Effects of Stocking Larger-Sized Fish on Water Quality, Growth Performance, and the Economic Yield of Nile Tilapia (Oreochromis niloticus L.) in Floating Cages
Abstract
1. Introduction
2. Materials and Methods
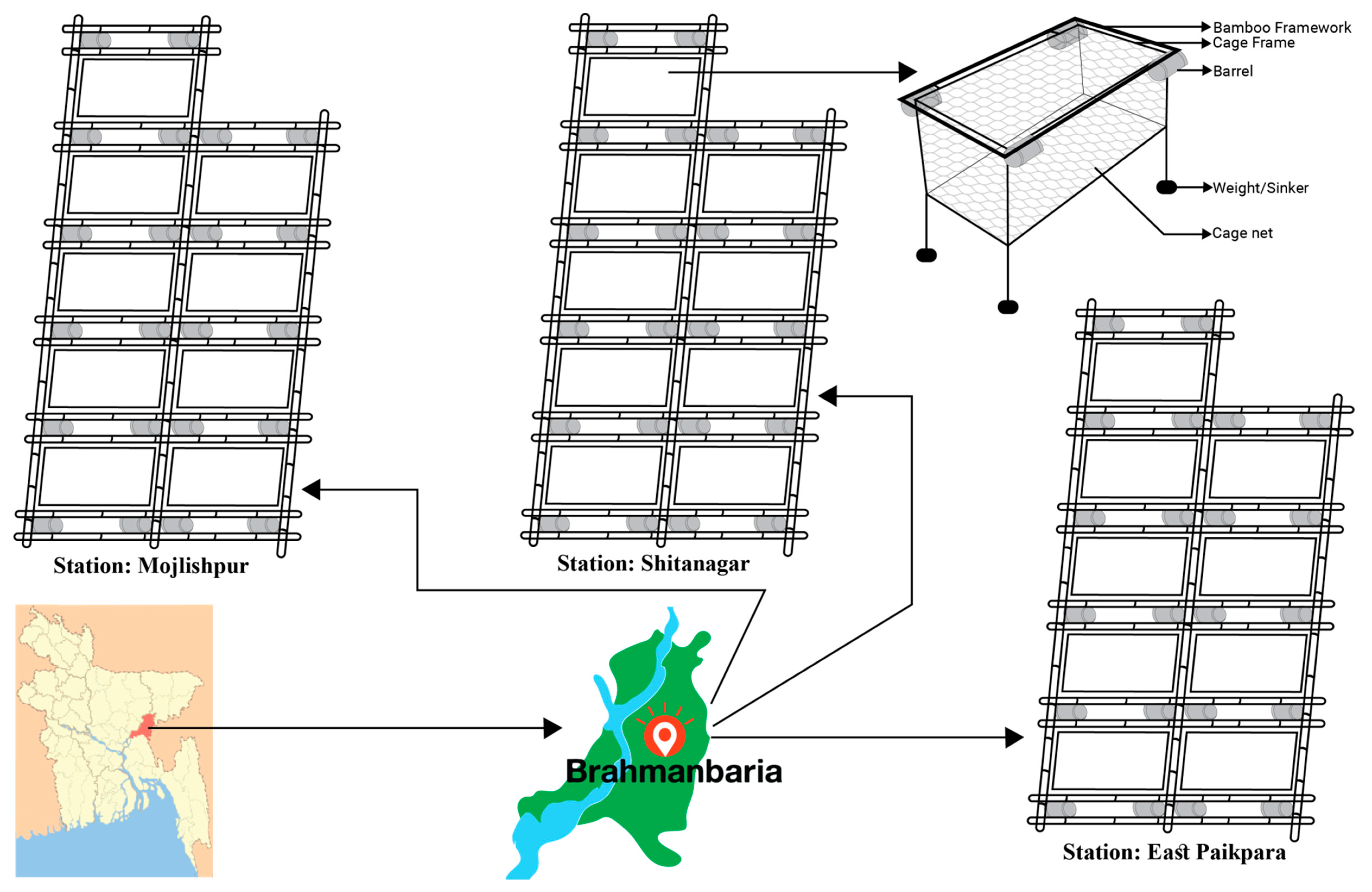
2.1. Study Area and Experimental Design
2.2. Feeding and Management
2.3. Growth Performance
2.4. Economic Yield
2.5. Business Feasibility
2.6. Statistical Analysis
3. Results and Discussions
3.1. Water Quality Parameters
3.2. Growth Performance and Yield
3.3. Economic and Business Feasibility Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; In brief; FAO: Rome, Italy, 2020. [Google Scholar]
- DoF. Yearbook of Fisheries Statistics of Bangladesh, 2018–2019; Bangladesh Government Press: Dhaka, Bangladesh, 2020; Volume 37, p. 135. [Google Scholar]
- FAO. Cultured Aquatic Species Information Program-Oreochromis niloticus (Linnaeus, 1758); Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Gupta, M.V.; Acosta, B.O. From drawing board to dining table: The success story of the GIFT project. NAGA WorldFish Cent. Q. 2005, 27, 4–14. [Google Scholar]
- Chowdhury, M.; Sukhan, Z.; Hannan, M. Climate change and its impact on fisheries resource in Bangladesh. In Proceedings of the International Conference on Environmental Aspects of Bangladesh (ICEAB10), Kitakyushu, Japan, 4 September 2010; pp. 95–98. [Google Scholar]
- Mbowa, S.; Odokonyero, T.; Munyaho, A. Harnessing Floating Cage Technology to Increase Fish Production in Uganda. Res. Ser. 2017, 262886, 44. [Google Scholar]
- Conte, L.; Sonoda, D.Y.; Shirota, R.; Cyrino, J.E.P. Productivity and economics of Nile tilapia Oreochromis niloticus cage culture in South-East Brazil. J. Appl. Aquac. 2008, 20, 18–37. [Google Scholar] [CrossRef]
- Beveridge, M.; Dabbadie, L.; Soto, D.; Ross, L.; Bueno, P. Climate Change and Aquaculture: Interactions with Fisheries and Agriculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Balkhande, J.V. Cage culture of Oreochromis Mossambicus (Tilapia) in back water of river Godavari, Nanded, Maharashtra India. MOJ Ecol. Environ. Sci. 2019, 4, 100–105. [Google Scholar] [CrossRef]
- Mcginty, A.S.; Rakocy, J.E. Cage Culture of Tilapia. The Fish Site, 1 November 2005. [Google Scholar]
- El-Sebai, A.; El-Murr, A.E.H.; Galal, A.A.A.; Abd El-Motaal, S.M.A. Effect of ginger dietary supplementation on growth performance, immune response and vaccine efficacy in Oreochromis niloticus challenged with Aeromonas hydrophila. Slov. Vet. Res. 2018, 55, 31–39. [Google Scholar] [CrossRef]
- Gibtan, A.; Getahun, A.; Mengistou, S. Effect of stocking density on the growth performance and yield of Nile tilapia [Oreochromis niloticus (L. 1758)] in a cage culture system in Lake Kuriftu, Ethiopia. Aquac. Res. 2008, 39, 1450–1460. [Google Scholar] [CrossRef]
- Rojas, A.; Wadsworth, S. A review of cage aquaculture: Latin America and the Caribbean. In Cage Aquaculture: Regional Reviews and Global Overview; FAO: Rome, Italy, 2007; pp. 77–100. [Google Scholar]
- Rahman, M.M.; Islam, M.S.; Halder, G.C.; Tanaka, M. Cage culture of sutchi catfish, Pangasius sutchi (Fowler 1937): Effects of stocking density on growth, survival, yield and farm profitability. Aquac. Res. 2006, 37, 33–39. [Google Scholar] [CrossRef]
- Osofero, S.A.; Otubusin, S.O.; Daramola, J.A. Effect of stocking density on tilapia Oreochromis niloticus Linnaeus 1757 growth and survival in bamboo—Net cages trial. Afr. J. Biotechnol. 2009, 8, 1322–1325. [Google Scholar] [CrossRef]
- Phan, L.T.; Bui, T.M.; Nguyen, T.T.T.; Gooley, G.J.; Ingram, B.A.; Nguyen, H.V.; Nguyen, P.T.; De Silva, S.S. Current status of farming practices of striped catfish, Pangasianodon hypophthalmus in the Mekong Delta, Vietnam. Aquaculture 2009, 296, 227–236. [Google Scholar] [CrossRef]
