Ameliorative Sexual Behavior and Phosphodiesterase-5 Inhibitory Effects of Spondias mangifera Fruit Extract in Rodents: In Silico, In Vitro, and In Vivo Study
Abstract
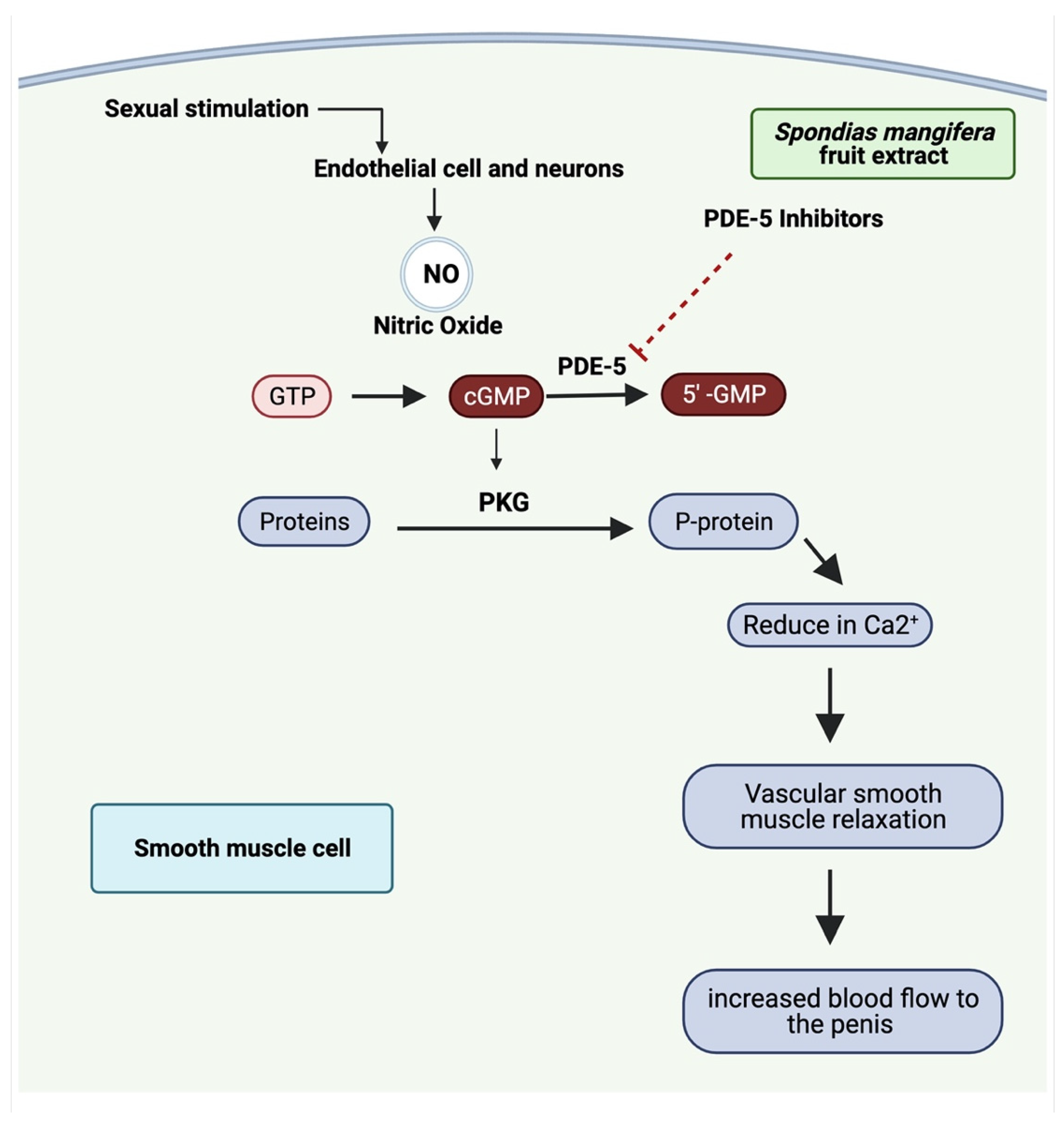
:1. Introduction
2. Materials and Methods
2.1. Plant Authentication
2.2. Chemicals
2.3. Molecular Docking
2.4. Molecular Dynamics (MD) Simulation
2.5. Preparation of Extracts
2.6. Animals
2.6.1. Experimental Protocol
2.6.2. Preparation of Male Animals
2.6.3. Preparation of Female Animals
2.7. Study Protocol for Male Sexual Behaviors
2.8. Determination of Phosphodiesterase-5 (PDE-5)
2.9. Testosterone Serum Preparation
2.10. Testosterone Analysis
2.11. Measurement of Nitric Oxide Level
3. Results
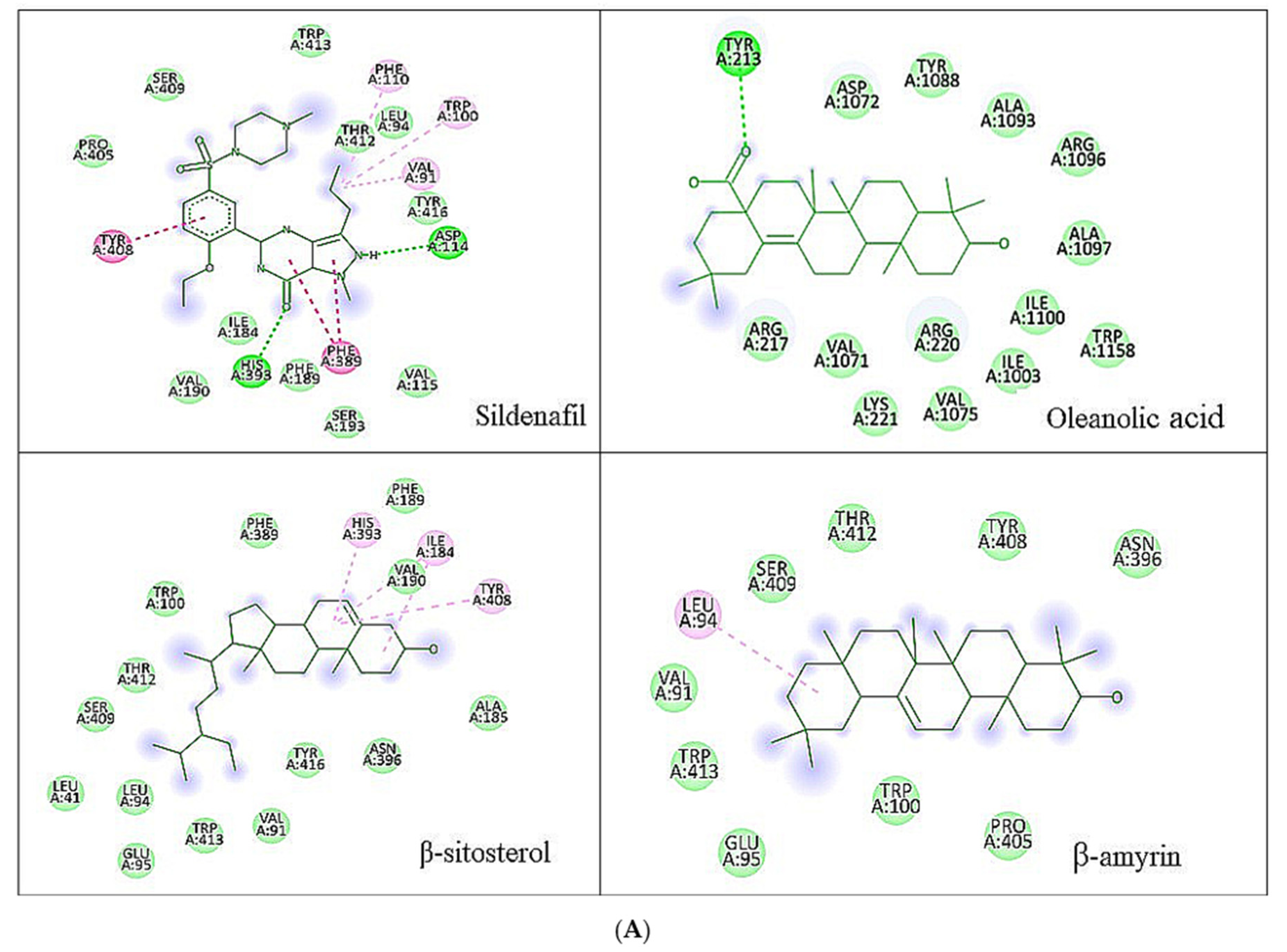
3.1. Molecular Docking Analysis
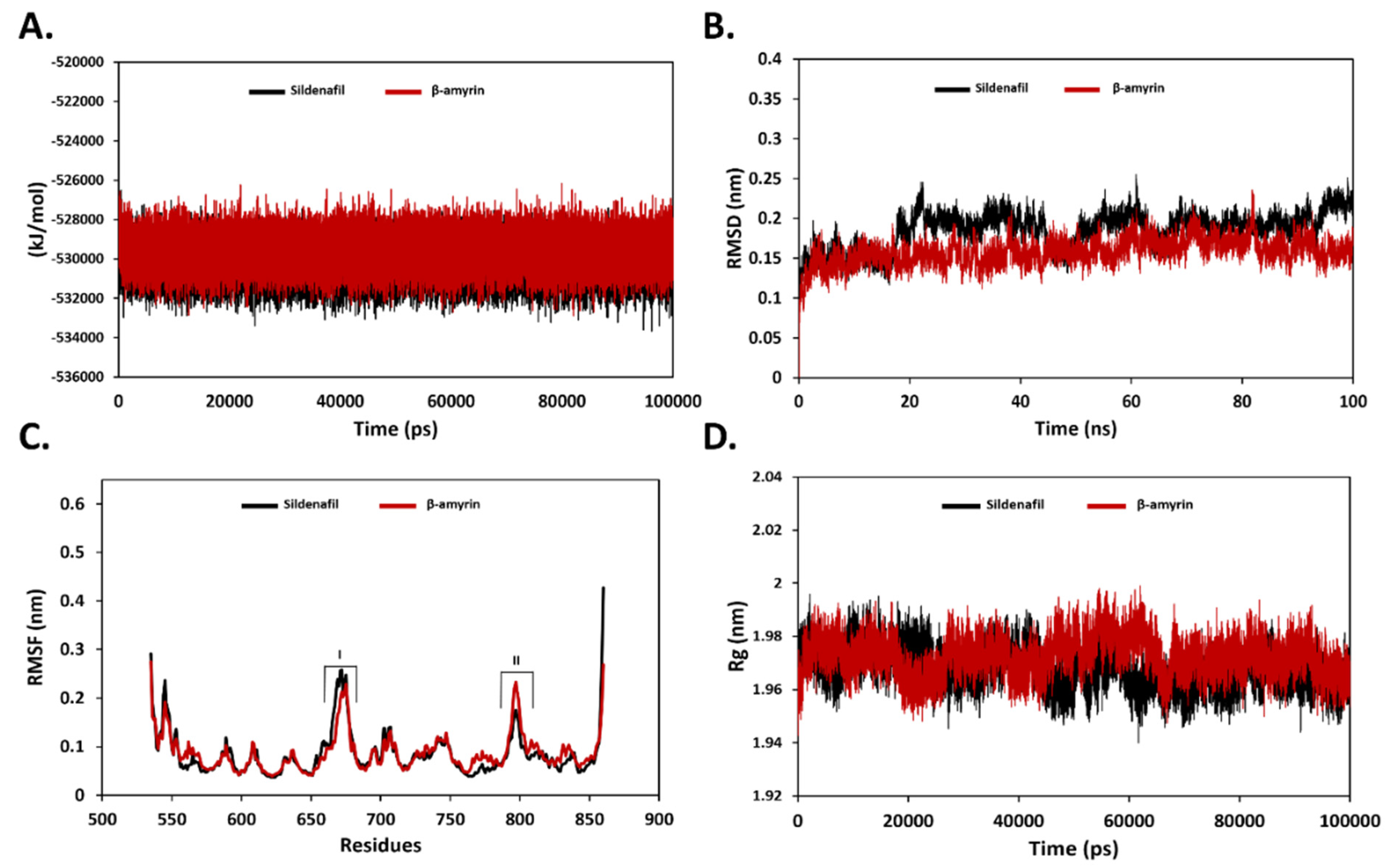
3.2. Binding Stability Analysis Using MD Simulation
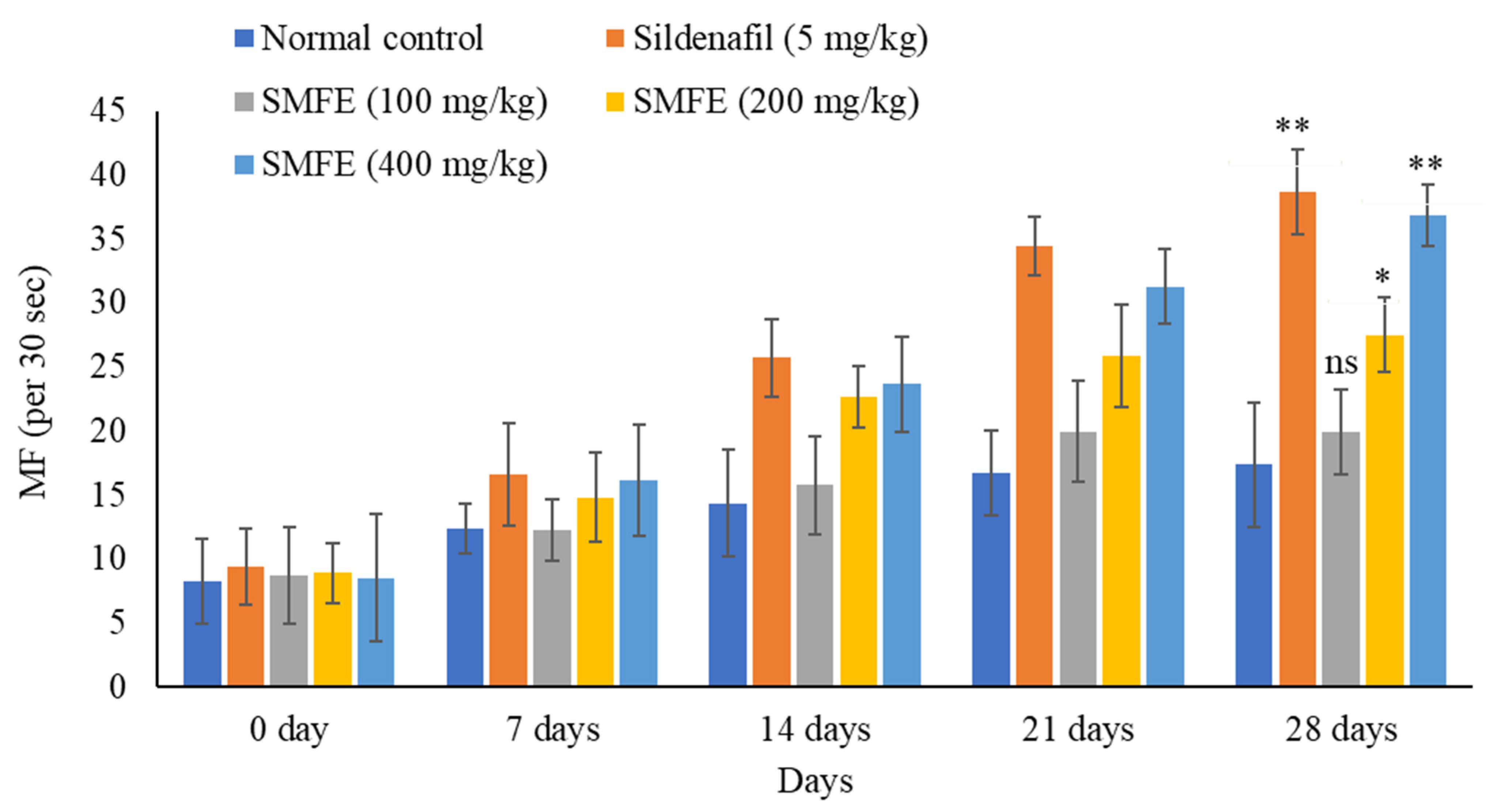
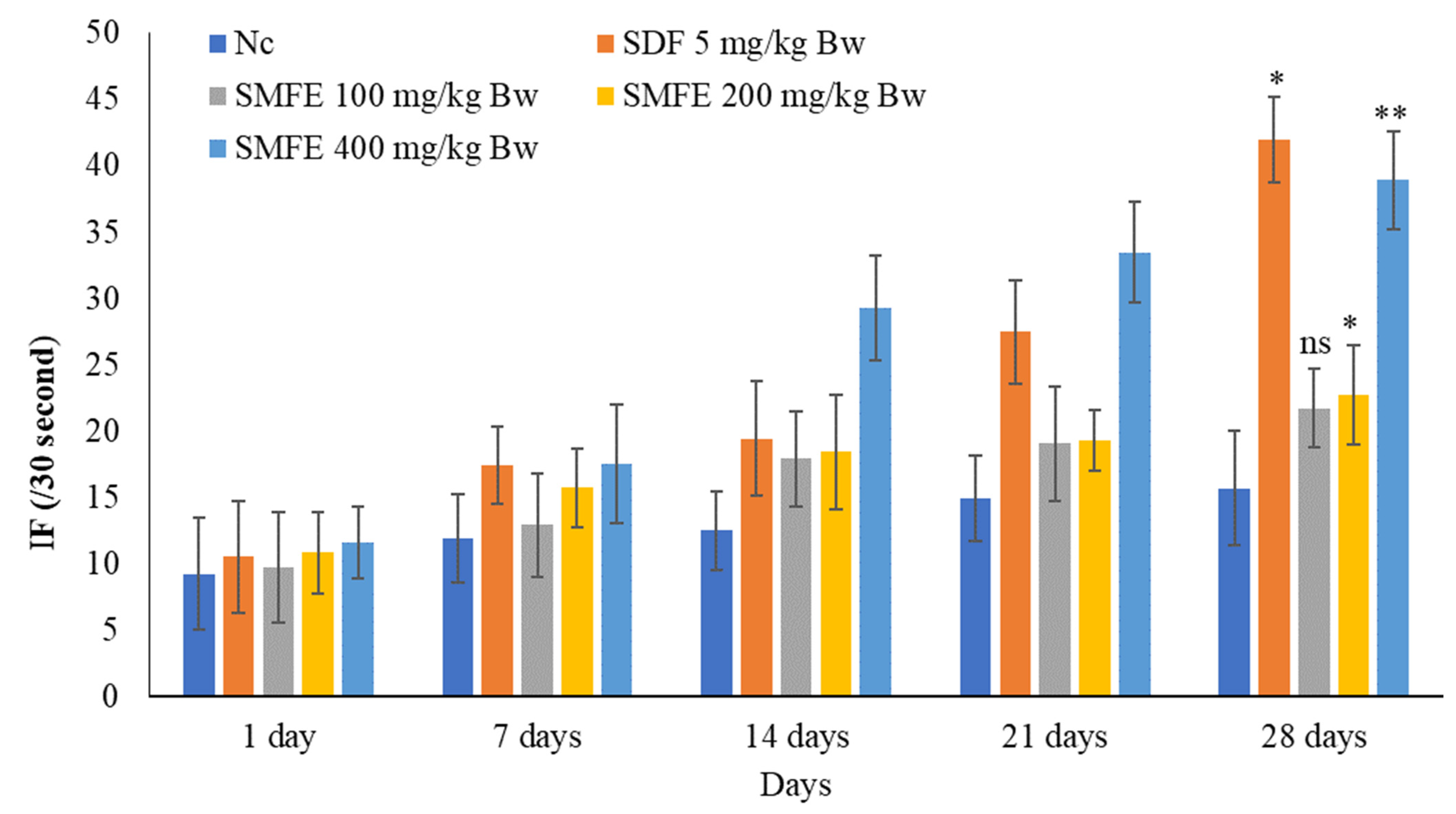
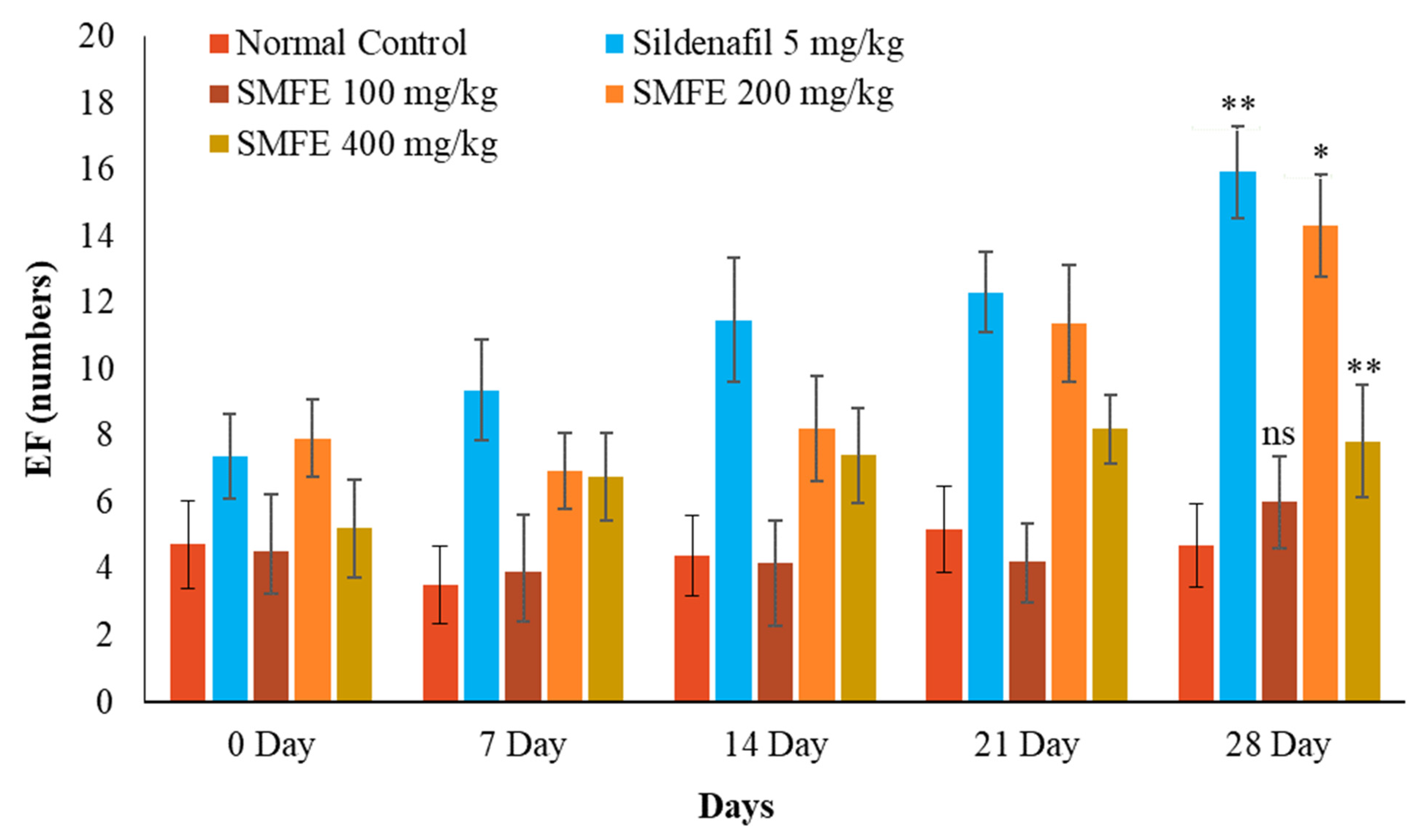
3.3. Effect of S. mangifera Ethanolic Extract on the Mount, Intermission, and Ejaculatory Frequencies
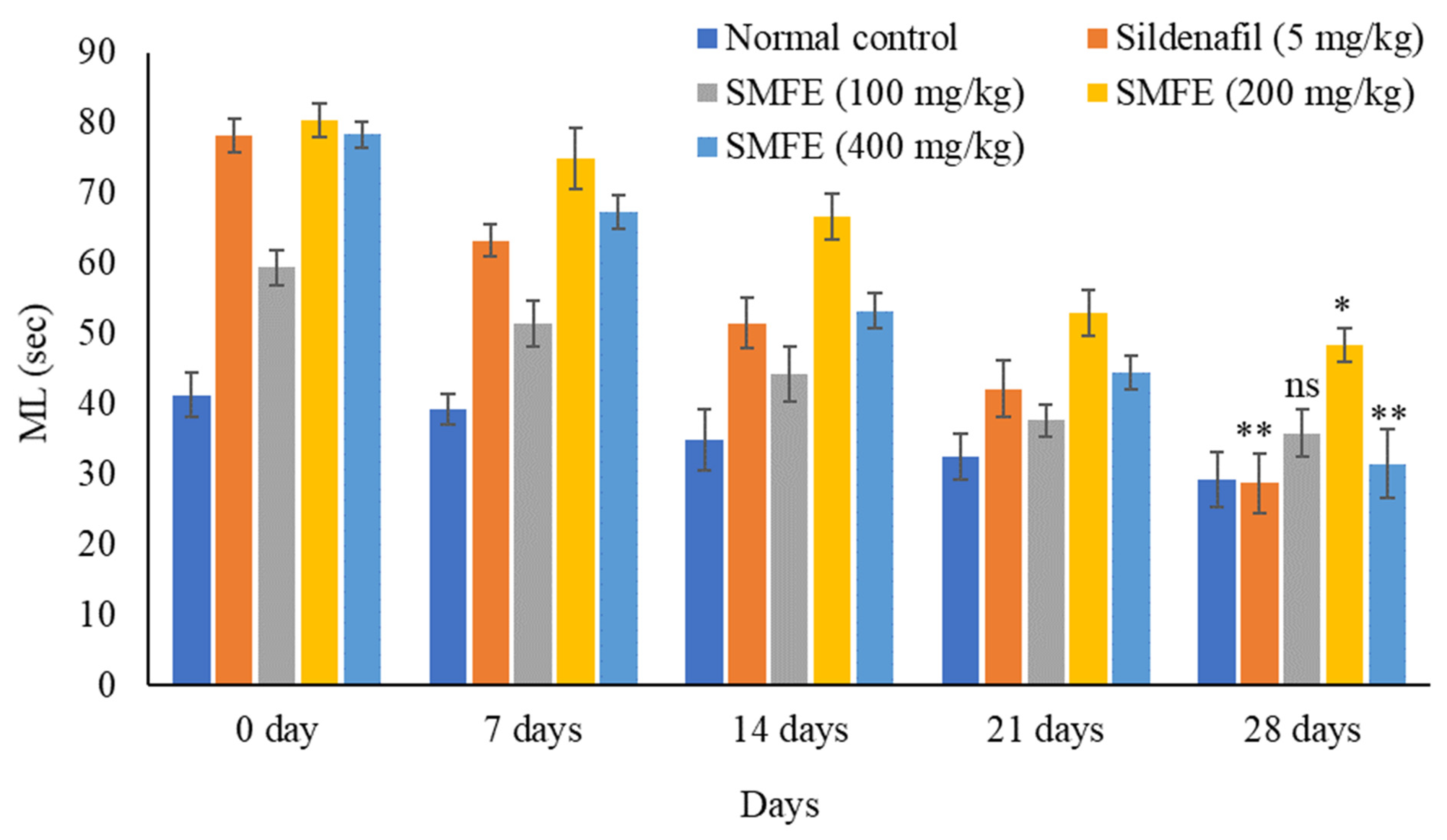
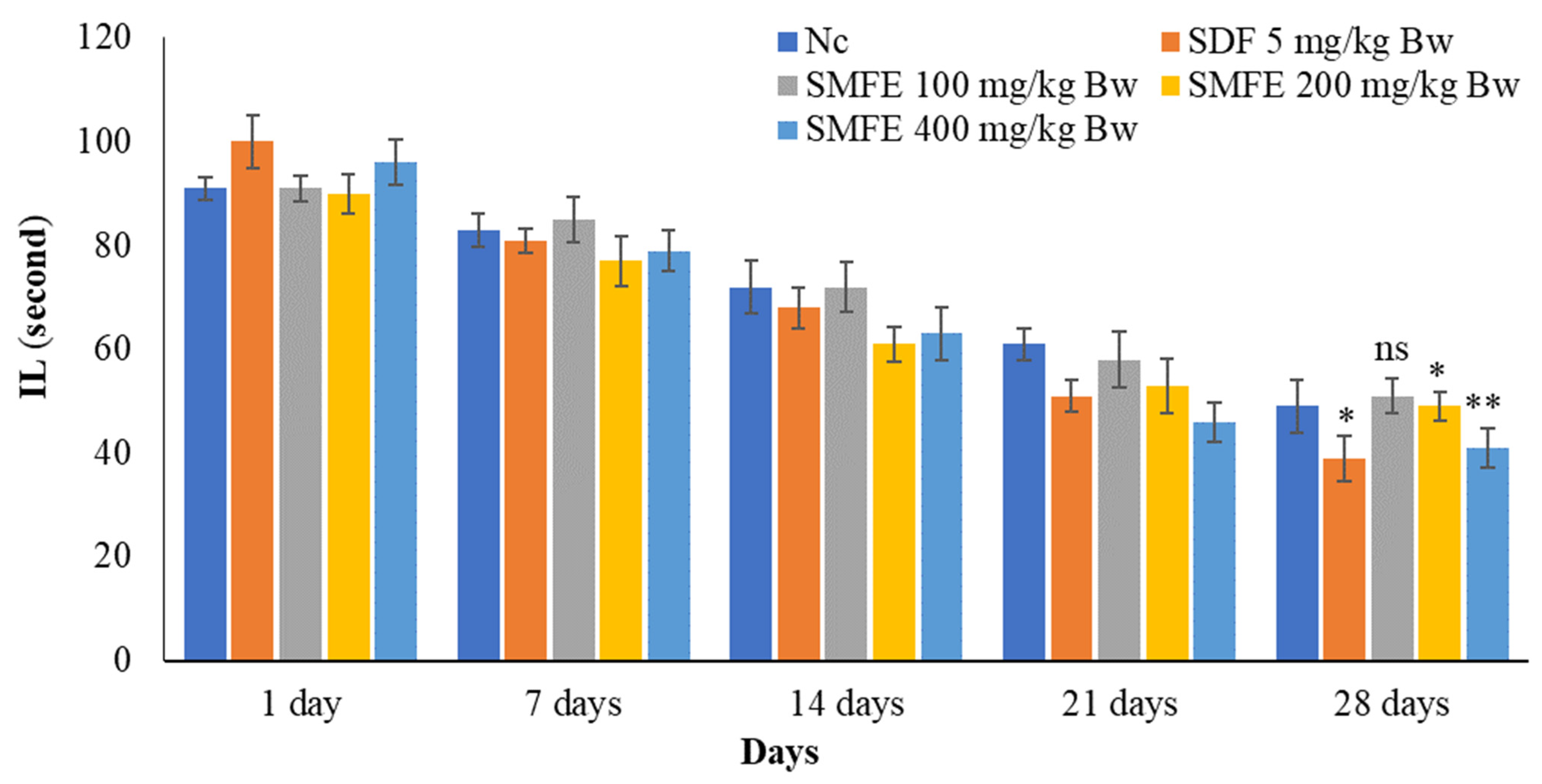
