Abstract
Essential molecules are embedded within the millenary crop Tropaeolum tuberosum (mashua); these compounds are critical for the Andean people’s traditional diet and extensively utilized by the pharmaceutical industry in Peru. In the Andean region, conventional cropping techniques generate microtubers susceptible to a viral infection, which substantially endangers mashua’s production. Therefore, we developed an innovative in vitro technique condition for enhancing the agriculture process for micro tubers production. The temporary immersion system (TIS) permits the production of high-quality microtubers in a reduced space, a lower amount of time, and in large quantities compared with tubers grown under traditional conditions. To obtain T. tuberosum’s microtubers via TIS, we propagated seedlings, utilizing TIS-RITA® vessels. A set of immersion frequency times were evaluated. Interestingly, results showed that immersion at 2 min every 3 h was more beneficial compared with 2 min every 5 h based on microtubers produced after 10 weeks from the treatments, revealing an efficient frequency setting which outputted improved microtubers quality and production.
1. Introduction
The mashua is a millenary crop that contains substantial nutritional and medical properties [1,2,3]. This crop originated from the Andean region [4], and is considered the fourth most crucial Andean root among other tubers such as potatoes, oca, and olluco [5]. The mashua is a propagation crop cultivated over the latest centuries across the Andean mountains in Peru, Bolivia, Ecuador, Venezuela, and Colombia [5,6]. This formidable tuber has managed to grow under nutrient-deprived soil conditions and at high altitudes without fertilizers or pesticides, outstanding for its resistance against harsh conditions in contrast to other contemporary crops [7,8].
Traditionally, the Andean mashua is propagated for production purposes as other tubers within the Andean crop fields [9]. Additionally, when the mashua is cultivated under field conditions, it necessitates between 6 and 8 months to properly attain its vegetative cycle stages, and in several cases, the tubers become virally infected, dramatically impacting the crop’s production [6]. Therefore, rural and local mashua production within the Andean region does not ensure suitable seed quality and necessitates alternative cropping techniques to improve biological features such as growth and vigor. In vitro techniques are utilized to improve crop production and decrease time constraints; therefore, sculpting innovative in vitro techniques for tuber cropping is needed.
One in vitro technique to harness the growth of seedlings is the modulation of the frequency and duration of immersion times [10] during the tuber early development. The TIS (temporary immersion system) strategy enables the rapid and efficient propagation of several plants with keen agricultural interest. The TIS enhances the growing speed and ensures the optimal quality of the plant tissue generated in vitro [11]. The TIS permits the production of high-quality pathogen-free seedlings and microtubers in vitro at any time throughout the year [12]. Moreover, it reduces large-scale crop production costs [13], automatizes the cropping process, and permits proper propagation by utilizing liquid media to ensure seedlings vigor [11].
The TIS enhances the growing speed and ensures the optimal quality of the plant tissue generated in vitro [11], enabling rapid and efficient propagation of several plants with strong agricultural interest. The TIS technique initiates by inducing air pressure flow through an air compressor; this de novo pressure elevates the liquid media permitting contact with the explants localized inside the chamber intermittently. As the air injection subsides within the system, and the media descends by gravity, the atmosphere remodels within the system, facilitating a robust growth and substantial improvement for the seedlings’ development [14]. Some critical factors defining the TIS technique are the following: the optimization of the total volume inside the vessels, the supplement in the media, the vitrification, the ethylene accumulation, and the carbon dioxide. Seedlings development and growth can be harnessed by modifying the frequency and duration of the immersion time [10].
Likewise, TIS-RITA® includes a structured container divided into two vessels: a superior vessel hosting the plants and an inferior vessel containing the media. The applied overpressure to the inferior vessel propels the media towards the superior compartment generating bubbles grazing the plant tissues. At this stage, seedlings temporarily submerge as overpressure is delivered. During the immersion period, the media falls by gravity. One result is the altered atmosphere inside the container [12]. The other critical parameter is the immersion time, involved in efficient sprout micropropagation, microtuberization, and somatic embryogenesis [12]. The TIS is regularly utilized for increasing in vitro propagation coefficients compared with field conditions. For example, these methods have been in used in other crop species such as bananas [15], anthurium [16], sugar cane [17], and potato microtubers [18].