- Pechsiri, J.; Yakupitiyage, A. A comparative study of growth and feed utilization efficiency of sex-reversed diploid and triploid Nile tilapia, Oreochromis niloticus L. Aquac. Res. 2005, 36, 45–51. [Google Scholar] [CrossRef]
- Cren, E.D. Le The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201. [Google Scholar] [CrossRef]
- Karnatak, G.; Das, B.K.; Mishal, P.; Tayung, T.; Kumari, S.; Sarkar, U.K.; Das, A.K.; Ali, Y. Impact of stocking density on growth, feed utilization and survival of cage reared minor carp, Labeo bata (Hamilton, 1822) in Maithon reservoir, India. Aquaculture 2021, 532, 736078. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Wahab, M.A.; Verdegem, M.C.J.; Adhikary, R.K.; Rahman, S.M.S.; Azim, M.E.; Verreth, J.A.J. Effects of carbohydrate source for maintaining a high C:N ratio and fish driven re-suspension on pond ecology and production in periphyton-based freshwater prawn culture systems. Aquaculture 2010, 301, 37–46. [Google Scholar] [CrossRef]
- Izmaniar, H.; Mahyudin, I.; Agusliani, E.; Ahmadi, A. The Business Prospect of Climbing Perch Fish Farming with Biofloc Technology at De’ Papuyu Farm Banjarbaru. Int. J. Environ. Agric. Biotechnol. 2018, 3, 1145–1153. [Google Scholar] [CrossRef]
- Hartley, H.O. Smallest Composite Designs for Quadratic Response Surfaces. Biometrics 1959, 15, 611. [Google Scholar] [CrossRef]
- Devi, P.; Padmavathy, P.; Srinivasan, A.; Jawahar, P. Environmental Impact of Cage Culture on Poondi Reservoir, Tamil Nadu. Curr. World Environ. 2015, 10, 1048–1054. [Google Scholar] [CrossRef]
- Lianthuamluaia, L.; Mishal, P.; Panda, D.; Sarkar, U.K.; Kumar, V.; Sandhya, K.M.; Karnatak, G.; Kumari, S.; Bera, A.K.; Das, S.; et al. Understanding spatial and temporal patterns of fish diversity and assemblage structure vis-a-vis environmental parameters in a tropical Indian reservoir. Environ. Sci. Pollut. Res. 2019, 26, 9089–9098. [Google Scholar] [CrossRef]
- Gatica, E.A.; Almeida, C.A.; Mallea, M.A.; Del Corigliano, M.C.; González, P. Water quality assessment, by statistical analysis, on rural and urban areas of Chocancharava River (Río Cuarto), Córdoba, Argentina. Environ. Monit. Assess. 2012, 184, 7257–7274. [Google Scholar] [CrossRef]
- Kashindye, B.B.; Nsinda, P.; Kayanda, R.; Ngupula, G.W.; Mashafi, C.A.; Ezekiel, C.N. Environmental impacts of cage culture in Lake Victoria: The case of Shirati Bay-Sota, Tanzania. SpringerPlus 2015, 4, 475. [Google Scholar] [CrossRef]
- Neto, R.M.; Nocko, H.R.; Ostrensky, A. Environmental characterization and impacts of fish farming in the cascade reservoirs of the Paranapanema River, Brazil. Aquac. Environ. Interact. 2015, 6, 255–272. [Google Scholar] [CrossRef][Green Version]
- Sarkar, U.K.; Sandhya, K.M.; Mishal, P.; Karnatak, G.; Lianthuamluaia, L.; Kumari, S.; Panikkar, P.; Palaniswamy, R.; Karthikeyan, M.; Mol, S.S.; et al. Status, Prospects, Threats, and the Way Forward for Sustainable Management and Enhancement of the Tropical Indian Reservoir Fisheries: An Overview. Rev. Fish. Sci. Aquac. 2018, 26, 155–175. [Google Scholar] [CrossRef]
- Wilson, R.P.; Corraze, G.; Kaushik, S. Nutrition and feeding of fish. Aquaculture 2007, 267, 1–2. [Google Scholar] [CrossRef]
- Ahmed, T.; Jahedul Hasan, S.; Robiul Awal Hossain, M.; Haidar, I.; Shafiqul Alam Rubel, A.; Hasan Pramanik, M. Assessment on Impact of Dietary Probiotic Supplementation on Growth Indices of Mono-Sex Tilapia (Oreochromis niloticus) Cage Culture at Dakatia River, Chandpur, Bangladesh. World J. Fish Mar. Sci. 2014, 6, 441–446. [Google Scholar] [CrossRef]
