Abstract
The objective was to evaluate the hematological and biochemical blood parameters and performance of Colossoma macropomum submitted to BFT maturation and under different feeding regimes. BFT maturation was carried out for 60 days (Phase 1). Feeding on six or seven days a week and feeding rates of 4% or 6% of biomass were tested (Phase 2). The water quality parameters were monitored throughout the experimental period. At the end of Phases 1 and 2, blood samples and zootechnical performance were evaluated. In Phase 1, total ammonia was higher on the 17th day (1.25 mg TAN L−1) and stabilized from the 21st day onwards. Nitrite reached a peak (9.67 mg L−1) on the 26th day. There was an increase in nitrate between the 25th and 60th day (1.79 ± 0.01 vs. 5.45 ± 0.01 mg N-NO3− L−1, respectively). FCR (1.90 ± 0.21), weight gain (9.81 ± 1.08 g), and SGR (1.26 ± 0.12%) were highest at 30 days of phase 1. The glucose level (118.23 ± 26.30 mg dL−1) was highest on the 30th day. The plasmatic protein (5.36 ± 0.30 g dL−1) and alanine aminotransferase (ALT) levels (27.58 ± 6.58 UI mL−1) were highest after 60 days. The hemoglobin level (5.77 ± 0.74 g dL−1) was lowest after 30 days. In Phase 2, the triglycerides, ALT, and hematocrit levels were different at the end of the experiment under all feeding regimes. Histological analysis of gills showed a normal condition for fish under BFT. It was possible to apply a feeding regime of six days a week and 4% biomass for juveniles, with 43 g on average.
1. Introduction
Biofloc technology (BFT) has been studied for many fish and shrimp species of commercial interest [1,2,3,4,5,6,7]. Classified as an intensive system with minimal water renewal, BFT depends on the generation of bioflocs, which are aggregates of microorganisms [8]. The primary function of the microorganisms in the system is the production of microbial biomass to recycle nutrients, especially nitrogen compounds, by microbial looping through heterotrophic bacteria [8,9] or chemoautotrophic microorganisms [8,10].
Nitrogenous substances such as ammonia, nitrite, and nitrate exist naturally in aquatic environments but generally present increased levels during cultivation [11,12,13,14,15,16]. Deamination of metabolic proteins produces ammonia, which represents 70–95% of the total of nitrogen excreted [17], thus constituting the main residue. Ammonia is also produced by deamination of microbial proteins in protein-rich organic matter, such as undigested food, feces, and dead animals [18]. In water, nitrifying bacteria can oxidize ammonia to nitrite and then nitrate, which can be reincorporated in microbial or vegetable protein [19]. Although at a different rate, three nitrogen compounds can negatively impact the metabolism and growth of organisms in cultivation [1,20,21,22,23]. In closed systems, until the establishment of the microbial biomass, total ammonia (TAN) and nitrite are expected to peak and can reach critical levels for aquatic organisms [8]. The increased concentration of nitrogen compounds brings losses to animal welfare and, consequently, to the growth performance of captive organisms [24,25,26].
Colossoma macropomum, known as tambaqui or black pacu, is native to the Amazon and represents the most commercially produced native species in South America, although it is also produced in Asia (China, Indonesia, Malaysia, Myanmar, and Vietnam) [27]. Interest in this species has been growing and includes a search for more efficient systems and feeding management. To improve the growth performance of fish, other factors beyond the production system, such as feeding management, need to be considered. Some such management involving feed restriction and rate have been tested in different systems and with different fish species to evaluate compensatory growth without harming welfare [28,29,30,31]. In nature, the fish species C. macropomum, Piaractus brachypomus, and Piaractus mesopotamicus go through long periods of feed restriction, and protocols of feed restriction in captivity have demonstrated improved growth performance [32,33,34,35] and reduced production cost with lower labor and feed cost [36,37,38]. Although the growth performance of juvenile C. macropomum in BFT systems has been described [39,40,41], the physiological condition and growth performance of C. macropomum during BFT maturation and when submitted to different feeding regimes with restriction in BFT have yet to be evaluated.
Therefore, this study aimed to investigate, through hematological analysis and growth performance, the physiological response of juvenile Colossoma macropomum during BFT maturation and when submitted to different feed regimes under BFT.
2. Materials and Methods
2.1. Experimental Conditions
The experiment was carried out in the Laboratório de Aquacultura at the Federal University of Minas Gerais (UFMG) following a protocol approved by the Committee for Ethics in Animals Use (CEUA-244/2020) and was divided into two experimental phases.
2.1.1. Phase 1—Growth Performance under Biofloc Systems
Phase 1 lasted 60 days and used 192 juvenile C. macropomum (21.25 ± 0.40 g, 11.19 ± 0.09 cm) distributed among 16 tanks (n = 12 fish tank−1) with volumes of 80 L each (3.22 kgm−3). The experimental tanks were arranged in a “macrocosm–microcosm” model [42], being interconnected in a 1000 L matrix recirculating macrocosm to keep the biofloc homogenized [38]. Submersible heater thermostats (at 28 °C) were used to control the water temperature. In addition, a submersible pump was installed in the matrix of the macrocosm to maintain the water circulation with water returned by gravity, maintaining a flow in each tank of 3.18 L min−1. An artificial substrate for fixing nitrifying bacteria was added to all tanks (20 × 35 cm Bedean®).
All tanks in the system were filled with clear water, and the photoperiod was maintained at 14 h L:10 h D. Unrefined sugar cane [43] was used as carbon source for BFT maturation, with the C/N ratio being maintained at 15:1 in the beginning and 6:1 thereafter [44]. Carbon (sugar) was added whenever ≥1 mg L−1 of total ammonia nitrogen was measured in the water [8,10]. The juveniles were fed manually two times a day (8 h and 16 h) with commercial feed extruded (Laguna Peixes Brasileiros-Socil, Brazil) with 4 mm of pellet diameter (crude protein—320 g kg−1; ethereal extract min.—50 (g kg−1); crude fiber—90 g kg−1; mineral matter—140 g kg−1; calcium min.—15 g kg−1; calcium max.—30 g kg−1; phosphorus min.—6000 g kg−1; humidity—1500/1000 g kg−1) at 3% of biomass [45], with feed adjustment at every biometric. Uneaten feed was collected 30 min after every feeding, dried in an oven (Nova Étic/Ethink) at 55 °C, and weighed to calculate consumption. Biometrics were performed, and survival was evaluated, at the end of Phase 1.
