-propelled spherical Janus micromotor: Self-diffusiophoresis and microbubble propulsion. Chin. Sci. Bull. 2016, 62, 167–185. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Hess, H. Chemically-powered swimming and diffusion in the microscopic world. Nat. Rev. Chem. 2021, 5, 500–510. [Google Scholar] [CrossRef]
- Naeem, S.; Naeem, F.; Mujtaba, J.; Shukla, A.K.; Mitra, S.; Huang, G.; Gulina, L.; Rudakovskaya, P.; Cui, J.; Tolstoy, V. Oxygen generation using catalytic nano/micromotors. Micromachines 2021, 12, 1251. [Google Scholar] [CrossRef]
- Hu, L.; Miao, J.; Grüber, G. Temperature effects on disk-like gold-nickel-platinum nanoswimmer’s propulsion fuelled by hydrogen peroxide. Sens. Actuators B Chem. 2017, 239, 586–596. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, X.; Wu, Z.; Mohwald, H.; He, Q. Self-propelled polymer multilayer Janus capsules for effective drug delivery and light-triggered release. ACS Appl. Mater. Interfaces 2014, 6, 10476–10481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, C.; Lin, Z.; Wang, D.; Li, Y.; Yuan, Y.; Zhu, B.; He, Q. Autonomous motion of bubble-powered carbonaceous nanoflask motors. Langmuir 2020, 36, 7039–7045. [Google Scholar] [CrossRef]
- Rao, D.V.; Reddy, N.; Fransaer, J.; Clasen, C. Self-propulsion of bent bimetallic Janus rods. J. Phys. D Appl. Phys. 2018, 52, 014002. [Google Scholar]
- Ye, H.; Ma, G.; Kang, J.; Sun, H.; Wang, S. Pt-Free microengines at extremely low peroxide levels. Chem. Commun. 2018, 54, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, Y.; Liu, X.; Xu, D.; Yuan, H.; Sun, H.; Wang, S.; Ma, X. Magnetically steerable iron oxides-manganese dioxide core–shell micromotors for organic and microplastic removals. J. Colloid Interface Sci. 2021, 588, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.H.; Wang, S.; Xian, L.; Zhou, X.; Chen, Y.; Lin, G.; Gao, Y. Highly efficient chemically-driven micromotors with controlled snowman-like morphology. Chem. Commun. 2020, 56, 15301–15304. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, K.; Zhou, Q.; Xu, C.; Gao, J.; Wang, Z.; Wang, F.; Chen, B.; Ye, Y.; Ou, J. Hydrogen-Powered Microswimmers for Precise and Active Hydrogen Therapy Towards Acute Ischemic Stroke. Adv. Funct. Mater. 2021, 31, 2009475. [Google Scholar] [CrossRef]
- Zhao, G.; Pumera, M. Magnetotactic artificial self-propelled nanojets. Langmuir 2013, 29, 7411–7415. [Google Scholar] [CrossRef]
- Reddy, N.K.; Clasen, C. Self-propelling micro-disks. Korea-Aust. Rheol. J. 2014, 26, 73–79. [Google Scholar] [CrossRef][Green Version]
- Mena-Giraldo, P.; Orozco, J. Polymeric Micro/Nanocarriers and Motors for Cargo Transport and Phototriggered Delivery. Polymers 2021, 13, 3920. [Google Scholar] [CrossRef]
- Wu, Z.; Si, T.; Gao, W.; Lin, X.; Wang, J.; He, Q. Superfast Near-Infrared Light-Driven Polymer Multilayer Rockets. Small 2016, 12, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Díez, P.; Lucena-Sánchez, E.; Escudero, A.; Llopis-Lorente, A.; Villalonga, R.; Martinez-Manez, R. Ultrafast Directional Janus Pt–Mesoporous Silica Nanomotors for Smart Drug Delivery. ACS Nano 2021, 15, 4467–4480. [Google Scholar] [CrossRef] [PubMed]
- de Avila, B.E.F.; Angsantikul, P.; Li, J.X.; Lopez-Ramirez, M.A.; Ramirez-Herrera, D.E.; Thamphiwatana, S.; Chen, C.R.; Delezuk, J.; Samakapiruk, R.; Ramez, V.; et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection (vol 8, 272, 2017). Nat. Commun. 2017, 8, 1299. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medina, M.; Ramos-Docampo, M.A.; Hovorka, O.; Salgueiriño, V.; Städler, B. Recent advances in nano-and micromotors. Adv. Funct. Mater. 2020, 30, 1908283. [Google Scholar] [CrossRef]