- Asase, A.; Ewusie Nunoo, F.K.; Klenam Attipoe, F.Y. Lake-Based Nursery Rearing of Nile Tilapia (Oreochromis niloticus) Fingerlings in Nylon Hapas: Effects of Stocking Density on Growth, Survival and Profitability. Agric. Sci. 2016, 07, 660–669. [Google Scholar] [CrossRef][Green Version]
- Ridha, M.T. Comparative study of growth performance of three strains of Nile tilapia, Oreochromis niloticus, L. at two stocking densities. Aquac. Res. 2006, 37, 172–179. [Google Scholar] [CrossRef]
- Kunda, M.; Pandit, D.; Harun-Al-Rashid, A. Optimization of stocking density for mono-sex Nile tilapia (Oreochromis niloticus) production in riverine cage culture in Bangladesh. Heliyon 2021, 7, E08334. [Google Scholar] [CrossRef]
- Moniruzzaman, M. Effects of Stocking Density on Growth, Body Composition, Yield and Economic Returns of Monosex Tilapia (Oreochromis niloticus L.) under Cage Culture System in Kaptai Lake of Bangladesh. J. Aquac. Res. Dev. 2015, 6, 8. [Google Scholar] [CrossRef]
- Ouattara, N. Aquaculture Potential of the Black-Chinned Tilapia, Sarotherodon Melanotheron (Cichlidae). Comparative Study of the Effect of Stocking Density on Growth Performance of Landlocked and Natural Populations under Cage Culture Conditions in Lake Ayame (Cote d’Ivoire); Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Liti, D.M.; Fulanda, B.; Munguti, J.M.; Straif, M.; Waidbacher, H.; Winkler, G. Effects of open-pond density and caged biomass of Nile Tilapia (Oreochromis niloticus L.) on growth, feed utilization, economic returns and water quality in fertilized ponds. Aquac. Res. 2005, 36, 1535–1543. [Google Scholar] [CrossRef]
- Bolivar, R.; Jimenez, E.T.; Sugue, J.R.A.; Brown, C.L. Effect of stocking sizes on the yield and survival of Nile tilapia (Oreochromis niloticus L.) on-grown in ponds. In Proceedings of the 6th International Symposium on Tilapia in Aquaculture, Manila, Philippines, 12–16 September 2004; pp. 574–583. [Google Scholar]
- Chambel, J.; Severiano, V.; Baptista, T.; Mendes, S.; Pedrosa, R. Effect of stocking density and different diets on growth of Percula Clownfish, Amphiprion percula (Lacepede, 1802). SpringerPlus 2015, 4, 183. [Google Scholar] [CrossRef]
- Ayoade, A.A. Length-weight relationship and diet of African carp labeo ogunensis (boulenger, 1910) in asejire lake southwestern Nigeria. J. Fish. Aquat. Sci. 2011, 6, 472–478. [Google Scholar] [CrossRef][Green Version]
- Yosuva, M.; Jeyapragash, D.; Manigandan, V.; Machendiranathan, M.; Saravanakumar, A. Length-weight relationship and relative condition factor of yellowfin tuna (Thunnus albacares) from Parangipettai coast, southeast coast of India. Zool. Ecol. 2018, 28, 94–99. [Google Scholar] [CrossRef]
- Anani, F.; Ofori-Danson, P.; Abban, E. Pen Culture of the Black-Chinned Tilapia, Sarotherodon Melanotheron in the Aglor Lagoon in Ghana. J. Ghana Sci. Assoc. 2010, 12. [Google Scholar] [CrossRef]
- Ahmed, E.O.; Ali, M.E.; Aziz, A.A. Length-Weight Relationships and Condition Factors of Six Fish Species in Atbara River and Khashm El-Girba Reservoir, Sudan. Int. J. Agric. Sci. 2011, 3, 65–70. [Google Scholar] [CrossRef]
- De Giosa, M.; Czerniejewski, P.; Rybczyk, A. Seasonal Changes in Condition Factor and Weight-Length Relationship of Invasive Carassius gibelio (Bloch, 1782) from Leszczynskie Lakeland, Poland. Adv. Zool. 2014, 2014, 678763. [Google Scholar] [CrossRef]
- Datta, S.N.; Kaur, V.I.; Dhawan, A.; Jassal, G. Estimation of length-weight relationship and condition factor of spotted snakehead Channa punctata (Bloch) under different feeding regimes. SpringerPlus 2013, 2, 436. [Google Scholar] [CrossRef]
