Functional Implications of Estrogen and Progesterone Receptors Expression in Adenomyosis, Potential Targets for Endocrinological Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissues Collection
2.2. Total RNA Isolation
2.3. Reverse Transcription and Real-Time PCR
2.4. Immunohistochemistry
2.5. Quantification of IHC Stainings in Myometrium and Adenomyosis Tissues
2.6. Primary Cells Isolation
2.7. Cell Proliferation Assay
2.8. Explants Stimulations
2.9. Prolactin Secretion Measurements
2.10. Statistics
3. Results
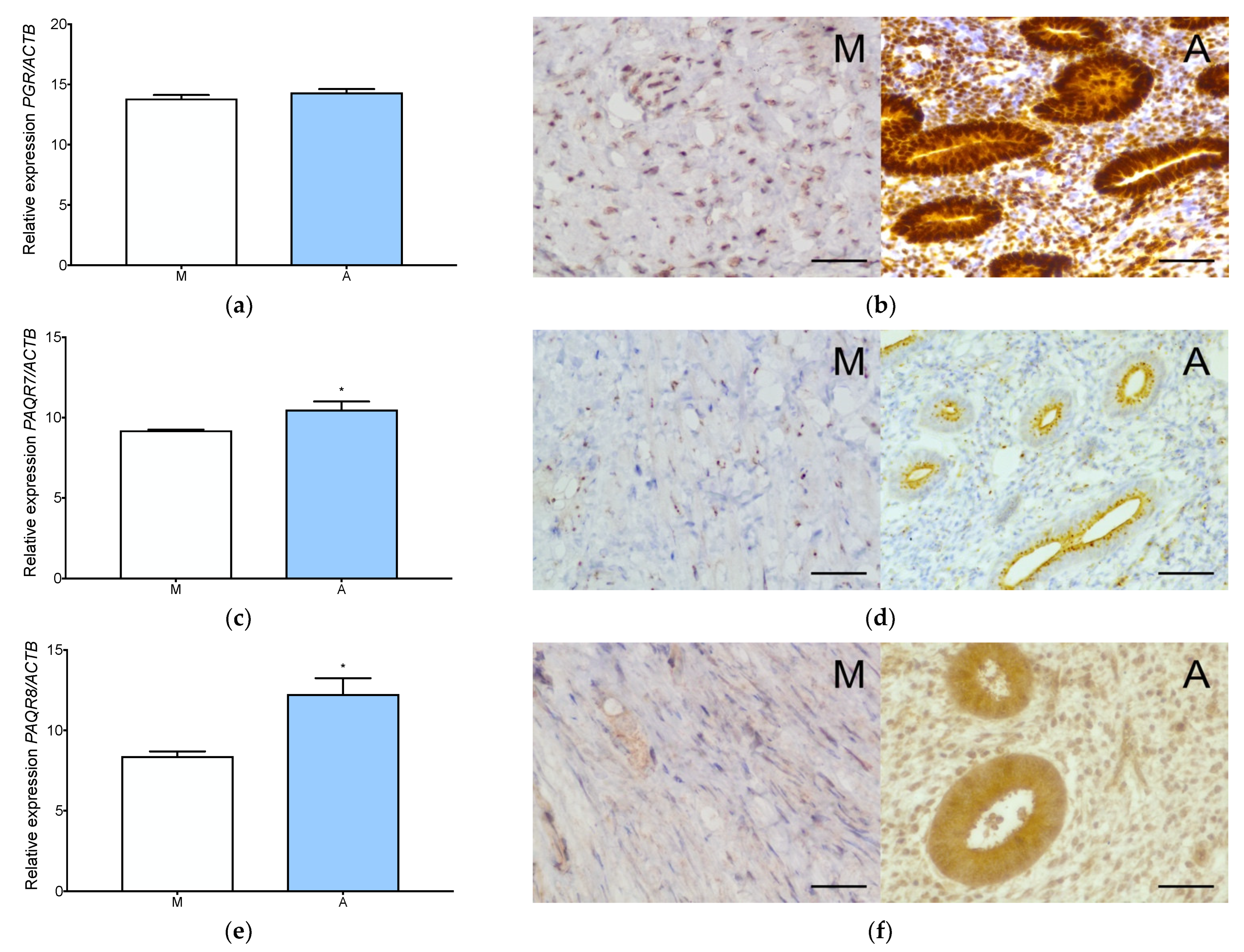
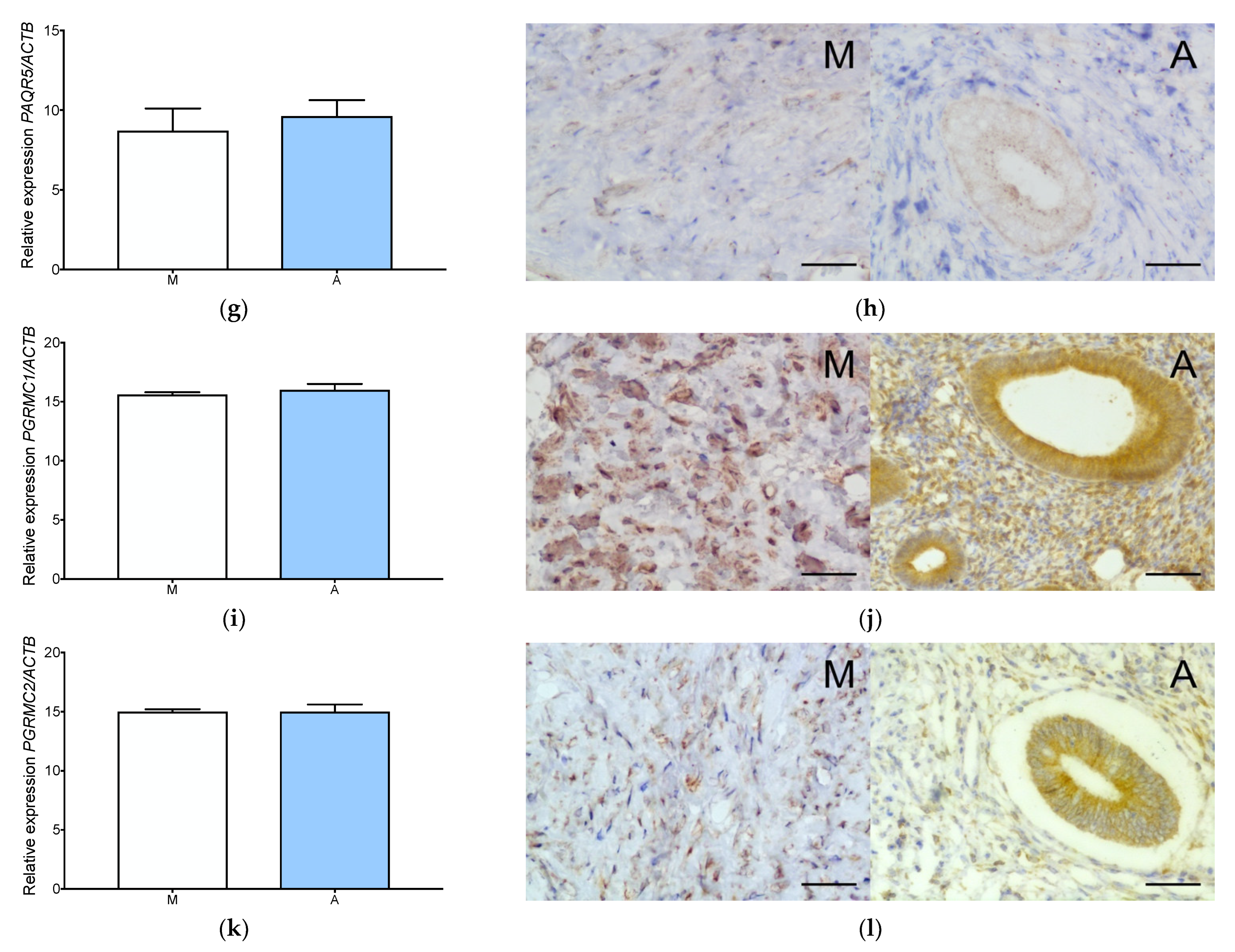
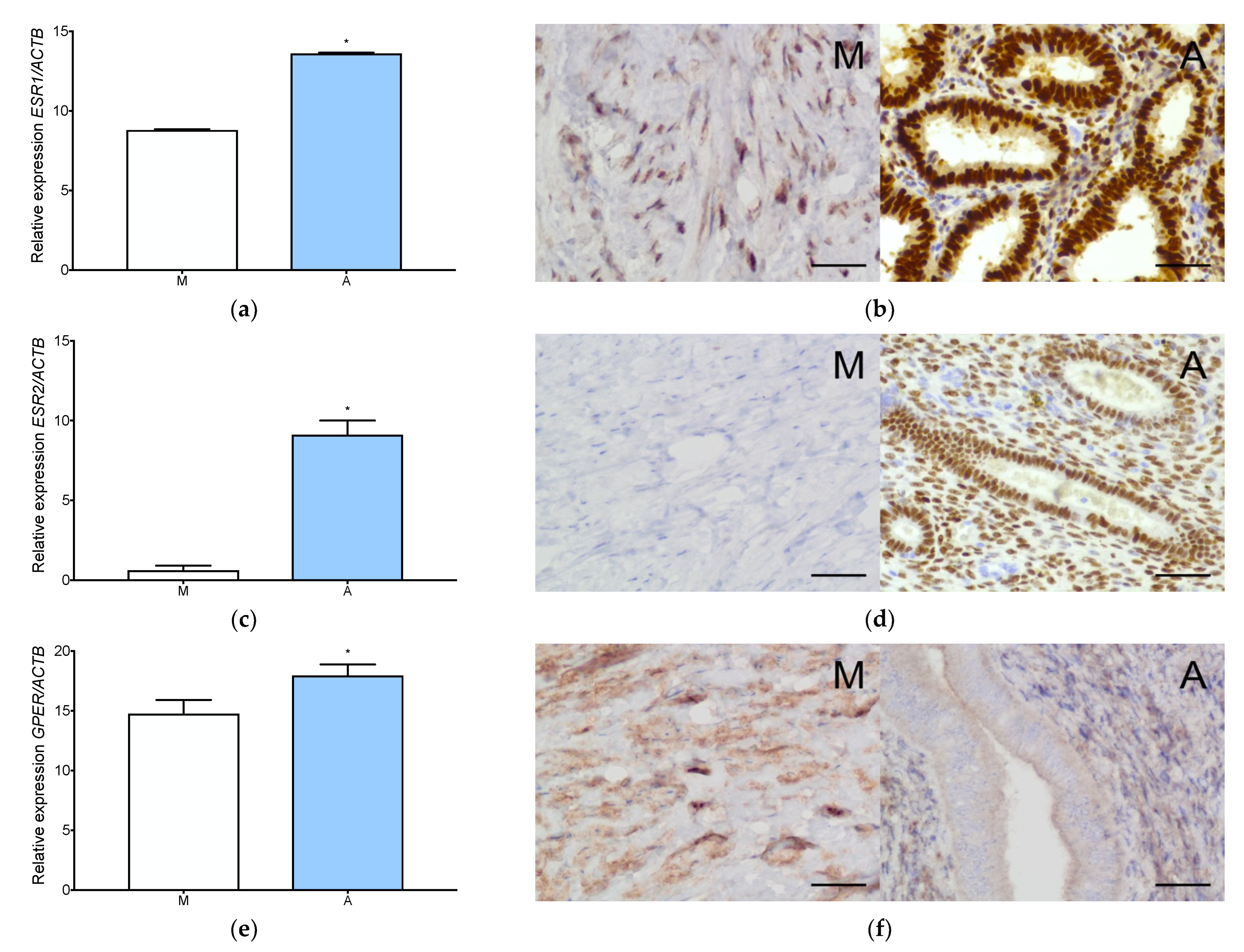
3.1. Estrogen and Progesterone Receptors Are Expressed in Adenomyosis and Normal Myometrium
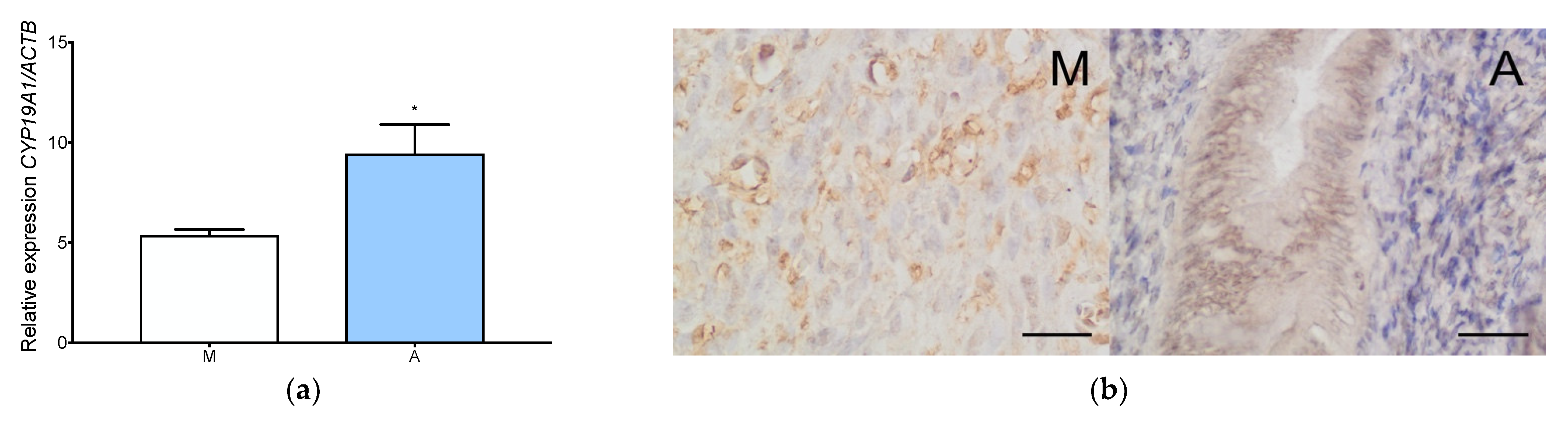
3.2. Aromatase Is Expressed Both in Adenomyosis and in the Normal Myometrium
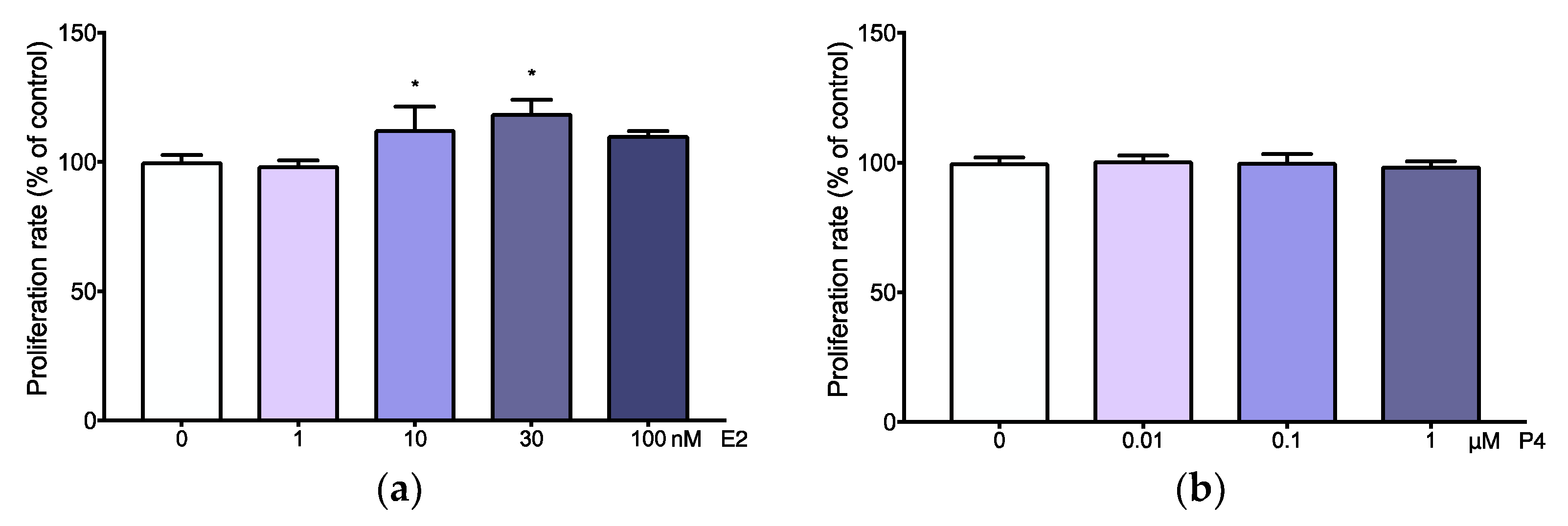
3.3. Adenomyotic Cell Proliferation Is Promoted by Physiological Levels of Estradiol
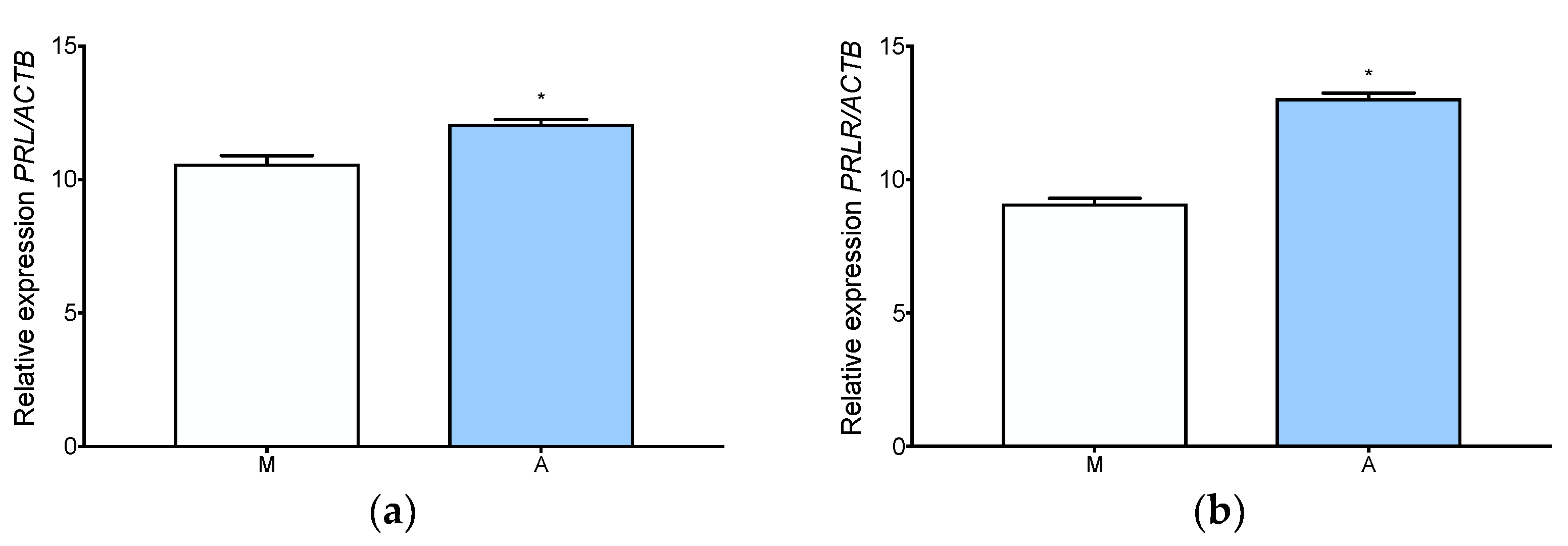
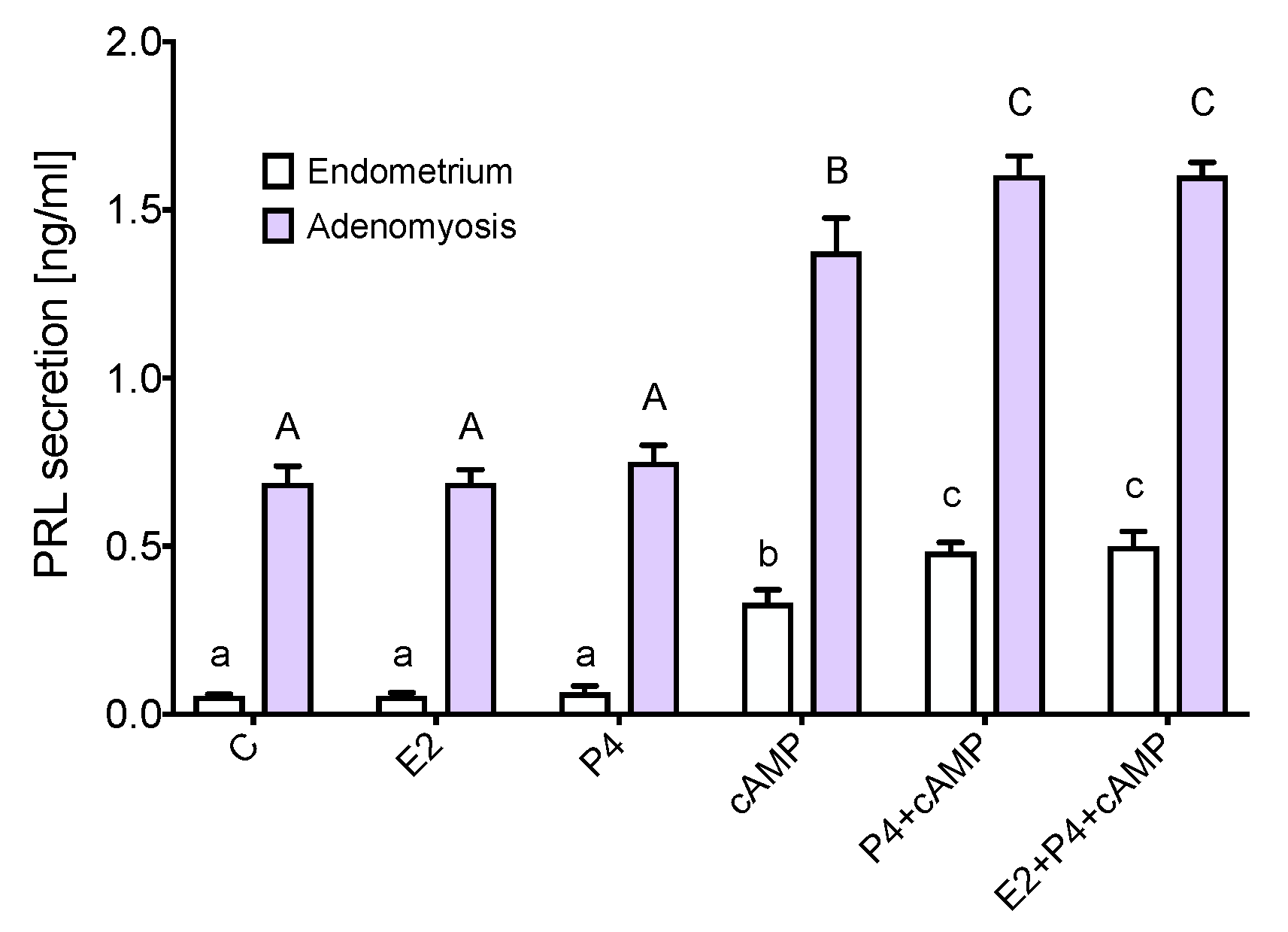
3.4. Prolactin Is Expressed and Secreted by Adenomyotic Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene | Primers Sequence (5′-3′) | Product Size (bp) | EMBL Accession Number |
|---|---|---|---|
| ACTB * | F: ACAGAGCCTCGCCTTTGC | 118 | NM_001101.3 |
| R: GAAGCCGGCCTTGCACAT | |||
| PGR | F: GAGCACTGGATGCTGTTGCT | 66 | NM_001202474.3 |
| R: GGCTTAGGGCTTGGCTTTC | |||
| MPRA | F: TGCCCTGCTGTGTGATCTTA | 113 | NM_178422.5 |
| R: ATAGCTGAGGCTCCTGGATG | |||
| MPRB | F: CGGTTGCATACCCTGTCCTG | 152 | NM_133367.4 |
| R: ATCTTGGGAAGCCCATCCTC | |||
| MPRG | F: ATTGTCCCAAGGCCTCAGAT | 108 | NM_001104554.1 |
| R: ATGCCATTCCAGTCAAATCC | |||
| PGRMC1 | F: TGCCTGGATAAGGAAGCACT | 120 | NM_006667.4 |
| R: GCCCACGTGATGATACTTGA | |||
| PGRMC2 | F: ATGGGAAAGTCTTCGACGTG | 106 | NM_006320.4 |
| R: CAAAATGTGGCCAGTCCTCT | |||
| ESR1 | F: CGCTACTGTGCAGTGTGCAAT | 64 | NM_000125 |