- Karshalev, E.; Zhang, Y.; de Avila, B.E.F.; Beltran-Gastelum, M.; Chen, Y.J.; Mundaca-Uribe, R.; Zhang, F.Y.; Nguyen, B.; Tong, Y.; Fang, R.H. Micromotors for active delivery of minerals toward the treatment of iron deficiency anemia. Nano Lett. 2019, 19, 7816–7826. [Google Scholar] [CrossRef]
- Lin, Z.; Gao, C.; Wang, D.; He, Q. Bubble-Propelled Janus Gallium/Zinc Micromotors for the Active Treatment of Bacterial Infections. Angew. Chem. Int. Ed. 2021, 60, 8750–8754. [Google Scholar] [CrossRef]
- Ismagilov, R.F.; Schwartz, A.; Bowden, N.; Whitesides, G.M. Autonomous Movement and Self-Assembly. Angew. Chem. Int. Ed. 2002, 41, 652–654. [Google Scholar] [CrossRef]
- Zhu, W.; Li, J.; Leong, Y.J.; Rozen, I.; Qu, X.; Dong, R.; Wu, Z.; Gao, W.; Chung, P.H.; Wang, J.; et al. 3D-Printed Artificial Microfish. Adv. Mater. 2015, 27, 4411–4417. [Google Scholar] [CrossRef]
- Yoshizumi, Y.; Suzuki, H. Self-Propelled Metal-Polymer Hybrid Micromachines with Bending and Rotational Motions. Acs Appl. Mater. Interfaces 2017, 9, 21355–21361. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Yang, Y.; Shen, Y. A fast and powerful swimming microrobot with a serrated tail enhanced propulsion interface. Nanoscale 2018, 10, 19673–19677. [Google Scholar] [CrossRef]
- Singh, A.V.; Sitti, M. Targeted Drug Delivery and Imaging Using Mobile Milli/Microrobots: A Promising Future Towards Theranostic Pharmaceutical Design. Curr. Pharm. Des. 2016, 22, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Burgner-Kahrs, J.; Rucker, D.C.; Choset, H. Continuum Robots for Medical Applications: A Survey. IEEE Trans. Robot. 2015, 31, 1261–1280. [Google Scholar] [CrossRef]
- Shi, C.; Luo, X.; Qi, P.; Li, T.; Song, S.; Najdovski, Z.; Fukuda, T.; Ren, H. Shape Sensing Techniques for Continuum Robots in Minimally Invasive Surgery: A Survey. IEEE Trans. Biomed. Eng. 2017, 64, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Sitti, M. Voyage of the microrobots. Nature 2009, 458, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Behkam, B.; Sitti, M. Bacterial flagella-based propulsion and on/off motion control of microscale objects. Appl. Phys. Lett. 2007, 90, 023902. [Google Scholar] [CrossRef]
- Simmchen, J.; Katuri, J.; Uspal, W.E.; Popescu, M.N.; Tasinkevych, M.; Sánchez, S. Topographical pathways guide chemical microswimmers. Nat. Commun. 2016, 7, 10598. [Google Scholar] [CrossRef]
- Rikken, R.S.; Nolte, R.J.; Maan, J.C.; van Hest, J.C.; Wilson, D.A.; Christianen, P.C. Manipulation of micro-and nanostructure motion with magnetic fields. Soft Matter 2014, 10, 1295–1308. [Google Scholar] [CrossRef]
- Kaynak, M.; Ozcelik, A.; Nourhani, A.; Lammert, P.E.; Crespi, V.H.; Huang, T.J. Acoustic actuation of bioinspired microswimmers. Lab Chip 2017, 17, 395–400. [Google Scholar] [CrossRef]
- Rao, K.J.; Li, F.; Meng, L.; Zheng, H.; Cai, F.; Wang, W. A force to be reckoned with: A review of synthetic microswimmers powered by ultrasound. Small 2015, 11, 2836–2846. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Liu, F.W.; Xiao, Z.D.; Sharma, N.; Cho, S.K.; Kim, K. Ultrasound Tracking of the Acoustically Actuated Microswimmer. IEEE Trans. Biomed. Eng. 2019, 66, 3231–3237. [Google Scholar] [CrossRef]