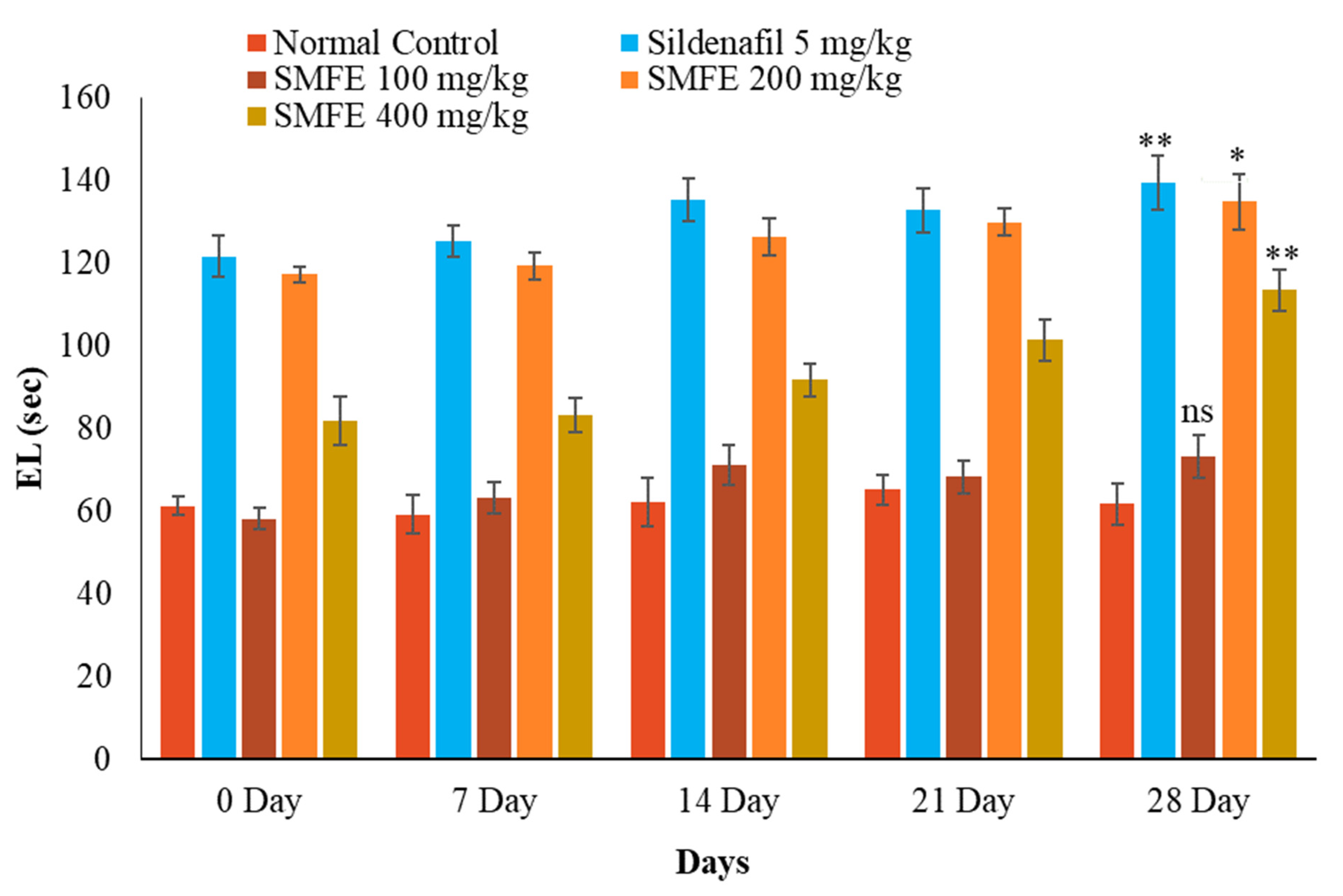
3.4. Effect of S. mangifera Ethanolic Extract on the Mount, Intermission, and Ejaculatory Latency
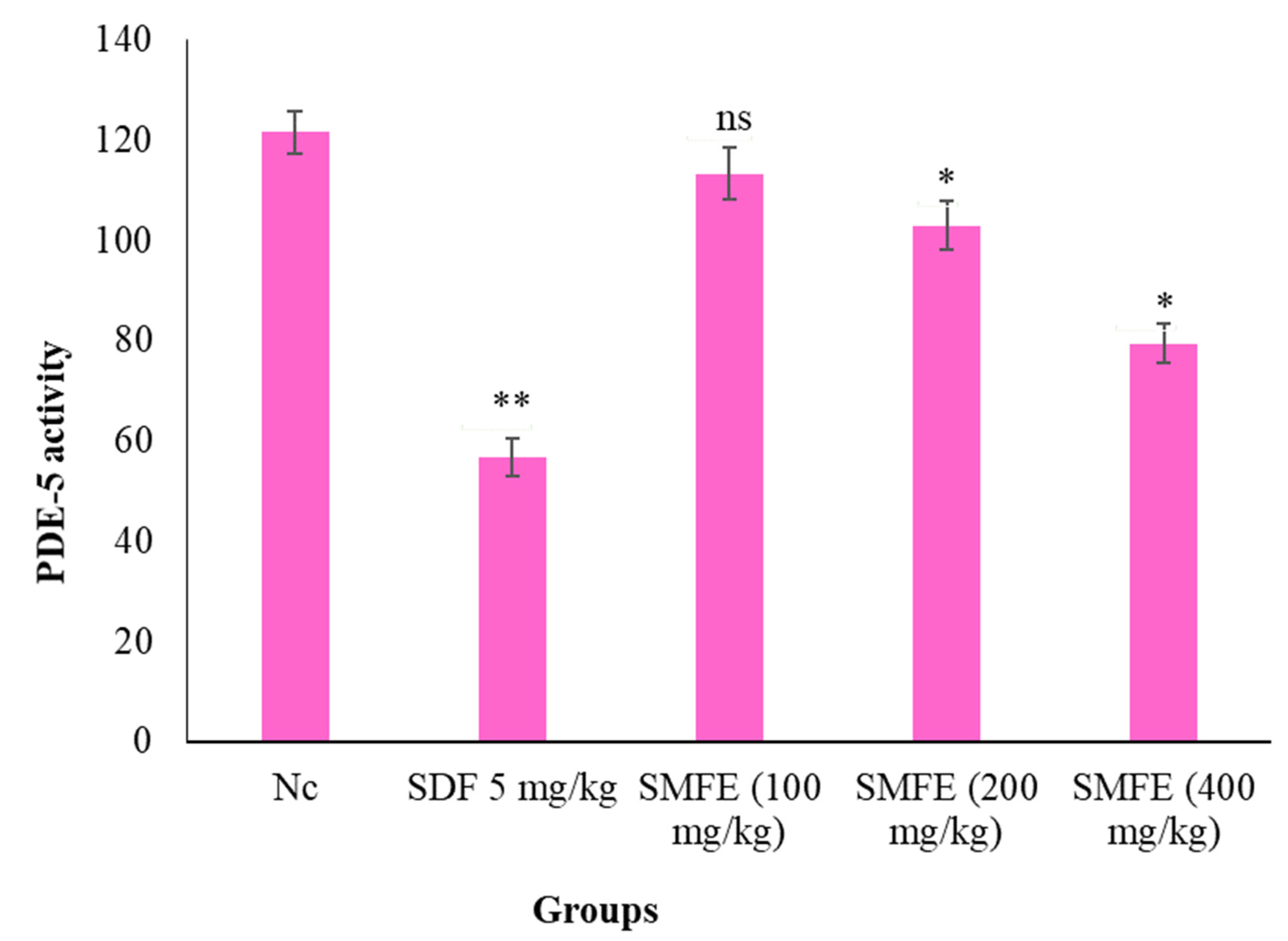
3.5. PDE-5 Activity
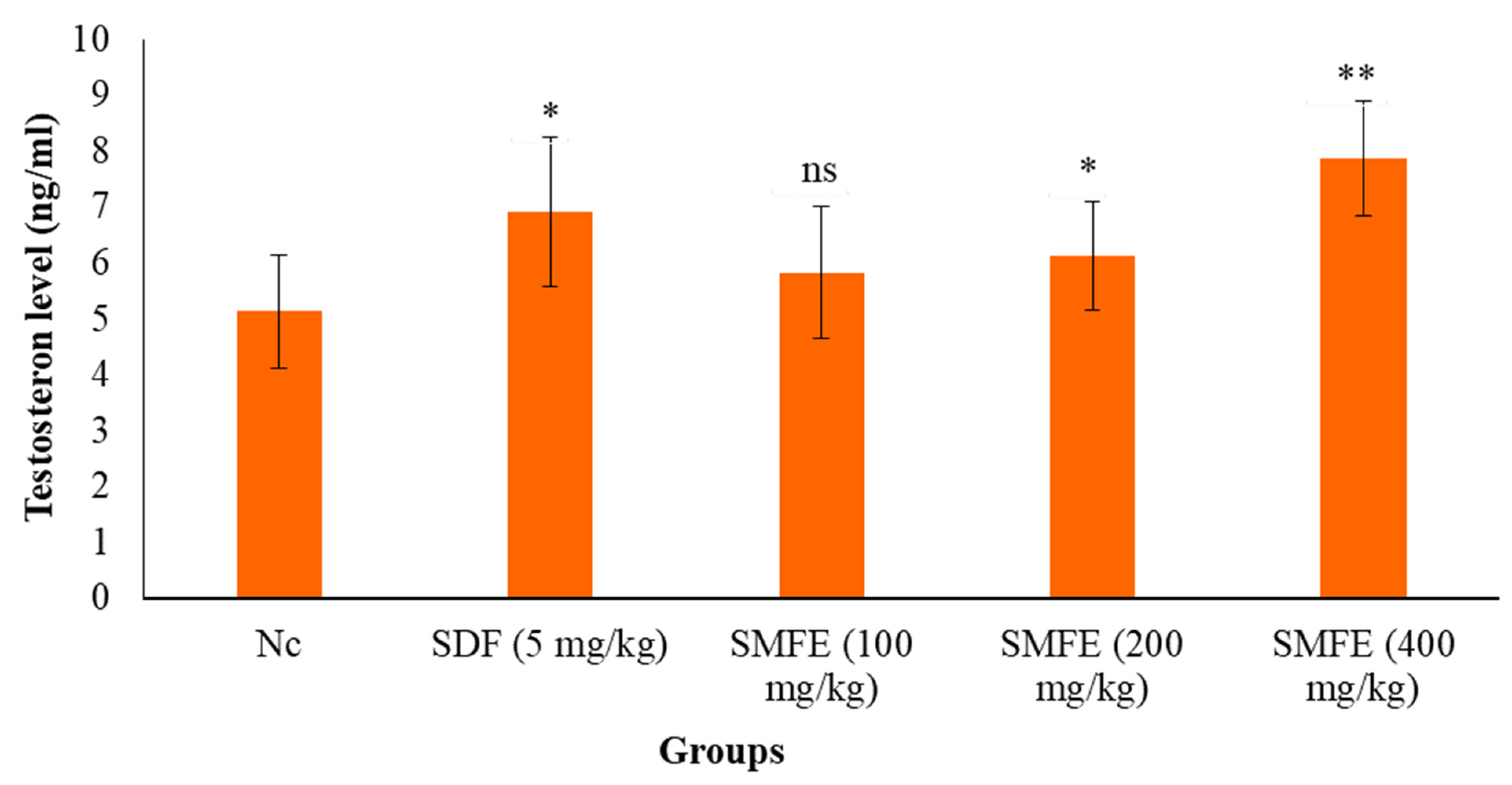
3.6. Testosterone Level
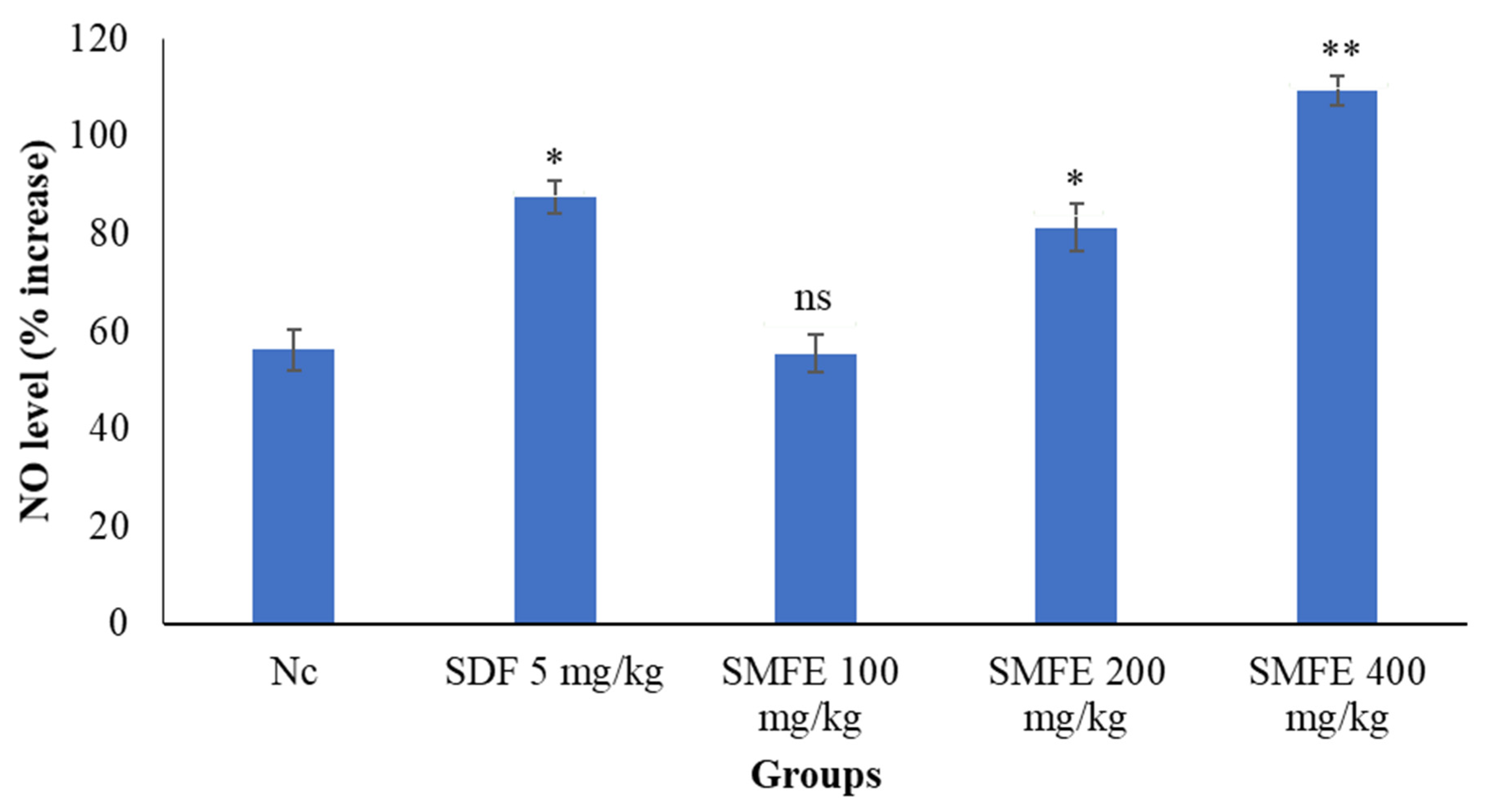
3.7. Nitric Oxide Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koloko, B.L.; Bushra, I.; Wankeu-Nya, M.; Ngaha Njila, M.I.; Kenmogne, H.; Nyonseu Nzeubang, D.C.; Nzangueu, C.B.; Dimo, T.; Dongmo, A.B.; Massoma Lembe, D. In Vivo Effects of Rauvolfia Vomitoria (Apocynaceae) Ethanolic Extract on Sexual Performance and Reproductive Activity in Male Rats. Andrologia 2020, 52, e13414. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.A.; Wahab, S.; Abullais, S.S.; Das, G.; Hani, U.; Ahmad, W.; Amir, M.; Ahmad, A.; Kandasamy, G.; Vasudevan, R. Pharmacological Efficacy of Tamarix Aphylla: A Comprehensive Review. Plants 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Anand Ganapathy, A.; Hari Priya, V.M.; Kumaran, A. Medicinal Plants as a Potential Source of Phosphodiesterase-5 Inhibitors: A Review. J. Ethnopharmacol. 2021, 267, 113536. [Google Scholar] [CrossRef] [PubMed]