In this study, we concentrated on the production of mashua MAC-3 morphotype via a novel in vitro procedure. MAC-3 morphotype (purple mashua) is known for its high antioxidant activity, total phenolic, tannins, total flavonoids, and total anthocyanins [19]. Recently, we had faithfully propagated Solanum tuberosum, Oxalis tuberosa, and Ullucus tuberosum in vitro utilizing TIS-RITA [20]. In a previous report, we found that a specific frequency condition in TIS-RITA substantially enhances the mashua’s microtuber propagation [21]. Therefore, we explored vital settings to generate high-quality seeds, utilizing a set of immersion frequencies to obtain improved seeds of the T. tuberosum MAC-3 morphotype that would positively impact the crop production for the Andean community in South America.
2. Materials and Methods
2.1. Micropropagation Study
Mashua seedlings (T. tuberosum Ruiz & Pav.) derived from a MAC-3 morphotype from the germplasm bank of the Cellular and Molecular Biology Laboratory (UNSCH, Ayacucho, Peru) were propagated in vitro (Figure 1). Seedlings were maintained using Murashige and Skoog 1962 (MS) solid medium. After 30 days of culture, the seedlings grown in solid medium were transferred to flasks containing 100 mL of MS liquid medium supplemented with 3% sucrose at a pH of 5.6 to obtain seedling vigor; the flasks were kept under constant agitation on an orbital shaker. Culture conditions were 19 ± 2 °C; 16 h of light and 8 h of darkness with a relative humidity between 60% and 70% during the multiplication phase.

Figure 1.
T. tuberosum Ruiz and Pav. “mashua” MAC-3 morphotype used in the TIS to obtain microtubers.
2.2. Production of Microtubers via TIS-RITA
TIS-RITA vessels were used according to Etienne et al. [12]. Murashige and Skoog (MS) liquid medium were prepared inside the RITA vessels, supplemented with 2 ppm BAP and 8% sucrose, at a pH 5.6; the vessels were sterilized at 121 °C for 15 min. This study evaluated one immersion time point and two frequencies: 2 min every 3 h and 2 min every 5 h. Next, TIS-RITA vessels were incubated under the constant temperature of 19 °C ± 2 °C, for a total of 10 weeks in total darkness. Produced microtubers were harvested off the culture vessels, and samples were rinsed off with tap water to remove any excess media. These microtubers were placed on trays covered with filter paper to remove humidity. Finally, the microtubers’ fresh weights (g) were evaluated using an analytical scale and a vernier ruler to measure the size (cm).
2.3. Data Analysis
The output data were statistically analyzed by using a random design format with double replicates. The variation analysis was performed to compare the size and weight of microtubers in a non-parametric U-Mann–Whitney test.
3. Results and Discussion
Using the RITA® temporary immersion system, it was possible to obtain mashua microtubers from MAC-3 morphotype using the two immersion frequencies. However, there was a significant difference between the two immersion frequencies in obtaining the size of the microtubers; a size of 1.09 cm was achieved at an immersion frequency of every three hours for two minutes, compared with 0.86 cm at a frequency of every five hours for two minutes respectively (Figure 2A), these findings being statistically significant (p = 0.0017; U-Mann Whitney test). According to Akita et al. [22], potato microtubers (Solanum tuberosum L.) were obtained using a laboratory-scale fermenter with a weight of more than 0.2 g. Montoya et al. [23] achieved the greatest number and size of Solanum tuberosum shoots using TIS with an immersion frequency of three hours. Escalona et al. [24] in the cultivation of Ananas commosus achieved a higher multiplication rate using an immersion time of two minutes and a frequency of three hours. Likewise, Cabrera et al. [25] obtained a greater number and size of Dioscorea alata microtubers with significant differences in relation to other immersion times using an immersion time of 15 min and after 18 weeks of culture. Etienne et al. [12] stated that Solanum tuberosum microtubers and Coffea arabica somatic embryos produced in temporary immersion bioreactors developed satisfactorily after planting.