| R: CCTCACAGGACCAGACTCCATAA | |||
| ESR2 | F: GGTTCAAAGAGGGA TGCTCACT | 59 | NM_001291 723.1 |
| R: TGATATCCCGATGCGTAATCG | |||
| CYP19A1 | F: ACCCTTCTGCGTCGTGTCA | 240 | NM_000103 |
| R: TCTGTGGAAATCCTGCGTCT | |||
| PRL | F: GACCCTTCGAGACCTGTTTG | 104 | NM_000948.6 |
| R: GGCCATGGGTATACCGTTTAT | |||
| PRLR | F: CTCAGCCTACATCCAGGACAG | 130 | NM_000949.7 |
| R: CGGTTGTATCATTCATGGTG |


References
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Bergeron, C.; Amant, F.; Ferenczy, A. Pathology and physiopathology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L. A new concept of endometriosis and adenomyosis: Tissue injury and repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011, 5, 125–142. [Google Scholar] [CrossRef] [PubMed]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Kitawaki, J. Adenomyosis: The pathophysiology of an oestrogen-dependent disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Stratopoulou, C.A.; Dolmans, M.M. Uterine Adenomyosis: From Disease Pathogenesis to a New Medical Approach Using GnRH Antagonists. Int. J. Environ. Res. Public Health 2021, 18, 9941. [Google Scholar] [CrossRef]
- Zhou, W.J.; Yang, H.L.; Shao, J.; Mei, J.; Chang, K.K.; Zhu, R.; Li, M.Q. Anti-inflammatory cytokines in endometriosis. Cell Mol. Life Sci. 2019, 76, 2111–2132. [Google Scholar] [CrossRef] [PubMed]
- Mechsner, S.; Grum, B.; Gericke, C.; Loddenkemper, C.; Dudenhausen, J.W.; Ebert, A.D. Possible roles of oxytocin receptor and vasopressin-1α receptor in the pathomechanism of dysperistalsis and dysmenorrhea in patients with adenomyosis uteri. Fertil. Steril. 2010, 94, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Mehasseb, M.K.; Panchal, R.; Taylor, A.H.; Brown, L.; Bell, S.C.; Habiba, M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil. Steril. 2011, 95, 2228–2235.e1. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pulido, E.I.; Muñoz-Valle, J.F.; Del Toro-Arreola, S.; Jave-Suárez, L.F.; Bueno-Topete, M.R.; Estrada-Chávez, C.; Pereira-Suárez, A.L. High expression of prolactin receptor is associated with cell survival in cervical cancer cells. Cancer Cell Int. 2013, 13, 103. [Google Scholar] [CrossRef]