- Aytaç, I.A.; McKinlay, J.B.; Krane, R.J. The Likely Worldwide Increase in Erectile Dysfunction between 1995 and 2025 and Some Possible Policy Consequences. BJU Int. 1999, 84, 50–56. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ojo, A.B.; Ajiboye, B.; Fadaka, A.; Imiere, O.D.; Adeyonu, O.; Olayide, I. Protective Influence of Ficus Asperifolia Miq Leaf Extract on Carbon Tetrachloride (CCL4)-Induced Testicular Toxicity in Rat’s Testes. J. Appl. Pharm. Sci. 2016, 6, 37–41. [Google Scholar] [CrossRef]
- Malviya, N.; Malviya, S.; Jain, S.; Vyas, S. A Review of the Potential of Medicinal Plants in the Management and Treatment of Male Sexual Dysfunction. Andrologia 2016, 48, 880–893. [Google Scholar] [CrossRef]
- Drewes, S.E.; George, J.; Khan, F. Recent Findings on Natural Products with Erectile-Dysfunction Activity. Phytochemistry 2003, 62, 1019–1025. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Afolayan, A.J. Effect of Aqueous Extract of Bulbine Natalensis (Baker) Stem on the Sexual Behaviour of Male Rats. Int. J. Androl. 2009, 32, 629–636. [Google Scholar] [CrossRef]