- Won, J.M.; Lee, J.H.; Lee, K.H.; Rhee, K.; Chung, S.K. Propulsion of water-floating objects by acoustically oscillating microbubbles. Int. J. Precis. Eng. Manuf. 2011, 12, 577–580. [Google Scholar] [CrossRef]
- Ahmed, D.; Lu, M.; Nourhani, A.; Lammert, P.E.; Stratton, Z.; Muddana, H.S.; Crespi, V.H.; Huang, T.J. Selectively manipulable acoustic-powered microswimmers. Sci. Rep. 2015, 5, 9744. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Dillinger, C.; Hong, A.; Nelson, B.J. Artificial Acousto-Magnetic Soft Microswimmers. Adv. Mater. Technol. 2017, 2, 1700050. [Google Scholar] [CrossRef]
- McNeill, J.M.; Nama, N.; Braxton, J.M.; Mallouk, T.E. Wafer-Scale Fabrication of Micro- to Nanoscale Bubble Swimmers and Their Fast Autonomous Propulsion by Ultrasound. ACS Nano 2020, 14, 7520–7528. [Google Scholar] [CrossRef]
- Qiu, T.; Palagi, S.; Mark, A.G.; Melde, K.; Adams, F.; Fischer, P. Active Acoustic Surfaces Enable the Propulsion of a Wireless Robot. Adv. Mater. Interfaces 2017, 4, 1700933. [Google Scholar] [CrossRef]
- Feng, J.; Yuan, J.; Cho, S.K. Micropropulsion by an acoustic bubble for navigating microfluidic spaces. Lab Chip 2015, 15, 1554–1562. [Google Scholar] [CrossRef]
- Feng, J.; Yuan, J.; Cho, S.K. 2-D steering and propelling of acoustic bubble-powered microswimmers. Lab Chip 2016, 16, 2317–2325. [Google Scholar] [CrossRef]
- Liu, F.W.; SCho, K. 3-D swimming microdrone powered by acoustic bubbles. Lab Chip 2021, 21, 355–364. [Google Scholar] [CrossRef]
- Bertin, N.; Spelman, T.A.; Stephan, O.; Gredy, L.; Bouriau, M.; Lauga, E.; Marmottant, P. Propulsion of Bubble-Based Acoustic Microswimmers. Phys. Rev. Appl. 2015, 4, 064012. [Google Scholar] [CrossRef]
- Bertin, N.; Spelman, T.A.; Combriat, T.; Hue, H.; Stephan, O.; Lauga, E.; Marmottant, P. Bubble-based acoustic micropropulsors: Active surfaces and mixers. Lab Chip 2017, 17, 1515–1528. [Google Scholar] [CrossRef]
- Spelman, T.A.; Stephan, O.; Marmottant, P. Multi-directional bubble generated streaming flows. Ultrasonics 2020, 102, 106054. [Google Scholar] [CrossRef] [PubMed]
- Louf, J.-F.; Bertin, N.; Dollet, B.; Stephan, O.; Marmottant, P. Hovering Microswimmers Exhibit Ultrafast Motion to Navigate under Acoustic Forces. Adv. Mater. Interfaces 2018, 5, 1800425. [Google Scholar] [CrossRef]
- Aghakhani, A.; Yasa, O.; Wrede, P.; Sitti, M. Acoustically powered surface-slipping mobile microrobots. Proc. Natl. Acad. Sci. USA 2020, 117, 3469–3477. [Google Scholar] [CrossRef]
- Aghakhani, A.; Pena-Francesch, A.; Bozuyuk, U.; Cetin, H.; Wrede, P.; Sitti, M. High shear rate propulsion of acoustic microrobots in complex biological fluids. Sci. Adv. 2022, 8, eabm5126. [Google Scholar] [CrossRef]
- Jang, D.; Jeon, J.; Chung, S.K. Acoustic bubble-powered miniature rotor for wireless energy harvesting in a liquid medium. Sens. Actuators A Phys. 2018, 276, 296–303. [Google Scholar] [CrossRef]
- Dincel, O.; Ueta, T.; Kameoka, J. Acoustic driven microbubble motor device. Sens. Actuators A Phys. 2019, 295, 343–347. [Google Scholar] [CrossRef]
- Mohanty, S.; Zhang, J.; McNeill, J.M.; Kuenen, T.; Linde, F.P.; Rouwkema, J.; Misra, S. Acoustically-actuated bubble-powered rotational micro-propellers. Sens. Actuators B Chem. 2021, 347, 130589. [Google Scholar] [CrossRef]