- Mensah, E.T.D.; Dankwa, H.R.; Torben, L.L.; Asmah, R.; Campion, B.B.; Edziyie, R. Effects of seasonal and environmental changes on aquaculture production in tropical Lake Volta, Ghana. Aquac. Int. 2018, 26, 1387–1400. [Google Scholar] [CrossRef]
- Arikan, M.; Aral, Y. Economic analysis of aquaculture enterprises and determination of factors affecting sustainability of the sector in Turkey. Ank. Üniversitesi Vet. Fakültesi Derg. 2019, 66, 59–66. [Google Scholar]
- Mohsin, A.B.M.; Islam, M.N.; Hossain, M.A.; Galib, S.M. Cost-Benefit Analyses of Carp Polyculture in Ponds: A Survey Study in Rajshahi and Natore Districts of Bangladesh. Bangladesh J. Environ. Sci. 2012, 23, 103–107. [Google Scholar]
- Jia, B.; St-Hilaire, S.; Singh, K.; Gardner, I.A. Farm-level returns and costs of yellow catfish (Pelteobagrus fulvidraco) aquaculture in Guangdong and Zhejiang provinces, China. Aquac. Rep. 2016, 4, 48–56. [Google Scholar] [CrossRef]
- Febrianty, I.; Mahreda, E.S.; Bachri, A. Fatmawati the Economies of Scale of Catfish Pond Culture in Banjar Regency, South Kalimantan. J. Biodivers. Environ. Sci. 2018, 13, 101–108. [Google Scholar]
- Sofia, L.A.; Nurlianti, S. The economic value of the resource utilization of wetlands: Comparative study of beje fisheries in North Hulu, Sungai Regency, South Kalimantan, Indonesia. AACL Bioflux. 2019, 12, 143–150. [Google Scholar]

| Test parameter | Production Cycle | |||||
|---|---|---|---|---|---|---|
| March–July | July–November | |||||
| Feed (Nursery) | Feed (Starter) | Feed (Grower) | Feed (Nursery) | Feed (Starter) | Feed (Grower) | |
| Crude protein (%) | 38.20 | 29.22 | 24.76 | 37.58 | 29.58 | 25.33 |
| Fat (%) | 3.50 | 4.39 | 3.68 | 3.69 | 4.33 | 3.57 |
| Crude fiber (%) | 10.80 | 8.88 | 11.13 | 11.06 | 9.21 | 7.96 |
| Crude ash (%) | 14.20 | 9.09 | 13.16 | 14.82 | 12.63 | 12.34 |
| Moisture (%) | 9.50 | 11.06 | 11.28 | 9.65 | 8.60 | 10.38 |
| Non-protein nitrogen (%) | 0.00 | 1.85 | 1.00 | 0.00 | 0.00 | 0.00 |
| Parameters | Production Cycle | Mojlishpur | Shitanagar | Paikpara | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cage Site | 50 m Away (Horizontal) | 50 m Away (Vertical) | p Value | Cage Site | 50 m Away (Horizontal) | 50 m Away (Vertical) | p Value | Cage Site | 50 m Away (Horizontal) | 50 m Away (Vertical) | p Value | ||
| Depth (m) | First cycle | 9.45 ± 0.06 a | 9.47 ± 0.08 a | 11.65 ± 0.07 b | 0.00 | 9.77 ± 0.05 a | 9.79 ± 0.06 a | 11.97 ± 0.07 b | 0.00 | 9.41 ± 0.07 a | 9.40 ± 0.09 a | 12.15 ± 0.07 b | 0.00 |
| Second cycle | 9.73 ± 0.02 a | 9.74 ± 0.03 a | 13.56 ± 0.03 b | 0.00 | 10.05 ± 0.0 a | 10.05 ± 0.03 a | 13.87 ± 0.02 b | 0.00 | 10.25 ± 0.03 a | 10.26 ± 0.04 a | 14.06 ± 0.03 b | 0.00 | |
| Transparency (cm) | First cycle | 31.72 ± 0.13 a | 31.74 ± 0.16 a | 32.94 ± 0.13 b | 0.00 | 31.67 ± 0.13 a | 31.69 ± 0.16 a | 32.82 ± 0.13 b | 0.00 | 31.62 ± 0.13 a | 31.64 ± 0.16 a | 32.75 ± 0.13 b | 0.00 |
| Second cycle | 32.38 ± 0.09 a | 32.40 ± 0.09 a | 33.47 ± 0.07 b | 0.00 | 32.30 ± 0.07 a | 32.30 ± 0.09 a | 33.41 ± 0.07 b | 0.00 | 32.25 ± 0.07 a | 32.25 ± 0.09 a | 33.35 ± 0.07 b | 0.00 | |
| Water temperature (°C) | First cycle | 29.43 ± 0.31 | 29.42 ± 0.39 | 29.41 ± 0.31 | 1.00 | 29.42 ± 0.32 | 29.42 ± 0.39 | 29.40 ± 0.32 | 1.00 | 29.47 ± 0.32 | 29.48 ± 0.39 | 29.47 ± 0.32 | 1.00 |
| Second cycle | 27.87 ± 0.13 | 27.81 ± 0.16 | 27.80 ± 0.13 | 0.92 | 27.90 ± 0.13 | 27.95 ± 0.16 | 27.85 ± 0.13 | 0.94 | 27.94 ± 0.12 | 27.89 ± 0.15 | 27.89 ± 0.12 | 0.96 | |
| pH | First cycle | 7.64 ± 0.02 | 7.66 ± 0.03 | 7.70 ± 0.02 | 0.20 | 7.59 ± 0.02 | 7.62 ± 0.02 | 7.64 ± 0.02 | 0.19 | 7.57 ± 0.02 | 7.59 ± 0.02 | 7.60 ± 0.02 | 0.27 |