- Alsayari, A.; Wahab, S. Genus Ziziphus for the Treatment of Chronic Inflammatory Diseases. Saudi J. Biol. Sci. 2021, 28, 6897–6914. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis- Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza Glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, M.T.; Akanji, M.A. Effect of Aqueous Extract of Massularia Acuminata Stem on Sexual Behaviour of Male Wistar Rats. Evid. Based Complement. Altern. Med. 2011, 2011, 738103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.N.; Liao, C.H.; Chen, K.C.; Liu, S.P.; Chiang, H.S. Effect of Ginkgo Biloba Extract (EGb-761) on Recovery of Erectile Dysfunction in Bilateral Cavernous Nerve Injury Rat Model. Urology 2015, 85, 1214.e7–1214.e15. [Google Scholar] [CrossRef]
- Kazmi, I.; Afzal, M.; Rahman, M.; Gupta, G.; Anwar, F. Aphrodisiac Properties of Polygonatum Verticillatum Leaf Extract. Asian Pac. J. Trop. Dis. 2012, 2, S841–S845. [Google Scholar] [CrossRef]
- Guohua, H.; Yanhua, L.; Rengang, M.; Dongzhi, W.; Zhengzhi, M.; Hua, Z. Aphrodisiac Properties of Allium Tuberosum Seeds Extract. J. Ethnopharmacol. 2009, 122, 579–582. [Google Scholar] [CrossRef]