- Pontis, A.; D’Alterio, M.N.; Pirarba, S.; de Angelis, C.; Tinelli, R.; Angioni, S. Adenomyosis: A systematic review of medical treatment. Gynecol. Endocrinol. 2016, 32, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Szubert, M.; Koziróg, E.; Olszak, O.; Krygier-Kurz, K.; Kazmierczak, J.; Wilczynski, J. Adenomyosis and Infertility-Review of Medical and Surgical Approaches. Int. J. Environ. Res. Public Health 2021, 18, 1235. [Google Scholar] [CrossRef] [PubMed]
- Osada, H. Uterine adenomyosis and adenomyoma: The surgical approach. Fertil. Steril. 2018, 109, 406–417. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sharara, F.I.; Kheil, M.H.; Feki, A.; Rahman, S.; Klebanoff, J.S.; Ayoubi, J.M.; Moawad, G.N. Current and Prospective Treatment of Adenomyosis. J. Clin. Med. 2021, 10, 3410. [Google Scholar] [CrossRef] [PubMed]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.M. Conservative Management of Uterine Adenomyosis: Medical vs. Surgical Approach. J. Clin. Med. 2021, 10, 4878. [Google Scholar] [CrossRef]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.M. Origin and Pathogenic Mechanisms of Uterine Adenomyosis: What Is Known So Far. Reprod. Sci. 2021, 28, 2087–2097. [Google Scholar] [CrossRef]
- Stratopoulou, C.A.; Camboni, A.; Donnez, J.; Dolmans, M.M. Identifying Common Pathogenic Features in Deep Endometriotic Nodules and Uterine Adenomyosis. J. Clin. Med. 2021, 10, 4585. [Google Scholar] [CrossRef]
- Garavaglia, E.; Audrey, S.; Annalisa, I.; Stefano, F.; Iacopo, T.; Laura, C.; Massimo, C. Adenomyosis and its impact on women fertility. Iran. J. Reprod. Med. 2015, 13, 327–336. [Google Scholar]