2.1.2. Phase 2—Effect of Different Feeding Regimes on Growth and Physiology Performance of C. macropomum Juveniles Reared in BFT
Phase 2 lasted 60 days and used 96 C. macropomum juveniles (43.34 ± 0.35 g, 14.49 ± 0.15 cm) from Phase 1 distributed in 16 tanks (80 L volume) containing 100% inoculum from Phase 1, with density adjusted to n = 6 juveniles tank−1 (3.25 kg m−3). The BFT system was disposed in the same “macrocosm–microcosm” model as in Phase 1, and the fish were submitted to the following different regimes of weekly feeding frequency and feeding rate in a 2 × 2 factorial, with four repetitions:T6 × 4: fed six days a week, 4% of biomass; T6 × 6: fed six days a week, 6% of biomass; T7 × 4: fed seven days a week, 4% of biomass; T7 × 6: fed seven days a week, 6% biomass.
Juveniles were fed twice a day (8 h and 16 h) with commercial extruded diet (32% crude protein, 4 mm diameter), with feed adjustment after every biometric as determined by biomass. Uneaten feed was collected 30 min after every feeding, dried in an oven at 55 °C, and weighed to calculate consumption. The juveniles were weighed and counted at the end of Phase 2 to calculate survival. The photoperiod was maintained at 14 h L:10 h D, and artificial substrate (20 × 35 cm Bedean®) was used in all treatment tanks.
2.2. Water Quality Analysis
In Phase 1, total ammonia, nitrite, pH, settleable solids, temperature, and dissolved oxygen were monitored daily, while nitrate was measured on days 25 and 60. In Phase 2, pH, settleable solids, temperature, and dissolved oxygen were monitored daily; total ammonia and nitrite, three times a week; and nitrate, at the beginning and at days 30 and 60. Total ammonia (TAN) [46], nitrite (N-NO2−) [47], and nitrate (N-NO3−) [48]; pH; temperature; and dissolved oxygen (DO) (model 550A YSI multiparameter—Ohio, USA) were monitored from microcosms in quadruplicate (four water samples), while settleable solids (SS) [8,49] (during 15 min) and alkalinity (ALPHA, 2012) were monitored from the macrocosm in duplicate.
For both phases, clarification was performed when settleable solids exceeded 10 mL L−1 [50]. Dolomitic limestone was used to adjust alkalinity when it reached ≤100 mg CaCO3− [51].
Water salinity was maintained at 2 g L−1 (checked with refractometer) during the Phase 1 and Phase 2 [52,53].
2.3. Blood Analysis
To determine any physiological effects on C. macropomum juveniles during biofloc maturation in Phase 1, blood was collected from 12 juveniles at 0 (basal), 30, and 60 days of rearing. To evaluate hematological and biochemical responses of C. macropomum juveniles reared in BFT under different feeding regimes in Phase 2, blood was collected from 12 juveniles at the beginning and end of the experimental period. Blood was collected from the caudal vein using heparinized syringes. Part of each blood sample was used to evaluate the hematocrit via capillary tubes [54]. Hemoglobin concentration was determined using 4 μL of blood in 1 mL of commercial colorimetric reagents (Bioclin® Brazil). Plasmatic protein was measured with a manual refractometer (RHC 200-ATC, Huake Instrument Co) after breaking the microhematocrit tube from the hematocrit. Biochemical levels of the glucose, triglycerides, and cholesterol, AST, and ALT were determined by centrifuging blood at 4000 rpm for 10 min for plasma separation and dosage in commercials kits (Bioclin® Minas Gerais, Brazil). All samples were read using a spectrophotometer (Libra S22 Biochrom Cambridge, UK).
2.4. Growth Performance
Growth performance was determined for all C. macropomum juveniles by measuring weight using a precision scale (0.01 g, AD5002 Marte Minas Gerais, Brazil) and length using an ichthyometer on days 1, 30, and 60 of Phase 1 and days 1 and 60 of Phase 2 to determine:
- Weight gain (WG; g) = final weight — initial weight;
- Specific growth rate (SGR; % / day) = ((ln final weight — ln initial weight)/days) × 100;
- Production (kg m−3) = biomass/tank volume in m3;
- Biomass (g) = total number of fish × final weight;
- Feed intake (g) = weight of offered feed — weight of uneaten feed;
- Feed conversion rate (FCR) = feed intake/weight gain;
- Survival (%) = (number of fish at the end of the experiment/number of initial fish) × 100.
2.5. Indexes
For phase 2, after blood collection, the 12 animals were euthanized (285 mg eugenol L−1) [55] and had their livers and adipose tissue removed to calculate their hepatosomatic and mesenteric fat indices as: Hepatosomatic index (HSI) = (liver weight/body weight) × 100; and Mesenteric fat index (MFI) = (mesenteric weight/body weight) × 100.
2.6. Histology
At the end of Phase 2, samples of gills from six fish of each treatment were fixed in Bouin’s fluid for histological processing. All samples were processed using LUPE PT05 automated equipment and were embedded in Paraplast®. Sections were cut at 3 µm with a LUPE MRP03 microtome. Histological sections were stained with hematoxylin–eosin. For classification purposes, light hyperplasia was designated to be 2 to 5 cell layers on the lamellae, moderate hyperplasia was designated to be 5 to 10, and severe hyperplasia was designated to be 10 or more [56].
2.7. Statistical Analysis
Data were tested for normality (Shapiro–Wilks) and homoscedasticity (Levene). Prior to analysis, percentage values for both phases were transformed by arc sin square root, while only untransformed data are shown. Growth performance data for Phase 1 were analyzed at days 30 and 60 during biofloc maturation by the Mann–Whitney test for nonparametric data and Student’s T-test for parametric data (α = 0.05). Blood data for Phase 1 were analyzed at days 0 (basal), 30, and 60 during biofloc maturation by one-way ANOVA and Tukey´s post hoc test (α = 0.05). Hepatosomatic and mesenteric fat indices for Phase 2 were also submitted to one-way ANOVA and Tukey´s post hoc test (α = 0.05). Growth performance and blood parameters were submitted to factorial (two-way) ANOVA and Tukey´s post hoc test (α = 0.05). Nonparametric data of both Phase 1 and Phase 2 were analyzed by the Kruskal–Wallis test (α = 0.05).
3. Results
3.1. Phase 1—Growth Performance under Biofloc Systems
Values for temperature, DO, alkalinity, and settleable solids during Phase 1 were 26.9 ± 0.99 °C, 5.97 ± 0.32 mg L−1, 109.47 ± 36.43 mg CaCO3 L−1, and 3.46 ± 4.35 mL L−1, respectively. Data for TAN, nitrite, and pH are shown Figure 1. The concentration of TAN was highest on day 17 (1.25 mg TAN L−1) and stabilized from day 21 onward, with means ≤ 0.25 mg L−1. Nitrite reached a peak (9.67 mg L−1) on day 26 and then reduced, reaching stability on day 43. Nitrate increased significantly (p < 0.05) between days 25 and 60 (1.79 ± 0.01 and 5.45 ± 0.01 mg N-NO3− L−1, respectively). During the same period of continuous nitrite increase, pH decreased until day 42, after which it increased. Data for DO are shown in Figure 1B. Dolomitic limestone was added to the BFT on days 33 to 46 to correct alkalinity. Settleable solids were detected starting on day 12 (0.2 mL L−1), with a peak (15 mL L−1) on day 45, the same period when the system was clarified. Partial renewal of water (10% of total volume, total of 1300 L) was necessary when nitrite reached ≥5 mg L−1.