- Kenmogne, H.; Koloko, B.; Hambe, C.; Domkam, J.; Ngaha Njila, M.; Bend, E.; Oundoum Oundoum, P.; Massoma Lembè, D.; Dimo, T. Effects of Aqueous Root Extract of Carpolobia Alba G. Don on Sexual Behaviour in Adult Male Rats. Andrologia 2016, 48, 908–914. [Google Scholar] [CrossRef]
- Kpomah, E.D.; Uwakwe, A.A.; Abbey, B.W. Aphrodisiac Studies of Diherbal Mixture of Zanthoxylum Leprieurii Guill & Perr. and Piper Guineense Schumach & Thonn. on Male Wistar Rats. Glob. J. Res. Med. Plants Indig. Med. 2012, 1, 381–390. [Google Scholar]
- Bansode, F.W.; Rajendran, S.M.; Singh, R.K. Dose-Dependent Effects of Ethanol Extract of Salvia Haematodes Wall Roots on Reproductive Function and Copulatory Behaviour in Male Rats. Andrologia 2015, 47, 266–275. [Google Scholar] [CrossRef]
- Alberto, A.V.P.; da Silva Ferreira, N.C.; Soares, R.F.; Alves, L.A. Molecular Modeling Applied to the Discovery of New Lead Compounds for P2 Receptors Based on Natural Sources. Front. Pharmacol. 2020, 11, 01221. [Google Scholar] [CrossRef]
- Home|Environment & Forest|Government of Assam, India. Available online: https://environmentandforest.assam.gov.in/ (accessed on 31 May 2022).