- Ponikwicka-Tyszko, D.; Chrusciel, M.; Stelmaszewska, J.; Bernaczyk, P.; Sztachelska, M.; Sidorkiewicz, I.; Doroszko, M.; Tomaszewski, J.; Tapanainen, J.S.; Huhtaniemi, I.; et al. Functional Expression of FSH Receptor in Endometriotic Lesions. J. Clin. Endocrinol. Metab. 2016, 101, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Murakami, T.; Uehara, S.; Canis, M.; Sasano, H.; Okamura, K. Expression of estrogen receptor alpha and beta in peritoneal and ovarian endometriosis. Fertil. Steril. 2001, 75, 1198–1205. [Google Scholar] [CrossRef]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of estrogen receptor-β in endometriosis. Semin. Reprod. Med. 2012, 30, 39–45. [Google Scholar] [CrossRef]
- Grings, A.O.; Lora, V.; Ferreira, G.D.; Brum, I.S.; Corleta, H.; Capp, E. Protein expression of estrogen receptors α and β and aromatase in myometrium and uterine leiomyoma. Gynecol. Obstet. Investig. 2012, 73, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Mehasseb, M.K.; Bell, S.C.; Brown, L.; Pringle, J.H.; Habiba, M. Phenotypic characterisation of the inner and outer myometrium in normal and adenomyotic uteri. Gynecol. Obstet. Investig. 2011, 71, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.E.; Peterson, T.J.; Tong, M.H.; Lee, E.J.; Pfaff, L.E.; Hewitt, S.C.; Korach, K.S.; Weiss, J.; Jameson, J.L. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J. Biol. Chem. 2006, 281, 26683–26692. [Google Scholar] [CrossRef] [PubMed]
- Sztachelska, M.; Ponikwicka-Tyszko, D.; Sokolowska, G.; Anisimowicz, S.; Czerniecki, J.; Lebiedzinska, W.; Zbucka-Kretowska, M.; Zygmunt, M.; Wołczynski, S.; Pierzynski, P. Oxytocin antagonism reverses the effects of high oestrogen levels and oxytocin on decidualization and cyclooxygenase activity in endometrial tissues. Reprod. Biomed. Online 2019, 39, 737–744. [Google Scholar] [CrossRef]
- Pupo, M.; Maggiolini, M.; Musti, A.M. GPER Mediates Non-Genomic Effects of Estrogen. Methods Mol. Biol. 2016, 1366, 471–488. [Google Scholar] [CrossRef]