| Second cycle | 7.82 ± 0.03 | 7.83 ± 0.03 | 7.81 ± 0.03 | 0.88 | 7.81 ± 0.02 | 7.83 ± 0.04 | 7.86 ± 0.03 | 0.49 | 7.77 ± 0.03 | 7.80 ± 0.04 | 7.81 ± 0.03 | 0.70 | |
| Dissolved Oxygen (mg/L) | First cycle | 5.97 ± 0.12 | 6.00 ± 0.15 | 6.03 ± 0.12 | 0.92 | 5.94 ± 0.12 | 5.97 ± 0.15 | 6.05 ± 0.12 | 0.81 | 5.88 ± 0.12 | 5.95 ± 0.15 | 6.01 ± 0.13 | 0.77 |
| Second cycle | 6.48 ± 0.08 | 6.49 ± 0.10 | 6.51 ± 0.08 | 0.95 | 6.45 ± 0.08 | 6.46 ± 0.09 | 6.49 ± 0.08 | 0.93 | 6.43 ± 0.08 | 6.45 ± 0.09 | 6.48 ± 0.08 | 0.92 | |
| Total Dissolved Solids (mg/L) | First cycle | 272.16 ± 6.52 | 270.41 ± 8.02 | 268.17 ± 6.75 | 0.91 | 275.60 ± 6.35 | 273.22 ± 7.79 | 270.88 ± 6.32 | 0.87 | 277.36 ± 6.25 | 275.51 ± 7.67 | 274.21 ± 6.18 | 0.93 |
| Second cycle | 237.37 ± 3.77 | 235.76 ± 4.63 | 270.88 ± 6.32 | 0.88 | 240.15 ± 3.96 | 238.95 ± 4.88 | 238.12 ± 3.94 | 0.94 | 242.99 ± 3.94 | 241.27 ± 4.80 | 239.76 ± 3.91 | 0.84 | |
| Ammonia (mg/L) | First cycle | 0.07 ± 0.004 a | 0.06 ± 0.003 a | 0.04 ± 0.002 b | 0.00 | 0.08 ± 0.004 a | 0.07 ± 0.003 a | 0.04 ± 0.001 b | 0.00 | 0.09 ± 0.003 a | 0.07 ± 0.003 a | 0.04 ± 0.002 b | 0.00 |
| Second cycle | 0.15 ± 0.010 | 0.15 ± 0.013 | 0.14 ± 0.011 | 0.81 | 0.17 ± 0.011 | 0.16 ± 0.014 | 0.16 ± 0.012 | 0.69 | 0.18 ± 0.012 | 0.17 ± 0.016 | 0.16 ± 0.013 | 0.51 | |
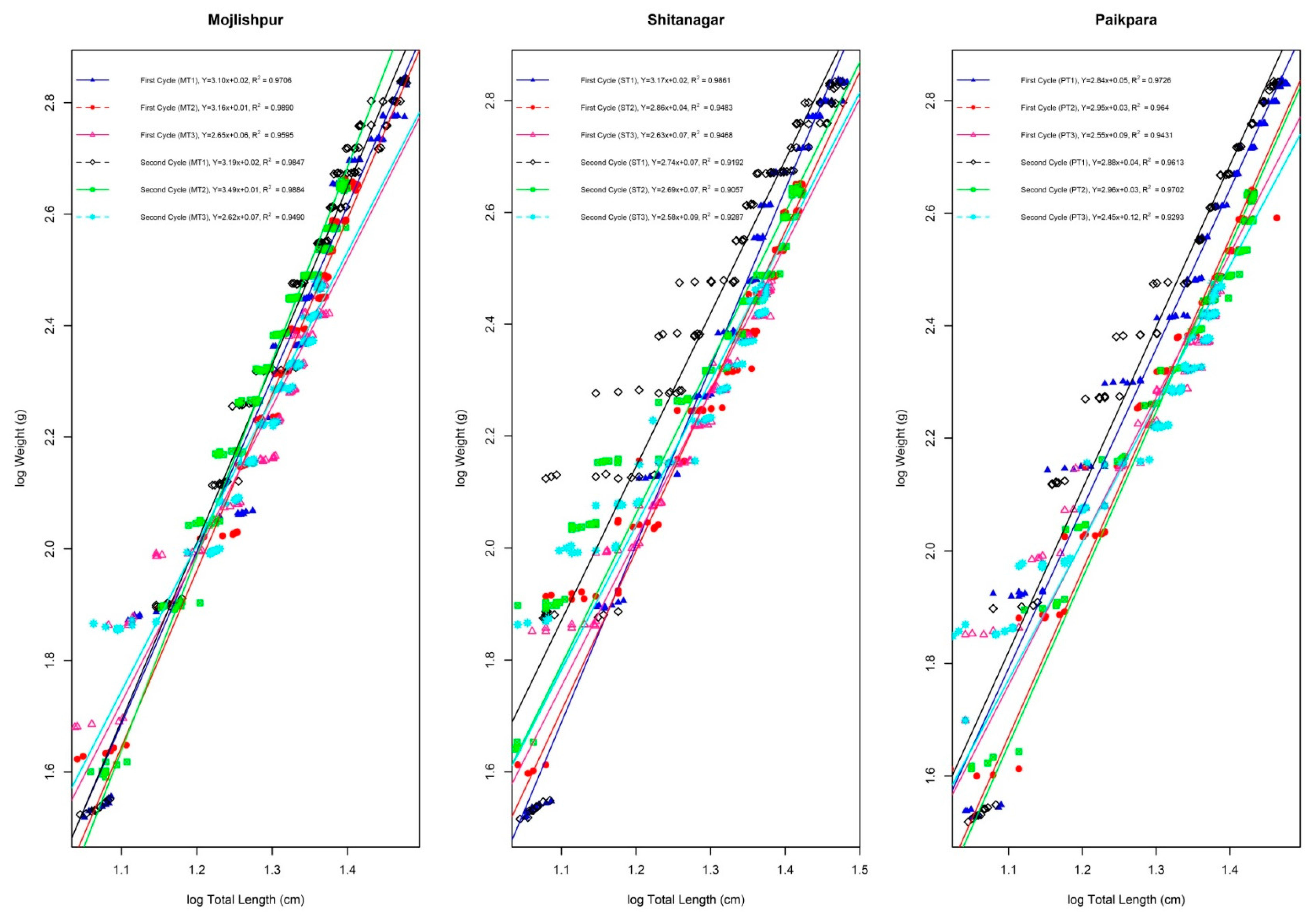
| Cage Site | Fish Size | Mean Initial Length (cm) | Mean Final Length (cm) | Mean Length Gain (cm) | % Length Gain | Mean Initial Weight (gm) | Mean Final Weight (gm) | Mean Weight Gain (gm) | % Weight Gain | SGR % Day | Average Daily Growth Rate (ADGR) | FCR | Survival Rate (%) | The Relative Condition Factor | Production (kg/Cage/120 Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mojlishpur | MT1 | 11.83 ± 0.05 a | 29.92 ± 0.06 b | 18.09 ± 0.10 b | 152.86 ± 1.43 c | 34.71 ± 0.29 a | 689.00 ± 1.58 a | 654.29 ± 1.85 a | 1885.31 ± 21.06 c | 2.49 ± 0.01c | 5.45 ± 0.02 a | 0.88 ± 0.00 i | 97.00 ± 0.42 a | 1.01 ± 0.01 | 334.17 ± 1.67 a |
| MT2 | 8.39 ± 0.16 b | 25.58 ± 0.08 f | 17.18 ± 0.10 h | 204.87 ± 4.98 b | 11.11 ± 0.31 b | 449.33 ± 2.80 d | 438.22 ± 3.10 d | 3951.98 ± 141.66b | 3.08 ± 0.03 b | 3.65 ± 0.03 d | 0.93 ± 0.01 g | 91.07 ± 0.29 d | 1.01 ± 0.01 | 204.59 ± 0.64 d | |
| MT3 | 6.36 ± 0.06 c | 23.13 ± 0.08 i | 16.78 ± 0.14 i | 264.08 ± 4.80 a | 5.52 ± 0.09 c | 299.67 ± 0.33 g | 294.14 ± 0.42 g | 5329.52 ± 91.95a | 3.33 ± 0.01 a | 2.45 ± 0.00 g | 1.03 ± 0.01 d | 86.20 ± 0.61 g | 1.02 ± 0.02 | 129.16 ± 0.88 g | |
| Shitanagar | ST1 | 11.61 ± 0.13 a | 29.98 ± 0.03 a | 18.38 ± 0.16 a | 158.42 ± 3.12 c | 34.21 ± 0.41 a | 680.89 ± 1.06 b | 646.68 ± 1.41 b | 1890.88 ± 26.54c | 2.49 ± 0.01 c | 5.39 ± 0.01 b | 0.91 ± 0.01 h | 93.80 ± 0.69 b | 1.01 ± 0.01 | 319.34 ± 2.63 b |
| ST2 | 8.81 ± 0.09 b | 26.22 ± 0.04 e | 17.41 ± 0.05 f | 197.65 ± 2.50 b | 11.02 ± 0.16 b | 441.33 ± 2.03 e | 430.31 ± 1.93 e | 3905.28 ± 45.92b | 3.08 ± 0.01 b | 3.59 ± 0.02 e | 1.00 ± 0.01 e | 89.07 ± 0.41 e | 1.03 ± 0.02 | 196.54 ± 1.56 e | |
| ST3 | 6.47 ± 0.05 c | 23.83 ± 0.10 h | 17.36 ± 0.06 g | 268.27 ± 2.00 a | 5.48 ± 0.04 c | 291.22 ± 3.36 h | 285.74 ± 3.33 h | 5216.22 ± 33.29a | 3.31 ± 0.01 a | 2.38 ± 0.03 h | 1.07 ± 0.01 c | 83.07 ± 0.37 h | 1.03 ± 0.03 | 120.95 ± 1.29 i | |
| Paikpara | PT1 | 11.47 ± 0.12 a | 29.54 ± 0.07 c | 18.08 ± 0.10 c | 157.68 ± 2.52 c | 34.37 ± 0.20 a | 675.78 ± 3.07 c | 641.41 ± 3.26 c | 1866.61 ± 20.18c | 2.48 ± 0.01 c | 5.35 ± 0.03 c | 0.93 ± 0.01 f | 92.87 ± 1.04 c | 1.01 ± 0.01 | 313.81 ± 4.58 c |
| PT2 | 9.00 ± 0.05 b | 26.83 ± 0.05 d | 17.83 ± 0.10 d | 198.17 ± 2.20 b | 11.21 ± 0.11 b | 429.22 ± 3.13 f | 418.01 ± 3.24 f | 3729.84 ± 65.15b | 3.04 ± 0.01 b | 3.48 ± 0.03 f | 1.07 ± 0.01 b | 86.80 ± 0.53 f | 1.02 ± 0.02 | 186.30 ± 2.42 f | |