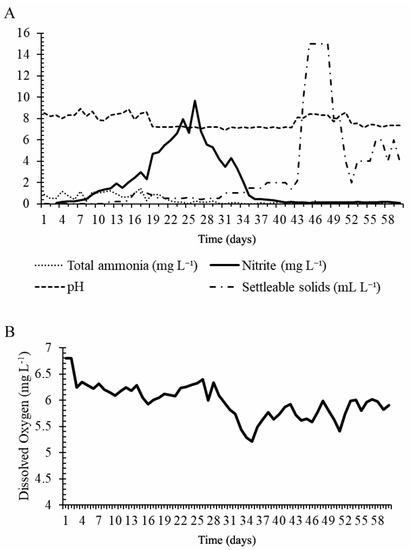
Figure 1.
(A) Curves for total ammonia (mg L−1), nitrite (mg L−1), settleable solids (mL L−1), and pH during biofloc maturation when rearing Colossoma macropomum juveniles. (B) Curve for dissolved oxygen (mg/L) during biofloc maturation when rearing Colossoma macropomum juveniles.
During biofloc maturation, the C. macropomum juveniles grew (in both weight and total length) and achieved their highest biomass, and consequently highest production (kg m−3), at the end of the experimental period (p < 0.05) (Table 1); however, the best performance for FCR, WG, and SGR (p < 0.05) was at day 30. Survival did not differ throughout the experimental period (p > 0.05).

Table 1.
Values (mean ± standard deviation) for weight, total length, biomass, feed conversion rate, weight gain, feed intake, specific growth rate, and survival of juvenile Colossoma macropomum at different times (days 30 and 60) during biofloc maturation.
Changes occurred in the blood parameters of the C. macropomum during biofloc maturation (Figure 2). Glucose concentration (Figure 2A) was highest on day 30 (p < 0.05). Plasmatic protein (Figure 2E) and ALT (Figure 2G) were highest at the end of the experimental period (p < 0.05). Hemoglobin (Figure 2C) differed significantly (p < 0.05) among basal, day 30, and day 60, being lowest on day 30. Hematocrit decreased (p < 0.05) on day 60 (Figure 2F). Metabolic triglycerides (Figure 2B), cholesterol (Figure 2D), and AST (Figure 2H) did not change significantly during biofloc maturation (p > 0.05).
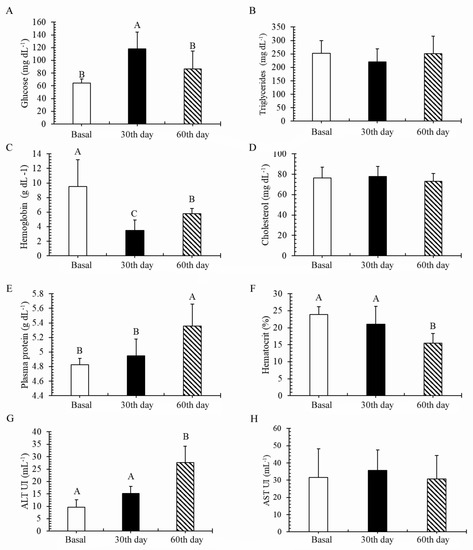
Figure 2.
Hematological, biochemical, and enzymatic blood parameters of the Colossoma macropomum juveniles (n = 12 pre collection) on days 0 (basal), 30, and 60 of rearing under biofloc maturation. Different letters on the bars indicate significant differences over time in glucose (A), cholesterol (D), plasmatic protein (E), hemoglobin (C), and ALT (G) according to Kruskal–Wallis test (p < 0.05). Different letters on the bars indicate significant differences over time in AST (H), triglycerides (B), and hematocrit (F), according to Tukey´s test (p < 0.05).
3.2. Phase 2—Effect of Different Feeding Regimes on Growth and Physiology Performance of C. macropomum Juveniles Reared in BFT
No differences (p > 0.05) were detected for the water quality parameters of temperature (28.78 ± 0.09 °C), pH (8.43 ± 0.33), TAN (0.12 ± 0.08 mg TAN L−1), nitrite (0.18 ± 0.06 mg N-NO2− L−1), DO (4.92 ± 0.18 mg OD L−1), alkalinity (153.08 ± 34.61 mg CaCO3 L−1), or settleable solids (5.69 ± 3.99 mL L−1) among different feeding regimes (p > 0.05). Nitrate did not differ significantly among feeding regime treatments (p > 0.05) at the beginning or on day 30 or 60 (5.45 ± 0.01, 19.74 ± 0.11, and 44.36 ± 0.11 mg N-NO3− L−1, respectively).
The parameters of weight, length, biomass, production, FCR, FI, WG, and SGR did not differ according to weekly feeding frequency or feeding rate after 30 and 60 days, and there was no interaction among them (p > 0.05) (Table 2). Survival was 100% for all treatments on day 30 and was 91.65 ± 9.64 (T6 × 4), 95.82 ± 8.35 (T6 × 6), 91.65 ± 9.64 (T7 × 4), and 87.50 ± 15.94 % (T7 × 6) on day 60, with no significant differences (p > 0.05).

Table 2.
Growth performance (mean ± SD) for weight (W, g), total length (L, cm), biomass (g), production (kg m−3), feed conversion rate (FCR), feed intake (FI, g), weight gain (WG, g), and specific growth rate (SGR, %) for Colossoma macropomum juveniles grown under BFT with different weekly feeding frequencies and feeding rates.
Plasmatic analysis found glucose (Figure 3A), hemoglobin (Figure 3D), cholesterol (Figure 3C), protein (Figure 3E), and AST (Figure 3H) not to differ among feeding regimes (p > 0.05). Triglycerides (Figure 3B), ALT (Figure 3G), and hematocrit (Figure 3F) were significantly different at the end of the experiment (p < 0.05) for all feeding regimes, with final concentrations of triglycerides and ALT being lower (p < 0.05), and hematocrit higher (p < 0.05), under all feeding regimes. There were no significant differences (p > 0.05) among treatments for HSI (1.48 ± 0.24) or MFI (2.46 ± 0.70).