- Plante, B.J.; Lessey, B.A.; Taylor, R.N.; Wang, W.; Bagchi, M.K.; Yuan, L.; Scotchie, J.; Fritz, M.A.; Young, S.L. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod. Sci. 2012, 19, 684–693. [Google Scholar] [CrossRef]
- Samartzis, N.; Samartzis, E.P.; Noske, A.; Fedier, A.; Dedes, K.J.; Caduff, R.; Fink, D.; Imesch, P. Expression of the G protein-coupled estrogen receptor (GPER) in endometriosis: A tissue microarray study. Reprod. Biol. Endocrinol. 2012, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Paul, J.W.; Read, M.; Chan, E.C.; Riley, S.C.; Nahar, P.; Smith, R. G-1-activated membrane estrogen receptors mediate increased contractility of the human myometrium. Endocrinology 2011, 152, 2448–2455. [Google Scholar] [CrossRef]
- Madej, P.; Plewka, A.; Plewka, D.; Paleń, P.; Nowaczyk, G.; Bogunia, E.; Marczyński, J.; Waloszek, J. The aromatase expression in myomas and myometriums of women in reproduction and perimenopausal age. Folia Histochem. Cytobiol. 2009, 47, 497–504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urabe, M.; Yamamoto, T.; Kitawaki, J.; Honjo, H.; Okada, H. Estrogen biosynthesis in human uterine adenomyosis. Acta Endocrinol. 1989, 121, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kitawaki, J.; Noguchi, T.; Amatsu, T.; Maeda, K.; Tsukamoto, K.; Yamamoto, T.; Fushiki, S.; Osawa, Y.; Honjo, H. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol. Reprod. 1997, 57, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. The Genetic Background of Endometriosis: Can ESR2 and CYP19A1 Genes Be a Potential Risk Factor for Its Development? Int. J. Mol. Sci. 2020, 21, 8235. [Google Scholar] [CrossRef]
- Maia, H., Jr.; Haddad, C.; Casoy, J. Correlation between aromatase expression in the eutopic endometrium of symptomatic patients and the presence of endometriosis. Int. J. Women’s Health 2012, 4, 61–65. [Google Scholar] [CrossRef][Green Version]