| PT3 | 6.36 ± 0.09 c | 24.01 ± 0.05 g | 17.66 ± 0.06 e | 277.93 ± 4.82 a | 5.39 ± 0.16 c | 290.22 ± 2.15 i | 284.83 ± 2.03 i | 5292.78 ± 126.69a | 3.32 ± 0.02 a | 2.37 ± 0.02 i | 1.11 ± 0.03 a | 82.87 ± 1.27 i | 1.03 ± 0.02 | 120.27 ± 2.70 h | |
| Two-way ANOVA (p value) | |||||||||||||||
| Site | 0.482 | 0.000 | 0.000 | 0.34 | 0.405 | 0.000 | 0.000 | 0.360 | 0.262 | 0.000 | 0.000 | 0.000 | 0.854 | 0.000 | |
| Size | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.378 | 0.000 | |
| Site X Size | 0.001 | 0.000 | 0.002 | 0.064 | 0.656 | 0.025 | 0.186 | 0.482 | 0.478 | 0.189 | 0.027 | 0.656 | 0.971 | 0.098 | |
| Cage Site | Fish Size | Mean Initial Length (cm) | Mean Final Length (cm) | Mean Length Gain (cm) | % Length Gain | Mean Initial Weight (gm) | Mean Final Weight (gm) | Mean Weight Gain (gm) | % Weight Gain | SGR % Day | Average Daily Growth Rate (ADGR) | FCR | Survival Rate (%) | The Relative Condition Factor | Production (kg/Cage/120 Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mojlishpur | MT1 | 11.69 ± 0.07 a | 29.84 ± 0.02 a | 18.15 ± 0.08 a | 155.29 ± 1.60 g | 34.39 ± 0.10 a | 690.00 ± 1.50 a | 655.61 ± 1.49 a | 1906.49 ± 6.52 c | 2.50 ± 0.00 c | 5.46 ± 0.01 a | 0.89 ± 0.00 i | 96.20 ± 0.23 a | 1.01 ± 0.01 | 331.89 ± 0.35 a |
| MT2 | 8.96 ± 0.02 b | 24.76 ± 0.06 f | 15.80 ± 0.05 i | 176.43 ± 0.59 f | 11.14 ± 0.06 b | 447.56 ± 0.78 d | 436.41 ± 0.79 d | 3916.19 ± 23.64 b | 3.08 ± 0.00 b | 3.64 ± 0.01 d | 0.98 ± 0.01 f | 89.53 ± 0.48 d | 1.01 ± 0.01 | 200.36 ± 1.41 d | |
| MT3 | 6.34 ± 0.02 c | 23.01 ± 0.03 i | 16.66 ± 0.04 h | 262.62 ± 1.41 c | 5.48 ± 0.09 c | 297.33 ± 0.51 g | 291.86 ± 0.48 g | 5331.07 ± 89.07 a | 3.33 ± 0.01 a | 2.43 ± 0.00 g | 1.02 ± 0.01 e | 84.67 ± 0.52 g | 1.03 ± 0.03 | 125.87 ± 0.86 g | |
| Shitanagar | ST1 | 11.59 ± 0.14 a | 29.26 ± 0.17 b | 17.67 ± 0.21 c | 152.61 ± 3.31 h | 34.14 ± 0.44 a | 674.33 ± 3.20 b | 640.19 ± 3.63 b | 1875.85 ± 35.12 c | 2.49 ± 0.01 c | 5.33 ± 0.03 b | 0.92 ± 0.02 h | 94.27 ± 0.75 b | 1.03 ± 0.02 | 317.86 ± 4.00 b |
| ST2 | 8.93 ± 0.07 b | 26.07 ± 0.10 e | 17.14 ± 0.16 f | 192.02 ± 3.13 e | 11.09 ± 0.09 b | 434.44 ± 2.19 e | 423.36 ± 2.27 e | 3818.62 ± 50.32 b | 3.06 ± 0.01 b | 3.53 ± 0.02 e | 1.04 ± 0.01 d | 88.20 ± 0.83 e | 1.04 ± 0.03 | 191.59 ± 2.03 e | |
| ST3 | 6.42 ± 0.05 c | 23.38 ± 0.09 h | 16.96 ± 0.10 g | 264.14 ± 3.00 b | 5.39 ± 0.08 c | 286.22 ± 3.08 i | 280.83 ± 3.08 i | 5213.53 ± 94.37 a | 3.31 ± 0.01 a | 2.34 ± 0.03 c | 1.08 ± 0.01 c | 83.00 ± 0.83 i | 1.04 ± 0.03 | 118.79 ± 1.94 h | |
| Paikpara | PT1 | 11.55 ± 0.10 a | 28.85 ± 0.10 c | 17.30 ± 0.16 e | 149.82 ± 2.51 i | 34.24 ± 0.25 a | 673.56 ± 3.04 c | 639.31 ± 3.21 c | 1867.19 ± 20.87 c | 2.48 ± 0.01 c | 5.33 ± 0.03 i | 0.95 ± 0.01 g | 90.73 ± 1.19 c | 1.01 ± 0.02 | 305.61 ± 5.38 c |
| PT2 | 8.95 ± 0.02 b | 26.87 ± 0.07 d | 17.92 ± 0.08 b | 200.19 ± 1.18 d | 11.17 ± 0.08 b | 426.56 ± 2.23 f | 415.38 ± 2.23 f | 3718.40 ± 33.15 b | 3.04 ± 0.01 b | 3.46 ± 0.02 f | 1.11 ± 0.01 b | 83.67 ± 0.35 h | 1.01 ± 0.02 | 178.45 ± 1.61 f | |
| PT3 | 6.39 ± 0.13 c | 24.02 ± 0.09 g | 17.63 ± 0.19 d | 276.24 ± 8.24 a | 5.41 ± 0.14 c | 289.44 ± 3.89 h | 284.03 ± 3.87 h | 5255.31 ± 144.36 a | 3.32 ± 0.02 a | 2.37 ± 0.03 h | 1.13 ± 0.02 a | 80.87 ± 1.57 b | 1.04 ± 0.03 | 117.01 ± 2.38 i | |
| Two-way ANOVA (p value) | |||||||||||||||
| Site | 0.901 | 0.000 | 0.000 | 0.006 | 0.696 | 0.000 | 0.000 | 0.183 | 0.086 | 0.000 | 0.000 | 0.000 | 0.38 | 0.000 | |
| Size | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.438 | 0.000 | |
| Site X site | 0.822 | 0.000 | 0.000 | 0.007 | 0.984 | 0.011 | 0.138 | 0.745 | 0.640 | 0.167 | 0.161 | 0.645 | 0.958 | 0.039 | |