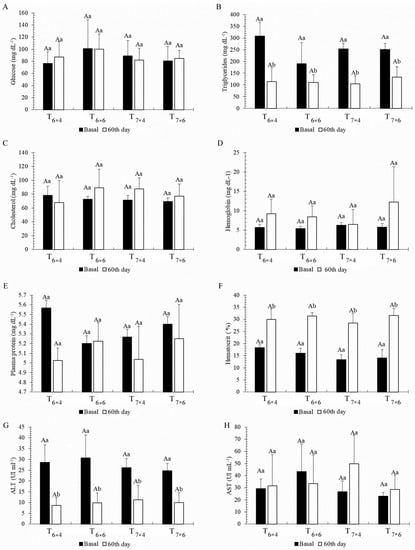
Figure 3.
Blood parameters (basal and on day 60, Phase I) for Colossoma macropomum juveniles under different feeding frequencies and feeding rates: glucose (A), triglycerides (B), cholesterol (C), hemoglobin (D), plasmatic protein (E), hematocrit (F), ALT (G), and AST (H). Capital letters indicate significant differences between feeding regimes (treatments); lowercase letters indicate significant differences over time (between basal and day 60). Two-way ANOVA, Tukey’s test (p < 0.05).
Histological analysis revealed hyperplasia for all treatments, all of them were classified as light to moderate (Figure 4B, T7 × 4). Epithelial lifting (Figure 4C, T6 × 6) and lamellar fusion also were observed. In general, all treatments exhibited normal predominances of primary and secondary lamellae (Figure 4A,D, T6 × 4 and T7 × 6).
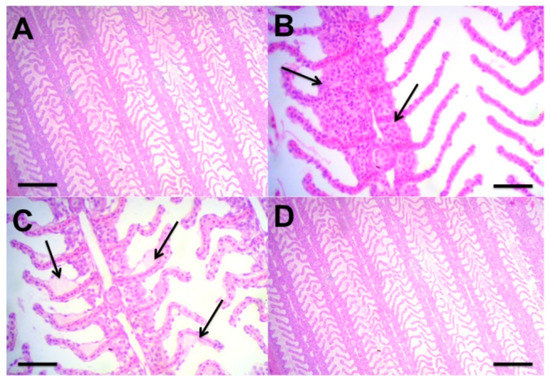
Figure 4.
Histology of fish gill structure of the Colossoma macropomum in BFT at the end of phase 2. (A) T6 × 4 and (D) T7 × 6, normal primary and secondary lamellae; (B) T7 × 4, moderate hyperplasia; and (C) T6 × 6, epithelium lifting. (A) H–E bar, 200 µ. (B) H–E bar, 50 µ. (C) H–E bar, 50 µ. (D) H–E bar, 200 µ.
4. Discussion
In Phase 1, the water quality parameters of temperature, DO, and pH remained within values indicated to the C. macropomum [57], and the alkalinity and SS remained within values indicated for fish [8,26].
Analysis of the curves for nitrogen compounds in Phase 1 revealed that they maintained the maturation pattern described in other works with BFT, with a nitrite peak followed by reduction [58,59,60]. Both TAN and N-NO3− remained at safe levels [61] for fish. Although N-NO2−, a nitrogenous compound that causes most of the problems for BFT [62], reached levels considered high for the species [63], in the present study, mortality was not recorded. This could be explained by the short exposure time associated with the use of salt (NaCl). Both Cl− and N-NO2− have the same inflow route, and increased chloride in water promotes reduction in the inflow of N-NO2−, thus decreasing the N-NO2− concentration in blood plasma [64,65]. The N-NO2− in the C. macropomum juveniles may not have caused mortality during the period, but effects of peak N-NO2−, such as hyperglycemia and changes in protein levels, which signal stressful situations for fish, were seen in blood analyses. In addition, effects of peak N-NO2− were detected in a reduction in hemoglobin. A similar response was described for tambacus (Piaractus mesopotamicus × Colossoma macropomum) submitted to toxic levels of N-NO2− [66]. This response is due to the oxygen binding site of hemoglobin becoming occupied by N-NO2−, transforming hemoglobin into methemoglobin and impairing oxygen transport [67].
In the present study, with a reduction in N-NO2− to safe levels (from the 35th day onwards), it was possible to verify that at the end of the Phase 1 experimental period, the juveniles were recovering in terms of their hemoglobin levels but did not reach their baseline levels, demonstrating a prolonged effect of N-NO2− on C. macropomum. At the end of Phase 1, the reduction in hematocrit could also be attributed to the prolonged toxic effect of N-NO2−, since in the peak period, the values were similar to baseline values. This same pattern of hemoglobin and hematocrit reduction was seen in Brycon cephalus; the researchers classified it as a picture of functional and hemolytic anemia caused by the toxic effect of N-NO2− [68]. Another prolonged effect attributed to the toxicity of this nitrogenous compound was an increase in ALT. The elevation of this enzyme in plasma is related to liver damage, and the same pattern has already been verified for Labeo rohita submitted to N-NO2− [69].
Regarding the growth parameters, although the animals showed increases in weight, total length, and consequently biomass and productivity at the end of Phase 1, performance (WG, SGR, and FCR) was worse, representing productive losses. This drop in performance has been reported in other studies involving N-NO2− toxicity [69] and in the BFT system for Brycon orbignianus [70]. In this phase, it is evident that the negative physiological effects and losses in performance caused by the BFT maturation process and its reflexes in production were prolonged, even being present 3.5 weeks after reducing the level of N-NO2− to a safe concentration for the species. The partial renewal of water during the peak in the maturation phase, the use of biofloc inoculum, and water salinization are some tested techniques that can minimize the toxicity of N-NO2− during the maturation of biofloc [24,70].
In Phase 2, the water quality parameters of DO, temperature, pH and alkalinity for the microcosm–macrocosm system remained the same among treatments and were considered ideal for the growth of tropical species [57,71,72]. With the mature BFT system, nitrogen compounds (TAN, N-NO2−, and N-NO3−) remained at safe levels [62], with no need for water changes, only replacement for evaporation and losses related to the clarification process (total of 1200 L), which kept SS at safe levels, especially to prevent gill occlusion and increased DO consumption by microorganisms present in the biofloc [8].
With the water quality parameters stabilized in this phase, and within the conditions suitable for the species, any difference in the performance of the animals could be attributed to the varied diet. However, performance did not differ among treatments. Studies with different diets reported that the highest feeding rates had the best performance for P. mesopotamicus [32] and Ictalurus punctatus [33]. Studying different feeding frequencies and feeding rates for C. macropomum in net tank [28] showed that the feeding frequency (two vs. three times a day) did not affect the fishes’ performance. However, the feeding rate of 10% weight/day showed best results for growth performance. On the other hand, as in the present study, juveniles of C. macropomum in RAS subjected to food restriction once a week performed the same as those subjected to daily feeding treatment [35]. Other species, such as Cichla monoculus under food restriction of one day [34] and Lophiosiluris alexandri under food restriction of both one and two days [73], did not show differences in performance. These results were similar to those in the present study with C. macropomum in BFT. The same was reported [37] with C. macropomum submitted to one or even three days of restriction, indicating recovery in growth with resumed feeding. It is possible that the same may have happened in the animals under restriction of one day (T6 × 4 and T6 × 6) in the present study without causing losses in performance.