- Mohanty, S.; Paul, A.; Matos, P.M.; Zhang, J.; Sikorski, J.; Misra, S. CeFlowBot: A Biomimetic Flow-Driven Microrobot that Navigates under Magneto-Acoustic Fields. Small 2021, 18, e2105829. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.K.; Cho, S.K. 3-D manipulation of millimeter- and micro-sized objects using an acoustically excited oscillating bubble. Microfluid. Nanofluidics 2008, 6, 261–265. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.H.; Won, J.M.; Rhee, K.; Chung, S.K. Micromanipulation using cavitational microstreaming generated by acoustically oscillating twin bubbles. Sens. Actuators A Phys. 2012, 188, 442–449. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Huang, Q.; Arai, T. Controlled rotation of micro-objects using acoustically driven microbubbles. Appl. Phys. Lett. 2021, 118, 063701. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Yan, J.; Guo, S.; Li, T. Soft-Contact Acoustic Microgripper Based on a Controllable Gas-Liquid Interface for Biomicromanipulations. Small 2021, 17, 2104579. [Google Scholar] [CrossRef]
- Kwon, J.O.; Yang, J.S.; Lee, S.J.; Rhee, K.; Chung, S.K. Electromagnetically actuated micromanipulator using an acoustically oscillating bubble. J. Micromech. Microeng. 2011, 21, 115023. [Google Scholar] [CrossRef]
- Park, I.S.; Lee, Y.R.; Hong, S.J.; Lee, K.Y.; Chung, S.K. On-chip enucleation using an untethered microrobot incorporated with an acoustically oscillating bubble. In Proceedings of the 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015. [Google Scholar]
- Park, I.S.; Shin, J.H.; Lee, Y.R.; Chung, S.K. On-chip micromanipulation using a magnetically driven micromanipulator with an acoustically oscillating bubble. Sens. Actuators A Phys. 2016, 248, 214–222. [Google Scholar] [CrossRef]
- Kwon, J.O.; Yang, J.S.; Chae, J.B.; Chung, S.K. A novel drug delivery method by using a microrobot incorporated with an acoustically oscillating bubble. In Proceedings of the 26th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Taipei, Taiwan, 20–24 January 2013. [Google Scholar]
- Kwon, J.O.; Yang, J.S.; Chae, J.B.; Chung, S.K. Micro-object manipulation in a microfabricated channel using an electromagnetically driven microrobot with an acoustically oscillating bubble. Sens. Actuators A Phys. 2014, 215, 77–82. [Google Scholar] [CrossRef]
- Jeon, J.P.; Hong, J.; Lee, Y.R.; Seo, J.H.; Oh, S.H.; Chung, S.K. Novel energy harvesting using acoustically oscillating microbubbles. In Proceedings of the 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 January 2015. [Google Scholar]
- Jeon, J.; Hong, J.; Lee, S.J.; Chung, S.K. Acoustically Excited Oscillating Bubble on a Flexible Structure and Its Energy-Harvesting Capability. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 531–537. [Google Scholar] [CrossRef]
- Jiang, L.; Erickson, D. Directed self-assembly of microcomponents enabled by laser-activated bubble latching. Langmuir 2011, 27, 11259–11264. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Jang, D.; Kim, D.; Lee, D.; Chung, S.K. Acoustic bubble-based drug manipulation: Carrying, releasing and penetrating for targeted drug delivery using an electromagnetically actuated microrobot. Sens. Actuators A Phys. 2020, 306, 111973. [Google Scholar] [CrossRef]
- Chung, S.K.; Zhao, Y.; Cho, S.K. On-chip creation and elimination of microbubbles for a micro-object manipulator. J. Micromech. Microeng. 2008, 18, 095009. [Google Scholar] [CrossRef]