- Kandali, R.; Kumar, B.K. Evaluation of Nutraceutical Potentiality of a Minor Fruit of Assam-Spondias Pinnata Kurz. In The Souvenir cum Abstract of “Vale Addition of Bio-Resources of NE India, Post-Harvest Technology and Cold Chain”; Department of Botany, Guwahati University: Guwahati, India, 2006. [Google Scholar]
- Arif, M.; Rahman, M.A.; Imran, M.; Khalid, M.; Khushtar, M. An Insight of Spondias Mangifera Willd: An Underutilized Medicinal Plant with Immense Nutraceutical and Therapeutic Potentials. Int. J. Res. Pharm. Sci. 2015, 6, 100–109. [Google Scholar]
- Kritikar, K.R.; Basu, B.D. Indian Medicinal Plants, M/S Bishen Singh, Mahendra Pal Singh. Int. B Distrib. Dehradune India 1975, 1, 672–675. [Google Scholar]
- Barua, U.; Hore, D.K.; Sarma, R. Wild Edible Plants of Majuli Island and Darrang Districts of Assam. Indian J. Tradit. Knowl. 2007, 6, 191–194. [Google Scholar]
- Rastogi, R.P.; Mehrotra, B.N. Compendium of Indian Medicinal Plants, Volume VI; Central Drug Research Institute and Publications & Information Directorate: Tamil Nadu, India, 1995; Volume 4, ISBN 9788185042053. [Google Scholar]
- CSIR’s The Wealth of India Encyclopaedia|Council of Scientific & Industrial Research|CSIR|GoI. Available online: https://www.csir.res.in/csir’s-wealth-india-encyclopaedia (accessed on 31 May 2022).