Food restriction could have caused the mobilization of energy reserves through viscerosomatic fat or the liver, reducing IGS and IHS, but no differences were observed in these indices, indicating that short periods of restriction with or without reduced feeding rates did not promote alteration or mobilization of the fat or liver of C. macropomum in BFT, a fact that indicates the possibility of less supply in the amount of food and restriction of one day (T6 × 4) for juveniles of C. macropomum in BFT with an initial average weight of 43 g.
The FCR values reported for C. macropomum in the literature on food restriction have varied. Comparatively, the FCR values of 2.06–4.14 described in [37] for C. macropomum in a net tank were close to those of the present study, whereas [35] presented lower FCR values of 0.57–0.8 for C. macropomum in RAS, demonstrating that it is possible to improve the efficiency of food use in intensive systems and that this point should be better studied in BFT.
The high survival rates in the present study were in agreement with other works with C. macropomum in RAS, net tanks, and nursery systems [28,35,74] demonstrating the plasticity that the species has in adapting to different production systems, including BFT, and different restriction regimes.
BFT has been used as a strategy for maintaining water quality, but it is known that the microorganisms that make up the biofloc can be used as food for fish and shrimp [25,75,76,77]. In the present study, the lower rates of feeding and/or restriction of one day may have also indicated that juveniles of C. macropomum took advantage of the biofloc as food because of the characteristics of the species’s feeding habits and the absence of feed supply, which would support the similar performance among treatments, although future studies are needed to confirm the use of biofloc by juveniles of C. macropomum.
There were no differences in blood parameters among treatments, even when the C. macropomum juveniles were subjected to one-day food restriction in Phase 2. At the end of the experimental period, all treatments showed reductions in triglycerides and ALT. Considering the two phases of the work, the fall in triglycerides and ALT in Phase 2 seemed to be related to recovery after coping with the N-NO2− peak in Phase 1 and not to the effect of different diets, since all treatments presented the same pattern of reductions in both triglycerides and ALT. Changes in glucose concentrations have been an important hematological parameter for defining the condition of fish [78]. In the case of food restriction, and depending on the time of restriction, fish may present hypo- or hyperglycemia [35]. In the present study, glucose levels remained stable among treatments at the beginning and end of the Phase 2 experimental period, and the normal gill histology condition suggested the adaptation of the species to the different feed regimes in BFT. Although damage was observed in the gills of C. macropomum, the damage was low and common in fish, e.g., carp [5], without prejudice to C. macropomum in BFT systems.
In general, it is possible to indicate the least frequent diet and the lowest feeding rate, T6 × 4, for C. macropomum in BFT, resulting directly in reductions in feeding per day and economic costs without damage to animals or production.
5. Conclusions
In Phase 1, hematological effects related to stress and performance loss were evident for C. macropomum when passing through the N-NO2− peak during BFT maturation. This demonstrates the importance of water management with techniques that reduce N-NO2− during this phase, such as more frequent water changes, increased salinity, and/or the use of biofloc inoculum. In Phase 2, the different feeding regimes demonstrated that it was possible to feed the C. macropomum juveniles (43 g) six days a week with a feeding rate of 4% of biomass without compromising their performance or hematological condition.
Author Contributions
C.L.N.: Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition. L.F.S.S.: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft preparation, visualization. F.A.C.d.S.: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, visualization. T.P.B.: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft preparation, visualization. G.C.F.: Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition. G.D.A.P.: Conceptualization, methodology, formal analysis, investigation, resources. N.F.A.C.d.M.: Conceptualization, methodology, formal analysis, investigation, resources. L.A.R.: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft preparation, visualization. R.K.L.: Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil—Procad 88887.200588/2018-00), and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil). LUZ, R.K. received a research grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Process 308547/2018-7).
Institutional Review Board Statement
The study was conducted in accordance with and approved by the Institutional Ethics Committee of Universidade Federal de Minas Gerais (protocol code on Animal Use is CEUA-244/2020).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil—Procad 88887.200588/2018-00), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-Brazil). LUZ, R.K. received a research grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Process 308547/2018-7).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo, G.; Gao, Q.; Wang, C.; Liu, W.; Sun, D.; Li, L.; Tan, H. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture 2014, 422–423, 1–7. [Google Scholar] [CrossRef]
- Fugimura, M.M.S.; dos Reis Flor, H.; de Melo, E.P.; da Costa, T.V.; Wasielesky, W.; Oshiro, L.M.Y. Brewery residues as a source of organic carbon in Litopenaeus schmitti white shrimp farms with BFT systems. Aquac. Int. 2015, 23, 509–522. [Google Scholar] [CrossRef]