| Cost Items | Unit | Price per unit | Total Price | Economic Life (year) | Depreciation Cost |
| Investment cost | |||||
| Cage net [(20 × 10 × 6) feet] | 27 | 1800 | 48,600 | 5 | 9720 |
| Plastic barrel (number) | 60 | 1000 | 60,000 | 10 | 6000 |
| Gi pipe for frame (1-inch diameter) (feet) | 1944 | 55 | 106,920 | 10 | 10,692 |
| Frame connecting angel (feet) | 189 | 100 | 18,900 | 10 | 1890 |
| Anchor (each 15 kg) (number) | 15 | 1350 | 20,250 | 10 | 2025 |
| Nylon rope (bundle) | 3 | 4000 | 12,000 | 5 | 2400 |
| Bamboo (number) | 54 | 350 | 18,900 | 5 | 3780 |
| Boat (number) | 3 | 12,000 | 36,000 | 10 | 3600 |
| Plastic bucket-cover 20 L | 3 | 120 | 360 | 3 | 120 |
| Scoop nets | 3 | 1000 | 3000 | 3 | 1000 |
| Testing kit | 1 | 5000 | 5000 | 2 | 2500 |
| Total investment cost | 329,930 | ||||
| The total investment cost for each treatment | 109,976.667 | ||||
| Fixed cost | |||||
| Total investment depreciation | 43,727 | ||||
| Total investment depreciation for each treatment | 14,575.66667 | ||||
| Cost Items | Production Cycle | Mojlishpur | Shitanagar | Paikpara Station | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MT1 | MT2 | MT3 | ST1 | ST2 | ST3 | PT1 | PT2 | PT3 | ||
| Fish seed (33.85–34.06 g) | First cycle | 2500.00 | 2500.00 | 2500.00 | ||||||
| Second cycle | 2500.00 | 2500.00 | 2500.00 | |||||||
| Fish seed (10.91–10.98 g) | First cycle | 1500.00 | 1500.00 | 1500.00 | ||||||
| Second cycle | 1500.00 | 1500.00 | 1500.00 | |||||||
| Fish seed (5.38–5.43 g) | First cycle | 1000.00 | 1000.00 | 1000.00 | ||||||
| Second cycle | 1000.00 | 1000.00 | 1000.00 | |||||||
| Feed (nursery) | First cycle | 796.61 | 745.21 | 803.84 | 757.09 | 810.33 | 719.33 | |||
| Second cycle | 768.67 | 701.55 | 816.34 | 710.54 | 791.52 | 690.08 | ||||
| Feed (starter-1, 2, 3) | First cycle | 8484.65 | 6391.96 | 6159.28 | 8227.80 | 6692.61 | 5953.02 | 8526.10 | 6782.40 | 6211.80 |
| Second cycle | 8607.08 | 6754.41 | 5922.06 | 8177.40 | 6821.86 | 5959.10 | 8378.26 | 6728.16 | 6193.80 | |
| Feed (grower-1, 2) | First cycle | 6546.11 | 2864.18 | 6603.30 | 2860.49 | 6367.83 | 2956.80 | |||
| Second cycle | 6462.35 | 2893.79 | 6753.60 | 2903.58 | 6483.87 | 2907.72 | ||||
| Transportation | First cycle | 3000.00 | 3000.00 | 3000.00 | 2500.00 | 2500.00 | 2500.00 | 2500.00 | 2500.00 | 2500.00 |
| Second cycle | 3000.00 | 3000.00 | 3000.00 | 2500.00 | 2500.00 | 2500.00 | 2500.00 | 2500.00 | 2500.00 | |
| Total variable cost without labor wage | First cycle | 20,530.77 | 14,552.75 | 10,904.49 | 19,831.10 | 14,356.95 | 10,210.11 | 19,893.93 | 14,549.53 | 10,431.13 |
| Second cycle | 20,569.42 | 14,916.86 | 10,623.61 | 19,931.00 | 14,541.78 | 10,169.65 | 19,862.13 | 14,427.40 | 10,383.88 | |
| Labor wage (share profit system) | First cycle | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 |
| Second cycle | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | 2000.00 | |
| Total variable cost with labor wage | First cycle | 22,530.77 | 16,552.75 | 12,904.49 | 21,831.10 | 16,356.95 | 12,210.11 | 21,893.93 | 16,549.53 | 12,431.13 |
| Second cycle | 22,569.42 | 16,916.86 | 12,623.61 | 21,931.00 | 16,541.78 | 12,169.65 | 21,862.13 | 16,427.40 | 12,383.88 | |
| Production Cycle | Stations | Treatments | Production (kg/cage) | Unit Price (BDT/kg) | Total Revenue (BDT) | Total Variable Cost (BDT) | Fixed Cost as Depreciation (BDT) | Total Cost (BDT) | Total Income as Profit (BDT) | Interest of Bank Loan as Investment (9%) (BDT) | Net Income (BDT) | Payback Period (PBP) (No Unit) | Break-Even Point (No Unit) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First cycle (March–July) | Mojlishpur | MT1 | 334.17 | 115.00 | 38,429.03 | 22,530.77 | 1619.52 | 24,150.29 | 14,278.74 | 1086.76 | 13,191.97 | 0.93 | 0.77 |