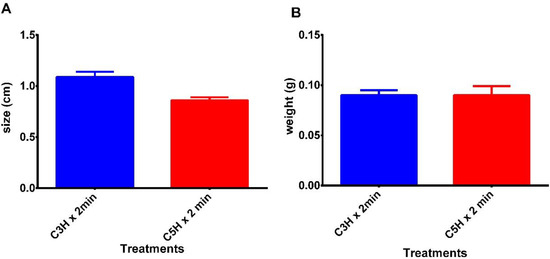
Figure 2.
(A) Mean comparison of the microtuber size and (B) weight from T. tuberosum “mashua” obtained in two TIS treatments: 2 min every 3 h (C3H × 2 min); 2 min every 5 h (C5H × 2 min).
On the other hand, using a TIS, 52 mashua microtubers from a MAC-3 morphotype were obtained at a frequency of every three hours of immersion for two minutes compared with a frequency of five hours for two minutes in which 39 microtubers were obtained. Moreover, Igarza et al. [26] acquired an average of between five and seven potato microtubers of the “Andinita” variety using a TIS. Montoya et al. [23] achieved greater efficiency in the in vitro tuberization of Solanum tuberosum variety Diacol Capiro when used in temporary immersion bioreactors and in MS medium supplemented with 1 ppm of 6-Benzylaminopurine (BAP) and 8% sucrose; in addition, the microtubers obtained in a TIS allowed the formation of tubers under field conditions. Gopal et al. [27]) concluded that microtubers produced in media without abscisic acid (ABA) during and containing high concentrations of sucrose and BAP can be stored for 12 months.
Regarding the fresh weight of the mashua, an average of 0.09 g was achieved in both immersion frequency treatments; therefore, there was no statistically significant difference in the results obtained between both treatments (Figure 2B). Igarza et al. [25] achieved an average fresh weight that did not exceed 3.5 g using an immersion system to obtain potato microtubers cv. “Andinita”.
The immersion system allows obtaining microtubers in two and a half months (Figure 3), significantly reducing the production time compared with the production of mashua tubers in the field, which generally requires between six to nine months. In addition, under this production system it is possible to obtain high-quality seeds, allowing ex situ conservation in a germplasm bank, and fundamentally for use in seed management and improvement programs.

Figure 3.
(a) Conservation in germplasm bank. (b) Micropropagation in solid medium. (c) Propagation in liquid medium. (d) Obtaining microtubers in a temporary immersion from T. tuberosum “mashua” MAC-3 after a 10-week culture. (e) The frequency: (Left): three hours for two minutes, (right): five hours for two minutes.
4. Conclusions
It was possible to obtain MAC-3 microtubers in the TIS RITA® using the Murashige and Skoog medium supplemented with 8% sucrose, 2 ppm BAP, and with an immersion frequency of every 3 h for 2 min. The TIS RITA® is an efficient alternative for the production of high-quality seeds. Furthermore, it would lead to obtaining virus-free microtubers as well as reducing the harvest time compared with traditional production techniques.