- Vilani, F.G.; Schveitzer, R.; Da Fonseca Arantes, R.; Do Nascimento Vieira, F.; Manoel do Espírito Santo, C.; Quadros Seiffert, W. Strategies for water preparation in a biofloc system: Effects of carbon source and fertilization dose on water quality and shrimp performance. Aquac. Eng. 2016, 74, 70–75. [Google Scholar] [CrossRef]
- Krummenauer, D.; Poersch, L.H.; Fóes, G.; Lara, G.; Wasielesky, W. Survival and growth of Litopenaeus vannamei reared in Bft System under different water depths. Aquaculture 2016, 465, 94–99. [Google Scholar] [CrossRef]
- Bakhshi, F.; Najdegerami, E.H.; Manaffar, R.; Tukmechi, A.; Farah, K.R. Use of different carbon sources for the biofloc system during the grow-out culture of common carp (Cyprinus carpio L.) fingerlings. Aquaculture 2018, 484, 259–267. [Google Scholar] [CrossRef]
- Kaya, D.; Genc, E.; Genc, M.A.; Aktas, M.; Eroldogan, O.T.; Guroy, D. Biofloc technology in recirculating aquaculture system as a culture model for green tiger shrimp, Penaeus semisulcatus: Effects of different feeding rates and stocking densities. Aquaculture 2020, 528, 735526. [Google Scholar] [CrossRef]
- Battisti, E.K.; Rabaioli, A.; Uczay, J.; Sutili, F.J.; Lazzari, R. Effect of stocking density on growth, hematological and biochemical parameters and antioxidant status of silver catfish (Rhamdia quelen) cultured in a biofloc system. Aquaculture 2020, 524, 735213. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guidebook, 2nd ed.; The Word Aquaculture Society: Baton Rouge, LA, USA, 2012; p. 271. [Google Scholar]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.M.; Burger, J.M.; Almeida, R.V.; Ayub, Z.; Harisanto, M.; Horowitz, A.; Brock, D.L. Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquac. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 2006, 58, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Yanbo, W.; Wenju, Z.; Weifen, L.; Zirong, X. Acute toxicity of nitrite on tilapia (Oreochromis niloticus) at different external chloride concentrations. Fish Phys. Bioch. 2006, 32, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xiang, F.; Liang, L.; Yang, Z. Toxicity of ammonia and its effects on oxidative stress mechanisms of juvenile crucian carp (Carassius auratus). J. Freshw. Ecol. 2010, 25, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, N.C.; Bonetti, C.; Seiffer, W.Q. Hydrological and water quality indices as management tools in marine shrimp culture. Aquaculture 2011, 318, 425–433. [Google Scholar] [CrossRef]
- Xian, J.A.; Wang, A.L.; Hao, X.M.; Miao, Y.T.; Li, B.; Ye, C.X.; Liao, S.A. In vitro toxicity of nitrite on haemocytes of the tiger shrimp Penaeus monodon, using flow cytometric analysis. Comp. Bioch. Phys. Part C 2012, 156, 75–79. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.J.; Hwang, I.K.; Han, J.M.; Kim, D.H.; Oh, C.W.; Lee, J.-S.; Kang, J.-C. Toxic effects of juvenile sablefish, Anoplopoma fimbria by ammonia exposure at different water temperature. Envir. Toxic. Pharm. 2017, 54, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, M.; Nizzoli, D.; Castaldelli, G.; Vialroli, P. Community metabolism and buffering capacity of nitrogen in a Ruppia cirrhosa meadow. J. Exp. Mar. Biol. Ecol. 2008, 360, 21–30. [Google Scholar] [CrossRef]
- Yun, L.; Yu, Z.; Li, Y.; Luo, P.; Jiang, X.; Tian, Y.; Ding, X. Ammonia nitrogen and nitrite removal by a heterotrophic Sphingomonas sp. strain LPN080 and its potential application in aquaculture. Aquaculture 2019, 500, 477–484. [Google Scholar] [CrossRef]
- Robles-Porchas, G.R.; Gollas-Galván, T.; Martínez-Porchas, M.; Martínez-Cordova, L.R.; Miranda-Baeza, A.; Vargas-Albores, F. The nitrification process for nitrogen removal in biofloc system aquaculture. Rev. Aquac. 2020, 12, 2228–2249. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Toxic effects of ammonia, nitrite, and nitrate to decapod crustaceans: A review on factors influencing their toxicity, physiological consequences, and coping mechanisms. Rev. Fish. Sci. 2013, 21, 1–21. [Google Scholar] [CrossRef]
- Long, L.N.; Yang, J.; Li, Y.; Guan, C.W.; Wu, F. Effect of biofloc technology on growth, digestive enzyme activity, hematology, and immune response of genetically improved farmed tilapia (Oreochromis niloticus). Aquaculture 2015, 448, 135–141. [Google Scholar] [CrossRef]
- Khatoon, H.; Banerjee, S.; Yuan, G.T.G.; Haris, N.; Ikhwanuddin, M.; Ambak, M.A.; Endut, A. Biofloc as a potential natural feed for shrimp postlarvae. Inter. Biodet. Biodeg. 2016, 113, 304–309. [Google Scholar] [CrossRef]
- Maciel, J.C.; Francisco, C.J.; Miranda, K.C. Compensatory growth and feed restriction in marine shrimp production, with emphasis on biofloc technology. Aqua. Int. 2018, 26, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Krummenauer, D.; Samocha, T.; Poersch, L.; Lara, G.; Wasielesky, W. The reuse of water on the culture of pacific white shrimp, litopenaeus vannamei, in BFT system. J. World Aquac. Soc. 2014, 45, 3–14. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Martínez-Córdova, L.R.; Martínez-Porchas, M.; Miranda-Baeza, A. Biofloc Technology (BFT): A Tool for Water Quality Management in Aquaculture. Water Qual. 2018, 5, 92–109. [Google Scholar] [CrossRef] [Green Version]
- Timmons, M.B.; Ebeling, J.M.; Wheaton, F.W.; Sommerfelt, S.T.; Vinci, B.J. Recirculating Aquaculture Systems, 2nd ed.; Caruga Aqua Ventures: New York, NY, USA, 2002. [Google Scholar]
- Woynárovich, A.; Anrooy, R.V. Field Guide to the Culture of Tambaqui (Colossoma Macropomum, Cuvier, 1816); FAO—Fisheries and Aquaculture Technical Paper, No. 624; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Silva, C.R.; Gomes, L.C.; Brandão, F.R. Effect of feeding rate and frequency on tambaqui (Colossoma macropomum) growth, production and feeding costs during the first growth phase in cages. Aquaculture 2007, 264, 135–139. [Google Scholar] [CrossRef]
- Abdel-Hakim, N.F.; State, H.A.A.; Al-Azab, A.A.; El-Kholy, K.F. Effect of Feeding Regimes on Growth Performance of Juvenile Hybrid Tilapia (Oreochromis niloticus × Oreochromis aureus). World J. Agric. Sci. 2019, 5, 49–54. [Google Scholar]