- Chung, S.K.; Rhee, K.; Cho, S.K. Bubble actuation by electrowetting-on-dielectric (EWOD) and its applications: A review. Int. J. Precis. Eng. Manuf. 2010, 11, 991–1006. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.H.; Won, J.M.; Rhee, K.; Chung, S.K. Mobile oscillating bubble actuated by AC-electrowetting-on-dielectric (EWOD) for microfluidic mixing enhancement. Sens. Actuators A Phys. 2012, 182, 153–162. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.H.; Chae, J.B.; Rhee, K.; Chung, S.K. On-chip micromanipulation by AC-EWOD driven twin bubbles. Sens. Actuators A Phys. 2013, 195, 167–174. [Google Scholar] [CrossRef]
- Yan, R.; Pham, R.; Chen, C.L. Activating Bubble’s Escape, Coalescence, and Departure under an Electric Field Effect. Langmuir 2020, 36, 15558–15571. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhuang, L.; Wei, M.; Sun, H.; Liu, F.; Tang, B.; Groenewold, J.; Zhou, G. Bubble Manipulation Driven by Alternating Current Electrowetting: Oscillation Modes and Surface Detachment. Langmuir 2021, 37, 6898–6904. [Google Scholar] [CrossRef]
- Hu, W.Q.; Fan, Q.H.; Tonaki, W.; Ohta, A.T. Bubble-Driven Light-Absorbing Hydrogel Microrobot for the Assembly of Bio-Objects. In Proceedings of the 35th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar]
- Quinto-Su, P.A.; Kuss, C.; Preiser, P.R.; Ohl, C.D. Red blood cell rheology using single controlled laser-induced cavitation bubbles. Lab Chip 2011, 11, 672–678. [Google Scholar] [CrossRef]
- Fan, Q.; Hu, W.; Ohta, A.T. Localized Single-Cell Lysis and Manipulation Using Optothermally-Induced Bubbles. Micromachines 2017, 8, 121. [Google Scholar] [CrossRef]
- Fan, Q.; Hu, W.; Ohta, A.T. Efficient single-cell poration by microsecond laser pulses. Lab Chip 2015, 15, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, C.; Zhong, P. Cell membrane deformation and bioeffects produced by tandem bubble-induced jetting flow. Proc. Natl. Acad. Sci. USA 2015, 112, E7039–E7047. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Kobayashi, K.; Kanaizuka, K.; Okamoto, T.; Toyabe, S.; Muneyuki, E.; Haga, M.-A. Manipulation of single DNA using a micronanobubble formed by local laser heating on a Au-coated surface. Chem. Lett. 2010, 39, 92–93. [Google Scholar] [CrossRef]
- Ceylan, H.; Giltinan, J.; Kozielski, K.; Sitti, M. Mobile microrobots for bioengineering applications. Lab Chip 2017, 17, 1705–1724. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, C. An optothermally generated surface bubble and its applications. Nanoscale 2017, 9, 6622–6631. [Google Scholar] [CrossRef]
- Takahashi, N.; Wang, Z.D.; Rahman, M.A.; Cheng, J.L.; Ohta, A.T. Automated Micro-object Caging Using Bubble Microrobots. In Proceedings of the 11th IEEE Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, 17–20 April 2016. [Google Scholar]
- Rahman, M.A.; Cheng, J.L.; Fan, Q.H.; Ohta, A.T. Automated Actuation of Multiple Bubble Microrobots Using Computer-Generated Holograms. In Proceedings of the Conference on Next-Generation Robotics II and Machine Intelligence and Bio-inspired Computation—Theory and Applications IX, Baltimore, MD, USA, 21–22 April 2015. [Google Scholar]
- Rahman, M.A.; Chong, J.L.; Ohta, A.T. Parallel Actuation and Independent Addressing of Many Bubble Microrobots. In Proceedings of the 11th IEEE Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, 17–20 April 2016. [Google Scholar]