- MacLean, J.A., 2nd; Hayashi, K. Progesterone Actions and Resistance in Gynecological Disorders. Cells 2022, 11, 647. [Google Scholar] [CrossRef]
- Arnett-Mansfield, R.L.; DeFazio, A.; Mote, P.A.; Clarke, C.L. Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J. Clin. Endocrinol. Metab. 2004, 89, 1429–1442. [Google Scholar] [CrossRef]
- Lim, C.S.; Baumann, C.T.; Htun, H.; Xian, W.; Irie, M.; Smith, C.L.; Hager, G.L. Differential localization and activity of the A- and B-forms of the human progesterone receptor using green fluorescent protein chimeras. Mol. Endocrinol. 1999, 13, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 2015, 21, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Jichan, N.; Xishi, L.; Guo, S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod. Sci. 2010, 17, 995–1005. [Google Scholar] [CrossRef]
- Che, X.; Wang, J.; He, J.; Guo, X.; Li, T.; Zhang, X. The new application of mifepristone in the relief of adenomyosis-caused dysmenorrhea. Int. J. Med. Sci. 2020, 17, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sinreih, M.; Knific, T.; Thomas, P.; Frković Grazio, S.; Rižner, T.L. Membrane progesterone receptors β and γ have potential as prognostic biomarkers of endometrial cancer. J. Steroid Biochem. Mol. Biol. 2018, 178, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Martínez, E.R.; Bello-Alvarez, C.; Hermenegildo-Molina, A.L.; Solís-Paredes, M.; Parra-Hernández, S.; Cruz-Orozco, O.; Silvestri-Tomassoni, J.R.; Escobar-Ponce, L.F.; Hernández-López, L.A.; Reyes-Mayoral, C.; et al. Expression of Membrane Progesterone Receptors in Eutopic and Ectopic Endometrium of Women with Endometriosis. BioMed Res. Int. 2020, 2020, 2196024. [Google Scholar] [CrossRef]