- Favero, G.C.; Boaventura, T.P.; Ferreira, A.L.; Silva, A.C.F.; Porto, L.A.; Luz, R.K. Fasting/re-feeding and water temperature promote the mobilization of body reserves in juvenile freshwater carnivorous catfish Lophiosilurus alexandri. Aquaculture 2019, 511, 734223. [Google Scholar] [CrossRef]
- Mozanzadeh, M.; Zabayeh Najafabadi, M.; Torfi, M.; Safari, O.; Oosooli, R.; Mehrjooyan, S.; Pagheh, E.; Javad Hoseini, S.; Saghavi, H.; Monem, J.; et al. Compensatory growth of Sobaity (Sparidentex hasta) and yellowfin seabreams (Acanthopagrus latus) relative to feeding rate during nursery phase. Aquac. Nutr. 2021, 27, 468–476. [Google Scholar] [CrossRef]
- Borghetti, J.R.; Canzi, C. The effect of water temperature and feeding rate on the growth rate of pacu (Piaractus mesopotamicus) raised in cages. Aquaculture 1993, 114, 93–101. [Google Scholar] [CrossRef]
- Robinson, E.H.; Li, M.H. Effect of dietary protein concentration and feeding rate on weight gain, feed efficiency, and body composition of pond-raised channel catfish Ictalurus punctatus. J. World Aquac. Soc. 1999, 30, 311–318. [Google Scholar] [CrossRef]
- Soares, E.C.; Pereira-Filho, M.; Roubach, R.; Silva, R.C.S. Condicionamento alimentar no desempenho zootécnico do tucunaré. Rev. Bras. Enga. Pesca. 2007, 2, 35–48. [Google Scholar]
- Assis, Y.P.A.S.; de Assis Porto, L.; de Melo, N.F.A.C.; Palheta, G.D.A.; Luz, R.K.; Favero, G.C. Feed restriction as a feeding management strategy in Colossoma macropomum juveniles under recirculating aquaculture system (RAS). Aquaculture 2020, 529, 735689. [Google Scholar] [CrossRef]
- Paz, A.L.; Pastrana, Y.M.; Brandão, L.V. Food deprivation does not affect growth performance of juvenile tambacu. Acta Amaz. 2018, 48, 207–210. [Google Scholar] [CrossRef]
- Santos, E.L.; Soares, A.C.L.; Tenório, O.L.D.; Soares, E.C.; Silva, T.J.; Gusmão Júnior, L.F.; Santos, E.L. Desempenho de tambaquis (Colossoma macropomum) submetidos a restrição alimentar e a realimentação em tanques—rede. Arq. Bras. Med. Vet. Zootec. 2018, 70, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Correa, A.D.S.; Pinho, S.M.; Molinari, D.; Pereira, K.D.R.; Gutiérrez, S.M.; Monroy-Dosta, M.D.C.; Emerenciano, M.G.C. Rearing of Nile tilapia (Oreochromis niloticus) juveniles in a biofloc system employing periods of feed deprivation. J. Appl. Aquac. 2020, 32, 139–156. [Google Scholar] [CrossRef]
- Abrar, W.A.; Pamukas, N.A.; Putra, I.; Penelitian, W. The effect of probiotic addition in feed towards growth performance and survival rate of tambaqui (Colossoma macropomum) using Bioflocs System. Jur. Perik. Kel. 2019, 24, 32–40. [Google Scholar]
- Oliveira, W.D.S.; Ferreira, J.D.N.; Gonçalves, M.D.S.; Castro, Í.P.; Marinho-Pereira, T.; Cavero, B.A.S. Resíduo De Macaxeira E De Banana Como Fontes De Carbono Na Criação De Tambaqui (Colossoma macropomum) Em Sistema Bioflocos No Estado Do Amazonas, Brasil / Cassava and Banana Residue As Carbon Sources in the Biofloc System Cultivation of Tambaqui (Colossoma macropomum) in Amazonas State, Brazil. Brazilian J. Dev. 2020, 6, 76711–76724. [Google Scholar] [CrossRef]
- Santos, R.B.; Izel-Silva, J.; Fugimura, M.M.S.; Suita, S.M.; Ono, E.A.; Affonso, E.G. Growth performance and health of juvenile tambaqui, Colossoma macropomum, in a biofloc system at different stocking densities. Aquac. Res. 2021, 52, 3549–3559. [Google Scholar] [CrossRef]
- Wasielesky, W.; Atwood, H.; Stokes, A.; Browdy, C.L. Effect of natural production in a zero-exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 2006, 258, 396–403. [Google Scholar] [CrossRef]
- García-Ríos, L.; Miranda-Baeza, A.; Coelho-Emerenciano, M.G.; Huerta-Rábago, J.A.; Osuna-Amarillas, P. Biofloc technology (BFT) applied to tilapia fingerlings production using different carbon sources: Emphasis on commercial applications. Aquaculture 2019, 502, 26–31. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guidebook; The World Aquaculture Society: Baton Rouge, LA, USA, 2009; p. 182. [Google Scholar]
- Corrêa, R.O.; Sousa, A.R.B.; Junior, H.M. Criação de Tambaquis; Embrapa: Brasília, Brasil, 2018; ISBN 9788570358226; Available online: http://www.embrapa.br/amazonia-oriental/publica%C3%A7%C3%B5es (accessed on 10 February 2022).
- UNESCO. Chemical Methods for Use in Marine Environmental Monitoring; Pages 29–36 in Manual and guides 12; Intergovernmental Oceanographic Commission: Paris, France, 1983. [Google Scholar]
- Aminot, A.; Chaussepied, M. Manuel des Analyses Chimiques en Milieu Marin; CNEXO: Paris, France, 1983; p. 395. [Google Scholar]
- Monteiro, M.I.C.; Ferreira, F.N.; de Oliveira, N.M.M.; Ávila, A.K. Simplified version of the sodium salicylate method for analysis of nitrate in drinking water. Anal. Chim. Acta. 2003, 477, 125–129. [Google Scholar] [CrossRef]
- Eaton, A.D.; Cleserci, L.S.; Greenberg, A.E. Standard Methods for the Examination of Water and Waste Water, 10th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Gaona, C.A.P.; Serra, F.P.; Furtado, P.S.; Poersch, L.H.; Wasielesky, W. Biofloc management with different flow rates for solids removal in the Litopenaeus vannamei BFT culture system. Aquac. Int. 2016, 24, 1263–1275. [Google Scholar] [CrossRef]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W. Effect of calcium hydroxide, carbonate and sodium bicarbonate on water quality and zootechnical performance of shrimp Litopenaeus vannamei reared in bioflocs technology (BFT) systems. Aquaculture 2011, 321, 130–135. [Google Scholar] [CrossRef]
- Jomori, R.K.; Luz, R.K.; Takata, R.; Perez Fabregat, T.E.H.; Portella, M.C. Água Levemente Salinizada Aumenta a Eficiência Da Larvicultura De Peixes Neotropicais. Pesqui. Agropecu. Bras. 2013, 48, 809–815. [Google Scholar] [CrossRef]
- Fiúza, L.S.; Aragão, N.M.; Ribeiro Junior, H.P.; de Moraes, M.G.; Rocha, Í.R.C.B.; Lustosa Neto, A.D.; de Sousa, R.R.; Madrid, R.M.M.; de Oliveira, E.G.; Costa, F.H.F. Effects of salinity on the growth, survival, haematological parameters and osmoregulation of tambaqui Colossoma macropomum juveniles. Aquac. Res. 2015, 46, 1–9. [Google Scholar] [CrossRef]