- Rao, B.G.; Raju, N.J. Investigation of hepatoprotective activity of spondias pinnata. Int. J. Pharma Sci. Res. 2010, 1, 193–198. [Google Scholar]
- Khalid, M.; Alqarni, M.H.; Shoaib, A.; Arif, M.; Foudah, A.I.; Afzal, O.; Ali, A.; Ali, A.; Alqahtani, S.S.; Altamimi, A.S.A. Anti-Arthritic and Anti-Inflammatory Potential of Spondias Mangifera Extract Fractions: An In Silico, In Vitro and In Vivo Approach. Plants 2021, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Colalto, C.; Delfino, D.V.; Iriti, M.; Izzo, A.A. Herbal Dietary Supplements for Erectile Dysfunction: A Systematic Review and Meta-Analysis. Drugs 2018, 78, 643–673. [Google Scholar] [CrossRef]
- Khalid, M.; Alqarni, M.H.; Alsayari, A.; Foudah, A.I.; Aljarba, T.M.; Mukim, M.; Alamri, M.A.; Abullais, S.S.; Wahab, S. Anti-Diabetic Activity of Bioactive Compound Extracted from Spondias Mangifera Fruit: In-Vitro and Molecular Docking Approaches. Plants 2022, 11, 562. [Google Scholar] [CrossRef]
- Rauf, A.; Khan, I.A.; Muhammad, N.; Al-Awthan, Y.S.; Bahattab, O.; Israr, M.; Mubarak, M.S. Phytochemical Composition, in Vitro Urease, α-Glucosidase and Phosphodiesterase Inhibatroy Potency of Syzygium Cumini (Jamun) Fruits. S. Afr. J. Bot. 2021, 143, 418–421. [Google Scholar] [CrossRef]
- Balhara, Y.P.S.; Sarkar, S.; Gupta, R. Phosphodiesterase-5 Inhibitors for Erectile Dysfunction in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Indian J. Endocrinol. Metab. 2015, 19, 451–461. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Huai, Q.; Cai, J.; Zoraghi, R.; Francis, S.H.; Corbin, J.D.; Robinson, H.; Xin, Z.; Lin, G.; et al. Multiple Conformations of Phosphodiesterase-5: Implications for Enzyme Function and Drug Development. J. Biol. Chem. 2006, 281, 21469–21479. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam—Topology and Parameters for Small Organic Molecules. J. Comput. Chem. 2012, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Siddiqui, H.H. Evaluation of Weight Reduction and Anti-Cholesterol Activity of Punarnava Root Extract against High Fat Diets Induced Obesity in Experimental Rodent. Asian Pac. J. Trop. Biomed. 2012, 2, S1323–S1328. [Google Scholar] [CrossRef]
- Warange, P.V.; Saravanan, J.; Praveen, T.K.; Rymbai, E.; Deepa, S. Evaluation of Aphrodisiac Activity of Allium Sativum in Male Rats. Int. J. Sci. Technol. Res. 2019, 8, 1683–1686. [Google Scholar]
- Singh, R.; Ali, A.; Jeyabalan, G.; Semwal, A. Jaikishan An Overview of the Current Methodologies Used for Evaluation of Aphrodisiac Agents. J. Acute Dis. 2013, 2, 85–91. [Google Scholar] [CrossRef]
- Abedi, A.; Parviz, M.; Karimian, S.M.; Rodsari, H.R.S.; Abedi, A.; Parviz, M.; Karimian, S.M.; Rodsari, H.R.S. Aphrodisiac Activity of Aqueous Extract of Phoenix Dactylifera Pollen in Male Rats. Adv. Sex. Med. 2013, 3, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Sharmila; Prabsattroo, T.; Wattanathorn, J.; Iamsa-Ard, S.; Muchimapura, S.; Thukhammee, W. Moringa Oleifera Leaves Extract Attenuates Male Sexual Dysfunction. Am. J. Neurosci. 2012, 3, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Padashetty, S.A.; Mishra, S.H. Aphrodisiac Studies of Tricholepis Glaberrima with Supportive Action from Antioxidant Enzymes. Pharm. Biol. 2007, 45, 580–586. [Google Scholar] [CrossRef] [Green Version]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide Biol. Chem. 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Akanji, M.A.; Oladiji, A.T. Aphrodisiac Potentials of the Aqueous Extract of Fadogia Agrestis (Schweinf. Ex Hiern) Stem in Male Albino Rats. Asian J. Androl. 2005, 7, 399–404. [Google Scholar] [CrossRef]
- Kumbhar, N.; Nimal, S.; Barale, S.; Kamble, S.; Bavi, R.; Sonawane, K.; Gacche, R. Identification of Novel Leads as Potent Inhibitors of HDAC3 Using Ligand-Based Pharmacophore Modeling and MD Simulation. Sci. Rep. 2022, 12, 1712. [Google Scholar] [CrossRef]
- Peluso, M.R. Flavonoids Attenuate Cardiovascular Disease, Inhibit Phosphodiesterase, and Modulate Lipid Homeostasis in Adipose Tissue and Liver. Exp. Biol. Med. 2006, 231, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Scepkowski, L.A. The Biologic Basis for Libido. Curr. Sex. Health Rep. 2005, 2, 95–100. [Google Scholar] [CrossRef]
- Schultz, W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol. Rev. 2015, 95, 853–951. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Schmeichel, B.E.; España, R.A. Noradrenergic Modulation of Wakefulness/Arousal. Sleep Med. Rev. 2012, 16, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njila, M.I.N.; Meng, G.Y.; Ebrahimi, M.; Awad, E.A.; Baiee, F.H.; Kenmogne, H.; Koloko, B.L.; Hambe, M.; Mandenguè, S.H.; Lembè, D.M. Effect of Methanolic Extract of Alchornea Cordifolia Leaves on the Sexual Behavior of Senescent and Sexually Inexperienced Rats. J. Phytopharm. 2018, 7, 471–476. [Google Scholar] [CrossRef]
- Bahmanpour, S.; Talaei, T.; Vojdani, Z.; Panjehshahin, M.R.; Poostpasand, A.; Zareei, S.; Ghaeminia, M. Effect of Phoenix Dactylifera Pollen on Sperm Parameters and Reproductive System of Adult Male Rats. Iran. J. Med. Sci. 2006, 31, 208–212. [Google Scholar]
- Cao, L.; Leers-Sucheta, S.; Azhar, S. Aging Alters the Functional Expression of Enzymatic and Non-Enzymatic Anti-Oxidant Defense Systems in Testicular Rat Leydig Cells. J. Steroid Biochem. Mol. Biol. 2004, 88, 61–67. [Google Scholar] [CrossRef]
- da Silva, C.V.; Borges, F.M.; Velozo, E.S. Phytochemistry of Some Brazilian Plants with Aphrodisiac Activity. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; IntechOpen: London, UK, 2012; ISBN 978-953-51-0296-0. [Google Scholar]