- Bunch, K.; Tinnemore, D.; Huff, S.; Hoffer, Z.S.; Burney, R.O.; Stallings, J.D. Expression patterns of progesterone receptor membrane components 1 and 2 in endometria from women with and without endometriosis. Reprod. Sci. 2014, 21, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Keator, C.S.; Mah, K.; Slayden, O.D. Alterations in progesterone receptor membrane component 2 (PGRMC2) in the endometrium of macaques afflicted with advanced endometriosis. Mol. Hum. Reprod. 2012, 18, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Salsano, S.; González-Martín, R.; Quiñonero, A.; Pérez-Debén, S.; Domínguez, F. Deciphering the Role of PGRMC1 During Human Decidualization Using an In Vitro Approach. J. Clin. Endocrinol. Metab. 2021, 106, 2313–2327. [Google Scholar] [CrossRef]
- Brar, A.K.; Frank, G.R.; Kessler, C.A.; Cedars, M.I.; Handwerger, S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 1997, 6, 301–307. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Del Vecchio, G.; Scairati, R.; Pirchio, R.; Liccardi, A.; Verde, N.; de Angelis, C.; Menafra, D.; Pivonello, C.; Conforti, A.; et al. The Interplay Between Prolactin and Reproductive System: Focus on Uterine Pathophysiology. Front. Endocrinol. 2020, 11, 594370. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Nagasawa, H.; Takahashi, S. The induction of adenomyosis in mice by intrauterine pituitary isografts. Life Sci. 1981, 29, 1277–1282. [Google Scholar] [CrossRef]
- Mori, T.; Nagasawa, H. Mechanisms of development of prolactin-induced adenomyosis in mice. Acta Anat. 1983, 116, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Łupicka, M.; Socha, B.M.; Szczepańska, A.A.; Korzekwa, A.J. Prolactin role in the bovine uterus during adenomyosis. Domest. Anim. Endocrinol. 2017, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gadó, K.; Pállinger, E.; Kovács, P.; Takács, E.; Szilvási, I.; Tóth, B.E.; Nagy, G.; Domján, G.; Falus, A. Prolactin influences proliferation and apoptosis of a human IgE secreting myeloma cell line, U266. Immunol. Lett. 2002, 82, 191–196. [Google Scholar] [CrossRef]