- Goldenfarb, P.B.; Bowyer, F.P.; Hall, E.; Brosious, E. Reproducibility in the hematology laboratory: The microhematocrit determination. Am. J. Clin. Path. 1971, 56, 35–39. [Google Scholar] [CrossRef]
- Mattioli, C.C.; Takata, R.; Paes Leme, F.D.O.; Costa, D.C.; Melillo Filho, R.; de Souza e Silva, W.; Luz, R.K. The effects of acute and chronic exposure to water salinity on juveniles of the carnivorous freshwater catfish Lophiosilurus alexandri. Aquaculture 2017, 481, 255–266. [Google Scholar] [CrossRef]
- Brittelli, M.R.; Hans, H.; Che, C.; Muska, C.F. Induction of brachial (gill) neoplasm in the Medaka fish (Oryzias latipes) by V-methyl-AT-nitrito-A-nitrosoguanidine. Cancer Res. 1985, 45, 3209–3214. [Google Scholar]
- Araújo-Lima, C.; Goulding, M. So Fruitful a Fish: Ecology, Conservation and Aquaculture of the Amazon´s Tambaqui; Columbia University Press: New York, NY, USA, 1997; p. 191. [Google Scholar]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bioflocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Emerenciano, M.; Cuzon, G.; Paredes, A.; Gaxiola, G. Evaluation of biofloc technology in pink shrimp Farfantepenaeus duorarum culture: Growth performance, water quality, microorganisms’ profile and proximate analysis of biofloc. Aquac. Int. 2013, 21, 1381–1394. [Google Scholar] [CrossRef]
- Becerril-Cortés, D.; Monroy-Dosta, M.D.C.; Emerenciano, M.G.C.; Sofia, B.; Bermúdez, S.; Correa, G.V.; Castro-Mejía, G.; Bermúdez, S.; Avnimelech, B.S. Effect on nutritional composition of produced bioflocs with different carbon sources (Molasses, coffee waste and rice bran) in Biofloc system. Int. J. Fish. Aquat. Stud. 2018, 6, 541–547. [Google Scholar]
- FAO; Eurofish. A Guide to Recirculation Aquaculture; Rep. 100; FAO: Rome, Italy; Eurofish: Copenhagen, Denmark, 2015. [Google Scholar]
- Abakari, G.; Luo, G.; Kombat, E.O. Dynamics of nitrogenous compounds and their control in biofloc technology (BFT) systems: A review. Aquac. Fish. 2021, 6, 441–447. [Google Scholar] [CrossRef]
- Costa, O.T.F.; Dos Santos Ferreira, D.J.; Presti Mendonça, F.L.; Fernandes, M.N. Susceptibility of the Amazonian fish, Colossoma macropomum (Serrasalminae), to short-term exposure to nitrite. Aquaculture 2004, 232, 627–636. [Google Scholar] [CrossRef]
- Perrone, S.J.; Meade, T.J. Protective effects of chloride on nitrite toxicity to coho salmon (Oncorhynchus kisutch). J. Fish. Res. Boar. Can. 1977, 34, 486–492. [Google Scholar] [CrossRef]
- Williams, E.M.; Eddy, F.B. Chloride uptake in freshwater teleosts and its relationship to nitrite uptake and toxicity. J. Comp. Phys. B 1986, 156, 867–872. [Google Scholar] [CrossRef]
- Moraes, G.; Avilez, I.M.; Hori, T.S.F. Comparison between biochemical responses of the teleost pacu and its hybrid tambacu (Piaractus mesopotamicus × Colossoma macropomum) to short term nitrite exposure. Braz. J. Biol. 2006, 66, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, P.K.; Jensen, F.B. Recovery from nitrite-induced methaemoglobinaemia and potassium balance disturbances in carp. Fish Physiol. Biochem. 1997, 16, 1–10. [Google Scholar] [CrossRef]
- Avilez, I.M.; Altran, A.E.; Aguiar, L.H.; Moraes, G. Hematological responses of the Neotropical teleost matrinxã (Brycon cephalus) to environmental nitrite. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 135–139. [Google Scholar] [CrossRef]
- Ciji, A.; Sahu, N.P.; Pal, A.K.; Akhtar, M.S.; Tincy, V.; Mishal, P.; Das, P. Effect of dietary vitamin e and nitrite exposure on growth and metabolic variables of Labeo rohita juveniles. Natl. Acad. Sci. Lett. 2014, 37, 123–129. [Google Scholar] [CrossRef]
- Sgnaulin, T.; de Mello, G.L.; Thomas, M.C.; Garcia, J.R.E.; de Oca, G.A.R.M.; Emerenciano, M.G.C. Biofloc technology (BFT): An alternative aquaculture system for piracanjuba Brycon orbignyanus? Aquaculture 2018, 485, 119–123. [Google Scholar] [CrossRef]
- Araújo-Lima, C.A.R.M. Tambaqui (Colossoma macropomum). In Espécies Nativas Para Piscicultura no BRASIL, 2nd ed.; Baldisserotto, B., Gomes, L.D.C., Eds.; UFSM: Santa Maria, Brasil, 2010; pp. 175–204. [Google Scholar]
- Santos, F.A.C.; Boaventura, T.P.; da Costa Julio, G.S.; Cortezzi, P.P.; Figueiredo, L.G.; Favero, G.C.; Palheta, G.D.A.; de Melo, N.F.A.C.; Luz, R.K. Growth performance and physiological parameters of Colossoma macropomum in a recirculating aquaculture system (RAS): Importance of stocking density and classification. Aquaculture 2021, 534, 736274. [Google Scholar] [CrossRef]
- Silva, W.D.S.; Hisano, H.; Mattioli, C.C.; Torres, I.F.A.; Paes-Leme, F.D.O.; Luz, R.K. Effects of cyclical short-term fasting and refeeding on juvenile Lophiosilurus alexandri, a carnivorous neotropical catfish. Aquaculture 2019, 505, 12–17. [Google Scholar] [CrossRef]
- Frisso, R.M.; de Matos, F.T.; Moro, G.V.; de Mattos, B.O. Stocking density of Amazon fish (Colossoma macropomum) farmed in a continental neotropical reservoir with a net cages system. Aquaculture 2020, 529, 735702. [Google Scholar] [CrossRef]
- Xu, W.J.; Pan, L.Q.; Zhao, D.H.; Huang, J. Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture 2012, 350–253, 147–153. [Google Scholar] [CrossRef]
- Ekasari, J.; Rivandi, D.R.; Firdausi, A.P.; Surawidjaja, E.H.; Zairin, M.; Bossier, P.; De Schryver, P. Biofloc technology positively affects Nile tilapia (Oreochromis niloticus) larvae performance. Aquaculture 2015, 441, 72–77. [Google Scholar] [CrossRef]
- Bossier, P.; Ekasari, J. Biofloc technology application in aquaculture to support sustainable development goals. Microb. Biotechnol. 2017, 10, 1012–1016. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Q.; Chen, Y.; Zhang, Y.; Zhou, C. Effects of dietary carbohydrate types on growth performance, innate immunity, antioxidant ability and glucose metabolism of brook trout Salvelinus fontinalis. Aquac. Res. 2020, 51, 4638–4648. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).