- Fouche, G.; Afolayan, A.J.; Wintola, O.A.; Khorombi, T.E.; Senabe, J. Effect of the Aqueous Extract of the Aerial Parts of Monsonia Angustifolia E. Mey. Ex A. Rich., on the Sexual Behaviour of Male Wistar Rats. BMC Complement. Altern. Med. 2015, 15, 343. [Google Scholar] [CrossRef] [Green Version]
- Ågmo, A. Male Rat Sexual Behavior. Brain Res. Protoc. 1997, 1, 203–209. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Akanji, M.A.; Oladiji, A.T.; Adesokan, A.A. Androgenic Potentials of Aqueous Extract of Massularia Acuminata (G. Don) Bullock Ex Hoyl. Stem in Male Wistar Rats. J. Ethnopharmacol. 2008, 118, 508–513. [Google Scholar] [CrossRef]
- Dasofunjo, K.; Asuk, A.A.; Ezugwu, H.C.; Nwodo, O.F.C.; Olatunji, T.L. Aphrodisiac Effect of Ethanol Extract of Piliostigma Thonningii Leaf on Male Albino Wistar Rats. J. Appl. Pharm. Sci. 2013, 3, 130–135. [Google Scholar] [CrossRef]
- Erhabor, J.O.; Idu, M.D. Aphrodisiac Potentials of the Ethanol Extract of Aloe Barbadensis Mill. Root in Male Wistar Rats. BMC Complement. Altern. Med. 2017, 17, 360. [Google Scholar] [CrossRef] [PubMed]
- Swaney, W.T.; Dubose, B.N.; Curley, J.P.; Champagne, F.A. Sexual Experience Affects Reproductive Behavior and Preoptic Androgen Receptors in Male Mice. Horm. Behav. 2012, 61, 472–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.H.; Morris, G.Z.; Corbin, J.D. Molecular Mechanisms That Could Contribute to Prolonged Effectiveness of PDE5 Inhibitors to Improve Erectile Function. Int. J. Impot. Res. 2008, 20, 333–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ademosun, A.O.; Adebayo, A.A.; Oboh, G. Anogeissus Leiocarpus Attenuates Paroxetine-Induced Erectile Dysfunction in Male Rats via Enhanced Sexual Behavior, Nitric Oxide Level and Antioxidant Status. Biomed. Pharmacother. 2019, 111, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Lugnier, C. Cyclic Nucleotide Phosphodiesterase (PDE) Superfamily: A New Target for the Development of Specific Therapeutic Agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef]
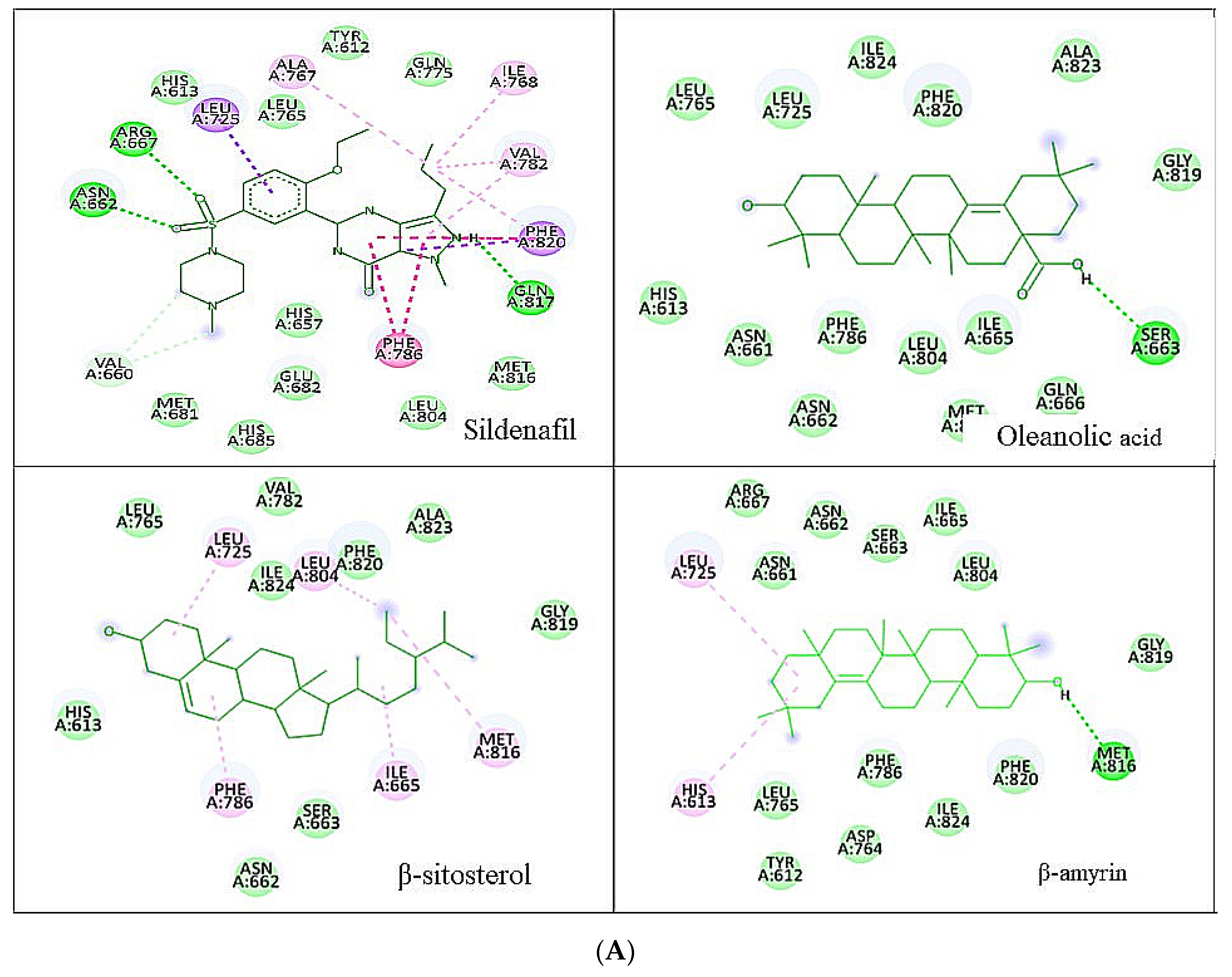


| Ligands | Chemical Structure | Binding Energy Score (kcal/mol) | H-Bond Interaction | Hydrophobic Interaction |
|---|---|---|---|---|
| Sildenafil |  | −10.01 | ARG A: 667, ASN A: 662, GLN A: 817 | HIS A: 613, LEU A: 725, LEU A: 765, ALN A: 765, TYR A: 612, GLN A: 725, ILE A: 768, VAL A: 782, PHE A: 820, PHE A: 786, MET A: 816, LEU A: 805, HIS A: 657, GLU A: 682, HIS A: 685, MET A: 681, VAL A: 660 |
| Oleanolic acid | 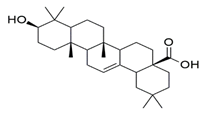 | −10.51 | SER A: 663 | LEU A: 765, LEU A: 725, ILE A: 824, PHE A: 820, ALA A: 823, GLY A: 819, GLN A: 666, MET A: 816, ASN A: 662, ILE A: 665, LEU A: 804, PHE A: 786, ASN A: 661, HIS A: 613 |
| β-sitosterol | 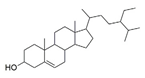 | −9.90 | -- | LEU A: 765, LEU A: 725, VAL A: 782, ILE A: 824, LEU A: 804, PHE A: 820 ALA A: 823, GLY A: 819, MET A: 816, ILE A: 665, SER A: 663, ASN A: 662, PHE A: 786, HIS A: 613 |
| β-amyrin | 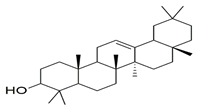 | −11.61 | MEH A: 816 | ARG A: 667, ASN A: 661, ASN A: 662, SER A: 663, ILE A: 665, LEU A: 804, GLY A: 819, PHE A: 820, ILE A: 824, PHE A: 786, LEU A: 765, TYR A: 612, ASP A: 764, HIS A: 613, LEU A: 725 |
| Ligands | Chemical Structure | Binding Energy Score (kcal/mol) | H-Bond Interaction | Hydrophobic Interaction |
|---|---|---|---|---|
| Sildenafil |  | −9.2 | ASP A: 114 HIS A: 393 | PRO A: 405, SERA: 409, TRPA: 413, PHEA: 110, LEUA: 94, TRPA: 100, THRA: 412, VAL A: 91, TYR A: 416, VAL A: 115, SER A: 193, PHE A: 389, PHE A: 189, VAL A: 190, ILE A: 184, TYR A: 408 |
| Oleanolic acid |  | −8.6 | TYR A: 213 | ASP A: 1072, TYR A: 1088, ALA A: 1093, ARG A: 1096, ALA A: 1097, ILE A: 1100, TRP A: 1158, ARG A: 220, ILE A: 1003, VAL A: 1075, LYS A: 221, VAL A: 1071, ARG A: 217 |
| β-Sitosterol |  | −8.9 | -- | VAL A: 91, TRP A: 413, GLU A: 95, LEU A: 94, LEU A: 41, SER A: 409, THR A: 412, TRP A: 100, PHE A: 489, HISA: 393, PHE A: 189, ILE A: 184, VAL A: 190, TYR A: 208, ALA A: 185, ASN A: 396, TYRA: 416, |
| β-Amyrin |  | −8.6 | TRP A: 413, VAL A: 91, LEU A: 94, SER A: 409, THR A: 412, TYR A: 402, ASN A: 396, PRO A: 405, TRP A: 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.; Alqarni, M.H.; Wahab, S.; Annadurai, S.; Alamri, M.A.; Foudah, A.I.; Aljarba, T.M.; Akhtar, J.; Badruddeen; Ahmad, S. Ameliorative Sexual Behavior and Phosphodiesterase-5 Inhibitory Effects of Spondias mangifera Fruit Extract in Rodents: In Silico, In Vitro, and In Vivo Study. J. Clin. Med. 2022, 11, 3732. https://doi.org/10.3390/jcm11133732
Khalid M, Alqarni MH, Wahab S, Annadurai S, Alamri MA, Foudah AI, Aljarba TM, Akhtar J, Badruddeen, Ahmad S. Ameliorative Sexual Behavior and Phosphodiesterase-5 Inhibitory Effects of Spondias mangifera Fruit Extract in Rodents: In Silico, In Vitro, and In Vivo Study. Journal of Clinical Medicine. 2022; 11(13):3732. https://doi.org/10.3390/jcm11133732
Chicago/Turabian StyleKhalid, Mohammad, Mohammed H. Alqarni, Shadma Wahab, Sivakumar Annadurai, Mubarak A. Alamri, Ahmed I. Foudah, Tariq M. Aljarba, Juber Akhtar, Badruddeen, and Sarfaraz Ahmad. 2022. "Ameliorative Sexual Behavior and Phosphodiesterase-5 Inhibitory Effects of Spondias mangifera Fruit Extract in Rodents: In Silico, In Vitro, and In Vivo Study" Journal of Clinical Medicine 11, no. 13: 3732. https://doi.org/10.3390/jcm11133732
APA StyleKhalid, M., Alqarni, M. H., Wahab, S., Annadurai, S., Alamri, M. A., Foudah, A. I., Aljarba, T. M., Akhtar, J., Badruddeen, & Ahmad, S. (2022). Ameliorative Sexual Behavior and Phosphodiesterase-5 Inhibitory Effects of Spondias mangifera Fruit Extract in Rodents: In Silico, In Vitro, and In Vivo Study. Journal of Clinical Medicine, 11(13), 3732. https://doi.org/10.3390/jcm11133732