Author Contributions
Conceptualization, G.P.-R. and R.C.-C.; methodology, G.P.-R.; formal analysis, V.A.-A.; investigation, G.P.-R., R.C.-C., V.A.-A. and O.H.-C.; writing—original draft preparation, V.A.L.; writing—review and editing, V.A.L.; visualization, O.H.-C.; project administration, G.P.-R.; funding acquisition, G.P.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CONCYTEC, Ministerio de Educacion, Perú. (MINEDU-CONCYTEC) project 199-2015-FONDECYT—UNSCH.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors would like to thank FONDECYT and the Department of Biology of the New York University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Apaza Ticona, L.N.; Tena Pérez, V.; Bermejo Benito, P. Local/Traditional Uses, Secondary Metabolites and Biological Activities of Mashua (Tropaeolum tuberosum Ruíz & Pavón). J. Ethnopharmacol. 2020, 247, 112152. [Google Scholar] [CrossRef]
- Apaza Ticona, L.; Arnanz Sebastián, J.; Serban, A.M.; Rumbero Sánchez, Á. Alkaloids Isolated from Tropaeolum tuberosum with Cytotoxic Activity and Apoptotic Capacity in Tumour Cell Lines. Phytochemistry 2020, 177, 112435. [Google Scholar] [CrossRef]
- Ticona, L.A.; Sánchez, Á.R.; Estrada, C.T.; Palomino, O.M. Identification of TRPV1 Ion Channels Agonists of Tropaeolum tuberosum in Human Skin Keratinocytes. Planta Med. 2021, 87, 383–394. [Google Scholar] [CrossRef]
- Campos, D.; Noratto, G.; Chirinos, R.; Arbizu, C.; Roca, W.; Cisneros-Zevallos, L. Antioxidant Capacity and Secondary Metabolites in Four Species of Andean Tuber Crops: Native Potato (Solanum Sp.), Mashua (Tropaeolum tuberosum Ruiz & Pavón), Oca (Oxalis Tuberosa Molina) and Ulluco (Ullucus Tuberosus Caldas). J. Sci. Food Agric. 2006, 86, 1481–1488. [Google Scholar] [CrossRef]
- Pissard, A.; Arbizu, C.; Ghislain, M.; Bertin, P. Influence of Geographical Provenance on the Genetic Structure and Diversity of the Vegetatively Propagated Andean Tuber Crop, Mashua (Tropaeolum tuberosum), Highlighted by Intersimple Sequence Repeat Markers and Multivariate Analysis Methods. Int. J. Plant Sci. 2008, 169, 1248–1260. [Google Scholar] [CrossRef]
- Grau, A.; Dueñas, R.O.; Cabrera, C.N.; Hermann, M. Mashua Tropaeolum Tuberosum Ruíz & Pav; Promoting the Conservation and Use of Underutilized and Neglected Crops 25; International Potato Center: Lima, Peru, 2003; Volume 52, p. 427. [Google Scholar]
- Ortega, O.R.; Duran, E.; Arbizu, C.; Ortega, R.; Roca, W.; Potter, D.; Quiros, C.F. Pattern of Genetic Diversity of Cultivated and Non-Cultivated Mashua, Tropaeolum tuberosum, in the Cusco Region of Perú. Genet. Resour. Crop. Evol. 2007, 54, 807–821. [Google Scholar] [CrossRef]
- Arbizu, C.; García, E.R. Catálogo de Los Recursos Fitogenéticos de Raíces y Tubérculos Andinos; Programa de Investigación de Cultivos andinos, Facultad de Ciencias Agrarias, Universidad Nacional San Cristóbal de Huamanga: Ayacucho, Peru, 1986. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants: Volume 12, Modified Stems, Roots, Bulbs; Universiteitsbibliotheek Gent: Ghent, Belgium, 2016; pp. 1–690. [Google Scholar] [CrossRef]
- Jäger, A.K.; Schottländer, B.; Smitt, U.W.; Nyman, U. Somatic Embryogenesis in Cell Cultures of Thapsia garganica: Correlation between the State of Differentiation and the Content of Thapsigargins. Plant Cell Rep. 1993, 12, 517–520. [Google Scholar] [CrossRef]
- Alvarenga Venutolo, S. Micropropagación Masiva de Stevia rebaudiana Bertoni en Sistemas de Inmersión Temporal. Cultiv. Trop. 2015, 36, 50–57. [Google Scholar]