- Rahman, M.A.; Cheng, J.; Wang, Z.; Ohta, A.T. Cooperative Micromanipulation Using the Independent Actuation of Fifty Microrobots in Parallel. Sci. Rep. 2017, 7, 3278. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, C.; Zhao, Y.; Li, S.; Rufo, J.; Yang, S.; Guo, F.; Huang, T.J. Optoacoustic tweezers: A programmable, localized cell concentrator based on opto-thermally generated, acoustically activated, surface bubbles. Lab Chip 2013, 13, 1772–1779. [Google Scholar] [CrossRef]
- Dai, L.; Jiao, N.; Wang, X.; Liu, L. A Micromanipulator and Transporter Based on Vibrating Bubbles in an Open Chip Environment. Micromachines 2017, 8, 130. [Google Scholar] [CrossRef]
- Shin, J.H.; Seo, J.; Hong, J.; Chung, S.K. Hybrid optothermal and acoustic manipulations of microbubbles for precise and on-demand handling of micro-objects. Sens. Actuators B Chem. 2017, 246, 415–420. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, H.; Li, J.; Shi, Q.; Cui, J.; Sun, T.; Huang, Q.; Fukuda, T. 3D Construction of Shape-Controllable Tissues through Self-Bonding of Multicellular Microcapsules. ACS Appl. Mater. Interfaces 2019, 11, 22950–22961. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Q.; Wang, H.; Sun, T.; Yu, N.; Huang, Q.; Fukuda, T. Automated Fluidic Assembly of Microvessel-Like Structures Using a Multimicromanipulator System. IEEE/ASME Trans. Mechatron. 2018, 23, 667–678. [Google Scholar] [CrossRef]
- Wang, H.; Cui, J.; Zheng, Z.; Shi, Q.; Sun, T.; Liu, X.; Huang, Q.; Fukuda, T. Assembly of RGD-Modified Hydrogel Micromodules into Permeable Three-Dimensional Hollow Microtissues Mimicking in Vivo Tissue Structures. ACS Appl. Mater. Interfaces 2017, 9, 41669–41679. [Google Scholar] [CrossRef]
- Dai, L.; Lin, D.; Wang, X.; Jiao, N.; Liu, L. Integrated Assembly and Flexible Movement of Microparts Using Multifunctional Bubble Microrobots. ACS Appl. Mater. Interfaces 2020, 12, 57587–57597. [Google Scholar] [CrossRef]
- Ge, Z.; Dai, L.; Zhao, J.; Yu, H.; Yang, W.; Liao, X.; Tan, W.; Jiao, N.; Wang, Z.; Liu, L. Bubble-based microrobots enable digital assembly of heterogeneous microtissue modules. Biofabrication 2022, 14, 025023. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Xiu, W.; Liu, Y.; Ren, L.; Xiao, H.; Yang, F.; Gao, Y.; Xu, C.; Wang, L. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci. Adv. 2020, 6, eaaz8204. [Google Scholar] [CrossRef] [PubMed]
- Jooss, V.M.; Bolten, J.S.; Huwyler, J.; Ahmed, D. In vivo acoustic manipulation of microparticles in zebrafish embryos. Sci. Adv. 2022, 8, eabm2785. [Google Scholar] [CrossRef] [PubMed]
- Wrede, P.; Degtyaruk, O.; Kalva, S.K.; Dean-Ben, X.L.; Bozuyuk, U.; Aghakhani, A.; Akolpoglu, B.; Sitti, M.; Razansky, D. Real-time 3D optoacoustic tracking of cell-sized magnetic microrobots circulating in the mouse brain vasculature. Sci. Adv. 2022, 8, 13. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Dai, L.; Jiao, N. Review of Bubble Applications in Microrobotics: Propulsion, Manipulation, and Assembly. Micromachines 2022, 13, 1068. https://doi.org/10.3390/mi13071068
Zhou Y, Dai L, Jiao N. Review of Bubble Applications in Microrobotics: Propulsion, Manipulation, and Assembly. Micromachines. 2022; 13(7):1068. https://doi.org/10.3390/mi13071068
Chicago/Turabian StyleZhou, Yuting, Liguo Dai, and Niandong Jiao. 2022. "Review of Bubble Applications in Microrobotics: Propulsion, Manipulation, and Assembly" Micromachines 13, no. 7: 1068. https://doi.org/10.3390/mi13071068
APA StyleZhou, Y., Dai, L., & Jiao, N. (2022). Review of Bubble Applications in Microrobotics: Propulsion, Manipulation, and Assembly. Micromachines, 13(7), 1068. https://doi.org/10.3390/mi13071068