- Gill, S.; Peston, D.; Vonderhaar, B.K.; Shousha, S. Expression of prolactin receptors in normal, benign, and malignant breast tissue: An immunohistological study. J. Clin. Pathol. 2001, 54, 956–960. [Google Scholar] [CrossRef]
- Yorganci, A.; Kadioğlu, N.; Gümgümcü, H.; Özyer, Ş.; Engin-Ustun, Y. Serum prolactin and CA 125 levels in uterine adenomyosis. J. Endometr. Pelvic Pain Disord. 2020, 12, 165–169. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztachelska, M.; Ponikwicka-Tyszko, D.; Martínez-Rodrigo, L.; Bernaczyk, P.; Palak, E.; Półchłopek, W.; Bielawski, T.; Wołczyński, S. Functional Implications of Estrogen and Progesterone Receptors Expression in Adenomyosis, Potential Targets for Endocrinological Therapy. J. Clin. Med. 2022, 11, 4407. https://doi.org/10.3390/jcm11154407
Sztachelska M, Ponikwicka-Tyszko D, Martínez-Rodrigo L, Bernaczyk P, Palak E, Półchłopek W, Bielawski T, Wołczyński S. Functional Implications of Estrogen and Progesterone Receptors Expression in Adenomyosis, Potential Targets for Endocrinological Therapy. Journal of Clinical Medicine. 2022; 11(15):4407. https://doi.org/10.3390/jcm11154407
Chicago/Turabian StyleSztachelska, Maria, Donata Ponikwicka-Tyszko, Lydia Martínez-Rodrigo, Piotr Bernaczyk, Ewelina Palak, Weronika Półchłopek, Tomasz Bielawski, and Sławomir Wołczyński. 2022. "Functional Implications of Estrogen and Progesterone Receptors Expression in Adenomyosis, Potential Targets for Endocrinological Therapy" Journal of Clinical Medicine 11, no. 15: 4407. https://doi.org/10.3390/jcm11154407
APA StyleSztachelska, M., Ponikwicka-Tyszko, D., Martínez-Rodrigo, L., Bernaczyk, P., Palak, E., Półchłopek, W., Bielawski, T., & Wołczyński, S. (2022). Functional Implications of Estrogen and Progesterone Receptors Expression in Adenomyosis, Potential Targets for Endocrinological Therapy. Journal of Clinical Medicine, 11(15), 4407. https://doi.org/10.3390/jcm11154407