| MT2 | 204.59 | 95.00 | 19,435.90 | 16,552.75 | 1619.52 | 18,172.26 | 1263.64 | 817.75 | 445.89 | 27.41 | 4.24 | ||
| MT3 | 129.16 | 85.00 | 10,978.23 | 12,904.49 | 1619.52 | 14,524.01 | −3545.78 | 653.58 | −4199.36 | −2.91 | −6.34 | ||
| Shitanagar | ST1 | 319.34 | 115.00 | 36,724.13 | 21,831.10 | 1619.52 | 23,450.62 | 13,273.51 | 1055.28 | 12,218.23 | 1.00 | 0.82 | |
| ST2 | 196.54 | 95.00 | 18,671.73 | 16,356.95 | 1619.52 | 17,976.46 | 695.27 | 808.94 | −113.67 | −107.50 | 5.28 | ||
| ST3 | 120.95 | 85.00 | 10,280.71 | 12,210.11 | 1619.52 | 13,829.63 | −3548.92 | 622.33 | −4171.25 | −2.93 | −6.33 | ||
| Paikpara | PT1 | 313.81 | 115.00 | 36,087.77 | 21,893.93 | 1619.52 | 23,513.45 | 12,574.32 | 1058.11 | 11,516.22 | 1.06 | 0.86 | |
| PT2 | 186.30 | 95.00 | 17,698.25 | 16,549.53 | 1619.52 | 18,169.05 | −470.81 | 817.61 | −1288.41 | −9.48 | 10.64 | ||
| PT3 | 120.27 | 85.00 | 10,223.37 | 12,431.13 | 1619.52 | 14,050.65 | −3827.29 | 632.28 | −4459.57 | −2.74 | −5.53 | ||
| Second cycle (July–November) | Mojlishpur | MT1 | 331.89 | 110.00 | 36,507.56 | 22,569.42 | 1619.52 | 24,188.94 | 12,318.62 | 1088.50 | 11,230.11 | 1.09 | 0.88 |
| MT2 | 200.36 | 90.00 | 18,032.34 | 16,916.86 | 1619.52 | 18,536.38 | −504.04 | 834.14 | −1338.18 | −9.13 | 10.95 | ||
| MT3 | 125.87 | 80.00 | 10,069.74 | 12,623.61 | 1619.52 | 14,243.13 | −4173.38 | 640.94 | −4814.33 | −2.54 | −4.78 | ||
| Shitanagar | ST1 | 317.86 | 110.00 | 34,964.44 | 21,931.00 | 1619.52 | 23,550.52 | 11,413.92 | 1059.77 | 10,354.15 | 1.18 | 0.94 | |
| ST2 | 191.59 | 90.00 | 17,243.06 | 16,541.78 | 1619.52 | 18,161.30 | −918.24 | 817.26 | −1735.49 | −7.04 | 17.42 | ||
| ST3 | 118.79 | 80.00 | 9503.08 | 12,169.65 | 1619.52 | 13,789.17 | −4286.09 | 620.51 | −4906.60 | −2.49 | −4.58 | ||
| Paikpara | PT1 | 305.61 | 110.00 | 33,616.64 | 21,862.13 | 1619.52 | 23,481.65 | 10,134.99 | 1056.67 | 9078.32 | 1.35 | 1.04 | |
| PT2 | 178.45 | 90.00 | 16,060.39 | 16,427.40 | 1619.52 | 18,046.92 | −1986.53 | 812.11 | −2798.64 | −4.37 | −33.29 | ||
| PT3 | 117.01 | 80.00 | 9361.11 | 12,383.88 | 1619.52 | 14,003.40 | −4642.29 | 630.15 | −5272.44 | −2.32 | −4.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamsuddin, M.; Hossain, M.B.; Rahman, M.; Kawla, M.S.; Tazim, M.F.; Albeshr, M.F.; Arai, T. Effects of Stocking Larger-Sized Fish on Water Quality, Growth Performance, and the Economic Yield of Nile Tilapia (Oreochromis niloticus L.) in Floating Cages. Agriculture 2022, 12, 942. https://doi.org/10.3390/agriculture12070942
Shamsuddin M, Hossain MB, Rahman M, Kawla MS, Tazim MF, Albeshr MF, Arai T. Effects of Stocking Larger-Sized Fish on Water Quality, Growth Performance, and the Economic Yield of Nile Tilapia (Oreochromis niloticus L.) in Floating Cages. Agriculture. 2022; 12(7):942. https://doi.org/10.3390/agriculture12070942
Chicago/Turabian StyleShamsuddin, Md, Mohammad Belal Hossain, Moshiur Rahman, Mst Salamun Kawla, Md. Farhan Tazim, Mohammed Fahad Albeshr, and Takaomi Arai. 2022. "Effects of Stocking Larger-Sized Fish on Water Quality, Growth Performance, and the Economic Yield of Nile Tilapia (Oreochromis niloticus L.) in Floating Cages" Agriculture 12, no. 7: 942. https://doi.org/10.3390/agriculture12070942
APA StyleShamsuddin, M., Hossain, M. B., Rahman, M., Kawla, M. S., Tazim, M. F., Albeshr, M. F., & Arai, T. (2022). Effects of Stocking Larger-Sized Fish on Water Quality, Growth Performance, and the Economic Yield of Nile Tilapia (Oreochromis niloticus L.) in Floating Cages. Agriculture, 12(7), 942. https://doi.org/10.3390/agriculture12070942