- Etienne, H.; Berthouly, M. Temporary Immersion Systems in Plant Micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- So Young, P.; Murthy, H.N.; Kee Yoeup, P. Mass Multiplication of Protocorm-like Bodies Using Bioreactor System and Subsequent Plant Regeneration in Phalaenopsis. Plant Cell Tissue Organ Cult. 2000, 63, 67–72. [Google Scholar] [CrossRef]
- Maldonado, E.R.; de Francisco, L.E.R.; Gómez, O.A.; Cerda, M.E.C. Diseño y Construcción de Un Sistema de Inmersión Temporal. Cent. Agríc. 2003, 30, 69–72. [Google Scholar]
- Pérez, M.B.; Pérez, M.B.; Vega, V.M.; Gálvez, E.O.; Delgado, M.T.; Torres, J.L.; Jova, M.C.; Pino, A.S.; Cabrera, A.R.; Toledo, M.B.; et al. Empleo de Sistemas de Inmersión Temporal Como Alternativa Para La Propagación in Vitro Del Cultivar de Plátano Vianda INIVITPV06-30 (Musa AAB). Biotecnol. Veg. 2012, 12, 53–57. [Google Scholar]
- Alamilla Magaña, J.C.; Caamal Velazquez, J.H.; Criollo Chan, M.A.; Vera Lopez, J.E.; Reyes Montero, J.A. Biofábricas y Biorreactores de Inmersión Temporal: Propagación in Vitro de Anthurium andreanum L., y Su Viabilidad Económica. Agro Product. 2019, 12, 23–29. [Google Scholar] [CrossRef]
- Villegas, A.B.; Villegas, A.B.; Aguila, Z.O.; Vázquez, M.J.; Fernández, O.R.; García-Aguila, L.; Feria, M. de Empleo de Los Sistemas de Inmersión Temporal Para La Producción de Vitroplantas de Caña de Azúcar. Biotecnol. Veg. 2002, 2, 201–206. [Google Scholar]
- Gautam, S.; Solis-Gracia, N.; Teale, M.K.; Mandadi, K.; da Silva, J.A.; Vales, M.I. Development of an in Vitro Microtuberization and Temporary Immersion Bioreactor System to Evaluate Heat Stress Tolerance in Potatoes (Solanum tuberosum L.). Front. Plant Sci. 2021, 12, 1659. [Google Scholar] [CrossRef]
- Rojas, G.P.; Rojas, G.P.; Sanchez, H.; Barahona, I.R.; Ayme, V.A.; Segura-Turkowsky, M.; Jimenez, R.E. Alternative Inputs for Micropropagation of Solanum tuberosum, Ullucus tuberosus and Oxalis tuberosa in Semisolid and Liquid Medium and Temporary Immersion System. Trop. Subtrop. Agroecosyst. 2020, 23, 41. [Google Scholar]
- Peña, G.; Peña, G.; Carhuaz, R.; Davalos, J.; Ayme, V.A. Use of Rita® Temporary Immersion System to Obtain Microtubers of Several Mashua (Tropaeolum tuberosum Ruiz & Pavón) Morphotypes. Trop. Subtrop. Agroecosyst. 2020, 23, 84. [Google Scholar]
- Akita, M.; Takayama, S. Stimulation of Potato (Solanum tuberosum L.) Tuberization by Semicontinuous Liquid Medium Surface Level Control. Plant Cell Rep. 1994, 13, 184–187. [Google Scholar] [CrossRef]
- Montoya, N.; Castro, D.; Díaz, J.; Ríos, D. Tuberización in Vitro de Papa (Solanum tuberosum L), Variedad Diacol Capiro, En Biorreactores de Inmersión Temporal y Evaluación de Su Comportamiento En Campo. Rev. Cienc. 2008, 16, 288–295. [Google Scholar]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) Micropropagation in Temporary Immersion Systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Cabrera, M.; Gómez, R.; Espinosa, E.; López, J.; Medero, V.; Basail, M.; Santos, A. Yam (Dioscorea alata L.) Microtuber Formation in Temporary Immersion System as Planting Material. Biotecnol. Apl. 2011, 28, 268–271. [Google Scholar]
- Igarza Castro, J.; Agramonte, D.; de Feria, M.; Jaime, J.; Pérez, M.; San Román, M. Obtención de Microtubérculos de Papa Cv. ‘Andinita’ En Sistemas de Inmersión Temporal. Biotecnol. Veg. 2011, 11, 59–62. [Google Scholar]
- Gopal, J.; Chamail, A.; Sarkar, D. In Vitro Production of Microtubers for Conservation of Potato Germplasm: Effect of Genotype, Abscisic Acid, and Sucrose. Vitr. Cell. Dev. Biol.-Plant 2004, 40, 485–490. [Google Scholar] [CrossRef]
- Moreno, M.; Oropeza, M. Efecto de Las Hormonas Vegetales y El Fotoperiodo En La Producción de Microtubérculos de Papa (Solanum tuberosum L.). Rev. Colomb. Biotecnol. 2017, 19, 29–38. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).